Abstract
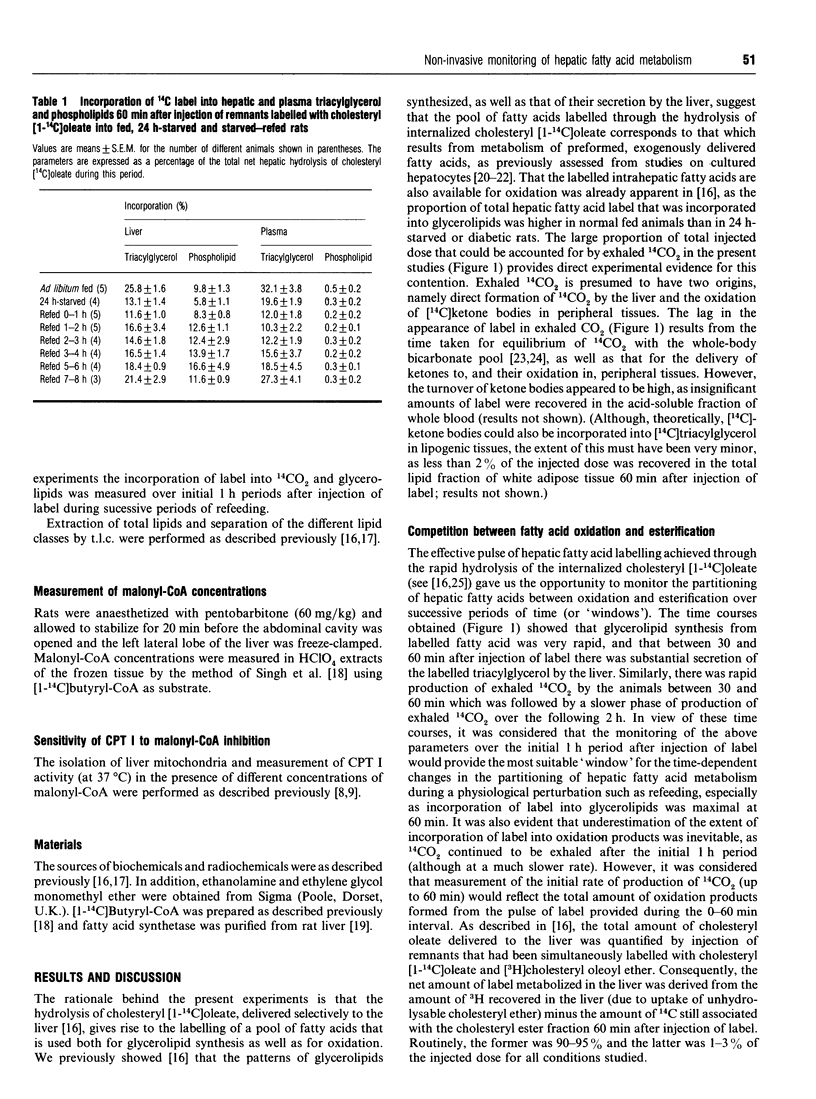
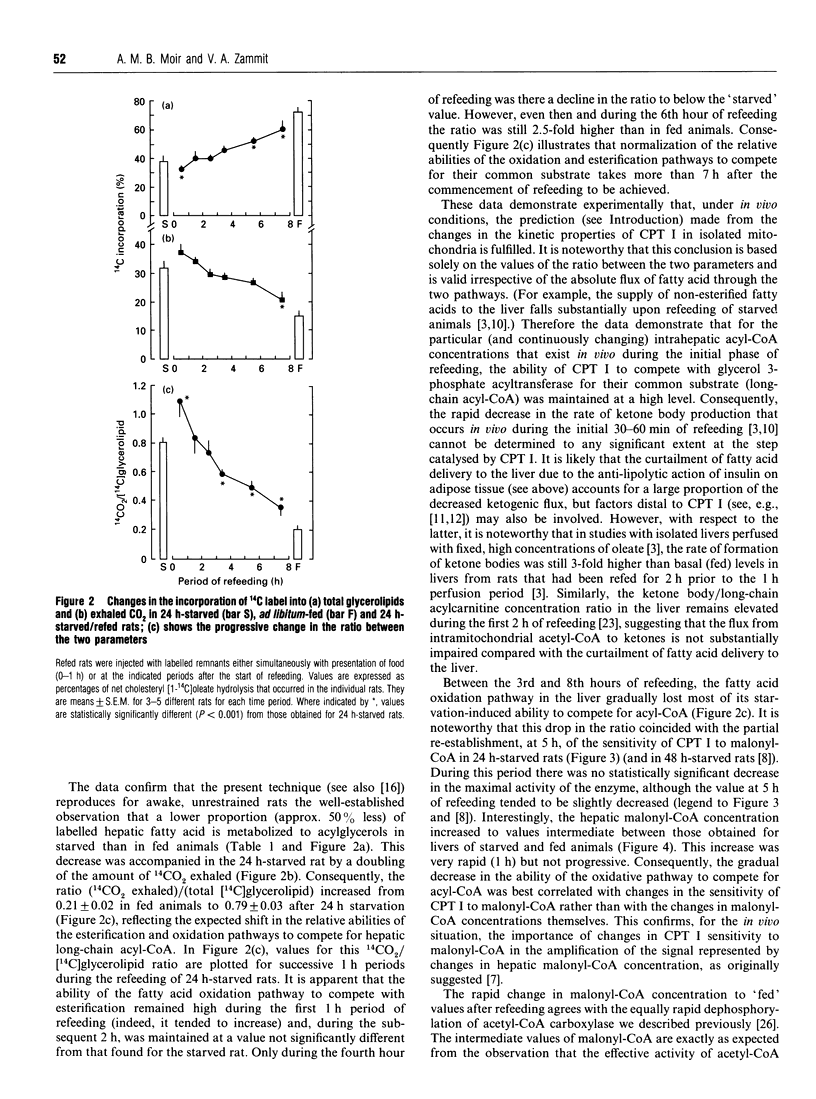
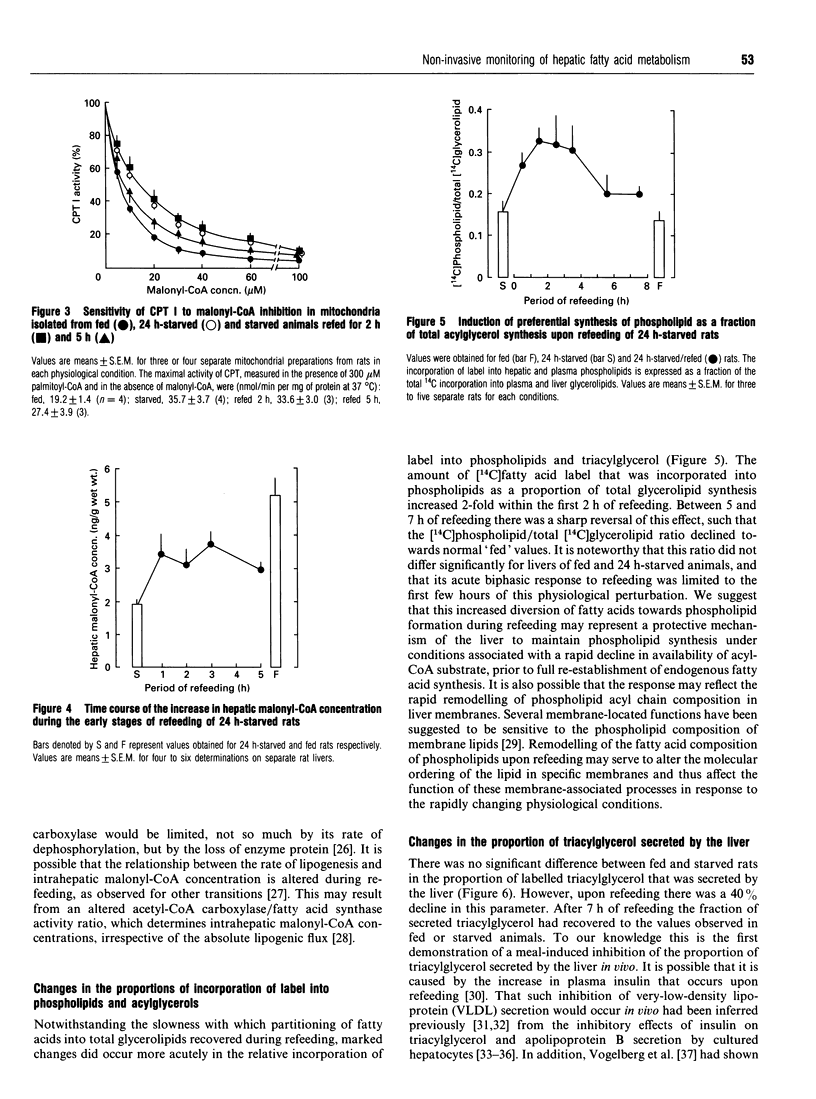
1. The technique of selective labelling of hepatic fatty acids in vivo [Moir and Zammit (1992) Biochem. J. 283, 145-149] has been used to monitor non-invasively the metabolism of fatty acids in the livers of awake unrestrained rats during the starved-to-refed transition. Values for the incorporation of labelled fatty acid into liver and plasma glycerolipids and into exhaled carbon dioxide after injection of labelled lipoprotein and Triton WR 1339 into rats with chronically cannulated jugular veins were obtained for successive 1 h periods from the start of refeeding of 24 h-starved rats. 2. Starvation for 24 h resulted in marked and reciprocal changes in the incorporation of label into glycerolipids and exhaled 14CO2, such that a 4-fold higher value was obtained for the oxidation/esterification ratio in livers of starved rats compared with fed animals. 3. Refeeding of starved rats did not return this ratio to the value observed for fed animals for at least 7 h; during the first 3 h of refeeding the ratio was at least as high as that for starved rats. Between 4 h and 6 h of refeeding the ratio was still approx. 70% of that in starved animals, and 2.5-fold higher than in fed rats. 4. These data support the hypothesis that the capacity of the liver to oxidize fatty acids is maintained at a high level during the initial stages of refeeding [Grantham and Zammit (1986) Biochem. J. 239, 485-488] and that control of the flux of hepatic fatty acids into the oxidative pathway is largely lost from the reaction catalysed by mitochondrial overt carnitine palmitoyltransferase (CPT I) during this phase of recovery from the starved state. 5. Refeeding also resulted in a rapid (< 1 h) increase in hepatic malonyl-CoA concentrations to values intermediate between those in livers of fed and starved animals. The sensitivity of CPT I to malonyl-CoA inhibition in isolated liver mitochondria was only partially reversed even after 5 h of refeeding. 6. Refeeding resulted in an acute 35% inhibition of the fraction of synthesized triacylglycerol that was secreted into the plasma; the maximal effect occurred 2-3 h after the start of refeeding. The inhibition of the fractional secretion rate was fully reversed after 5 h of refeeding. 