Abstract
Background & Aims
Wilson disease (WD) is caused by accumulation of copper primarily in the liver and brain. During maintenance therapy of WD with D-penicillamine, current guidelines recommend on-treatment ranges of urinary copper excretion (UCE) of 200-500 μg/24 h and serum non-ceruloplasmin-bound copper (NCC) of 50-150 μg/L. We compared NCC (measured by two novel assays) and UCE from patients with clinically stable WD on D-penicillamine therapy with these recommendations.
Methods
This is a secondary analysis of data from the Chelate trial (NCT03539952) that enrolled physician-selected patients with clinically stable WD on D-penicillamine maintenance therapy (at an unaltered dose for at least 4 months). We analyzed laboratory samples from the first screening visit, prior to interventions. NCC was measured by either protein speciation (NCC-Sp) using anion exchange high-performance liquid chromatography protein speciation followed by copper determination with inductively coupled plasma mass spectroscopy or as exchangeable copper (NCC-Ex). NCC-Sp was also analyzed in healthy controls (n = 75).
Results
In 76 patients with WD with 21.3±14.3 average treatment-years, NCC-Sp (mean±SD: 56.6±26.2 μg/L) and NCC-Ex (mean±SD: 57.9±24.7 μg/L) were within the 50-150 μg/L target in 61% and 54% of patients, respectively. In addition, 36% and 31%, respectively, were even below the normal ranges (NCC-Sp: 46-213 μg/L, NCC-Ex: 41-71 μg/L). NCC-Ex positively correlated with NCC-Sp (r2 = 0.66, p <0.001) but with systematic deviation. UCE was outside the 200-500 μg/24 h target range in 58%. Only 14/69 (20%) fulfilled both the NCC-Sp and UCE targets. Clinical or biochemical signs of copper deficiency were not detected.
Conclusion
Clinically stable patients with WD on maintenance D-penicillamine therapy frequently have lower NCC-Sp or higher UCE than current recommendations without signs of overtreatment. Further studies are warranted to identify appropriate target ranges of NCC-Sp, NCC-Ex and UCE in treated WD.
Impact and implications:
Chelator treatment of patients with Wilson disease (WD) is currently guided by measurements of non-ceruloplasmin-bound copper (NCC) and 24 h urinary copper excretion (UCE) but validation is limited. In 76 adults with ≈21 years history of treated WD and clinically stable disease on D-penicillamine therapy, NCC was commonly found to be below normal values and recommended target ranges whether measured by protein speciation (NCC-Sp) or as exchangeable copper (NCC-Ex), while UCE values were above the recommended target range in 49%. Common wisdom would suggest overtreatment in these cases, but no clinical or biochemical signs of copper deficiency were observed. Exploratory analysis of liver enzymes suggested that NCC below levels seen in controls may be beneficial, while the relation to UCE was less clear. The data calls for critical re-evaluation of target