7. The amount of 14C label that was incorporated into phospholipids as a fraction of total glycerolipid synthesis was doubled within 2 h of the start of refeeding.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brindle N. P., Zammit V. A., Pogson C. I. Regulation of carnitine palmitoyltransferase activity by malonyl-CoA in mitochondria from sheep liver, a tissue with a low capacity for fatty acid synthesis. Biochem J. 1985 Nov 15;232(1):177–182. doi: 10.1042/bj2320177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C. D., Brindle N. P., Wang T. W., Hales C. N. Interaction of non-esterified fatty acid and insulin in control of triacylglycerol secretion by Hep G2 cells. Biochem J. 1991 Nov 15;280(Pt 1):99–104. doi: 10.1042/bj2800099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N., Williams D. L., Alaupovic P. Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res. 1989 Sep;30(9):1365–1373. [PubMed] [Google Scholar]
- Duerden J. M., Bartlett S. M., Gibbons G. F. Long-term maintenance of high rates of very-low-density-lipoprotein secretion in hepatocyte cultures. A model for studying the direct effects of insulin and insulin deficiency in vitro. Biochem J. 1989 Nov 1;263(3):937–943. doi: 10.1042/bj2630937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Storage, mobilization and secretion of cytosolic triacylglycerol in hepatocyte cultures. The role of insulin. Biochem J. 1990 Dec 15;272(3):583–587. doi: 10.1042/bj2720583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem J. 1990 May 15;268(1):1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Burnham F. J. Effect of nutritional state on the utilization of fatty acids for hepatitic triacylglycerol synthesis and secretion as very-low-density lipoprotein. Biochem J. 1991 Apr 1;275(Pt 1):87–92. doi: 10.1042/bj2750087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. C., Zammit V. A., Robinson D. S. The preferential uptake of very-low-density lipoprotein cholesteryl ester by rat liver in vivo. Biochem J. 1990 Dec 15;272(3):735–741. doi: 10.1042/bj2720735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Schofield P. S., Sugden M. C. The relationship between fat synthesis and oxidation in the liver after re-feeding and its regulation by thyroid hormone. Biochem J. 1987 Nov 1;247(3):621–626. doi: 10.1042/bj2470621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Sugden M. C. Hepatic glycogen synthesis on carbohydrate re-feeding after starvation. A regulatory role for pyruvate dehydrogenase in liver and extrahepatic tissues. Biochem J. 1986 Apr 15;235(2):441–445. doi: 10.1042/bj2350441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej M. P., Zammit V. A. Sensitivity of inhibition of rat liver mitochondrial outer-membrane carnitine palmitoyltransferase by malonyl-CoA to chemical- and temperature-induced changes in membrane fluidity. Biochem J. 1990 Dec 1;272(2):421–425. doi: 10.1042/bj2720421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C. Purification and crystallization of rat liver fatty acid synthetase. Arch Biochem Biophys. 1981 Jul;209(2):613–619. doi: 10.1016/0003-9861(81)90320-9. [DOI] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. Succinylation and inactivation of 3-hydroxy-3-methylglutaryl-CoA synthase by succinyl-CoA and its possible relevance to the control of ketogenesis. Biochem J. 1985 Nov 15;232(1):37–42. doi: 10.1042/bj2320037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS B., SIMPSON-MORGAN M. W. THE EXCRETION OF 14CO2 DURING THE CONTINUOUS INTRAVENOUS INFUSION OF NAH-14CO3 IN UNANAESTHETIZED RATS. J Physiol. 1963 Dec;169:713–728. doi: 10.1113/jphysiol.1963.sp007291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Changes in the properties of cytosolic acetyl-CoA carboxylase studied in cold-clamped liver samples from fed, starved and starved-refed rats. Biochem J. 1990 Dec 1;272(2):511–517. doi: 10.1042/bj2720511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Selective labelling of hepatic fatty acids in vivo. Studies on the synthesis and secretion of glycerolipids in the rat. Biochem J. 1992 Apr 1;283(Pt 1):145–149. doi: 10.1042/bj2830145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner C. B., Gilboe D. P., Nuttall F. Q. Metabolic effects of oral glucose in the liver of fasted rats. Am J Physiol. 1984 Jan;246(1 Pt 1):E89–E94. doi: 10.1152/ajpendo.1984.246.1.E89. [DOI] [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quant P. A., Tubbs P. K., Brand M. D. Treatment of rats with glucagon or mannoheptulose increases mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase activity and decreases succinyl-CoA content in liver. Biochem J. 1989 Aug 15;262(1):159–164. doi: 10.1042/bj2620159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. S., Sugden M. C., Corstorphine C. G., Zammit V. A. Altered interactions between lipogenesis and fatty acid oxidation in regenerating rat liver. Biochem J. 1987 Jan 15;241(2):469–474. doi: 10.1042/bj2410469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Stakkestad J. A., Bremer J., Borrebaek B. Determination of malonyl-coenzyme A in rat heart, kidney, and liver: a comparison between acetyl-coenzyme A and butyryl-coenzyme A as fatty acid synthase primers in the assay procedure. Anal Biochem. 1984 Apr;138(1):107–111. doi: 10.1016/0003-2697(84)90776-0. [DOI] [PubMed] [Google Scholar]
- Sparks J. D., Sparks C. E. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem. 1990 May 25;265(15):8854–8862. [PubMed] [Google Scholar]
- Sparks J. D., Sparks C. E., Miller L. L. Insulin effects on apolipoprotein B production by normal, diabetic and treated-diabetic rat liver and cultured rat hepatocytes. Biochem J. 1989 Jul 1;261(1):83–88. doi: 10.1042/bj2610083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelberg K. H., Gries F. A., Moschinski D. Hepatic production of VLDL-triglycerides. Dependence of portal substrate and insulin concentration. Horm Metab Res. 1980 Dec;12(12):688–694. doi: 10.1055/s-2007-999233. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Burke J. F. Effect of burn trauma on glucose turnover, oxidation, and recycling in guinea pigs. Am J Physiol. 1977 Aug;233(2):E80–E85. doi: 10.1152/ajpendo.1977.233.2.E80. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M. The roles of different protein kinases and of calmodulin in the effects of Ca2+ mobilization on 3-hydroxy-3-methylglutaryl-CoA reductase activity in isolated rat hepatocytes. Biochem J. 1991 Jan 15;273(Pt 2):485–488. doi: 10.1042/bj2730485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]


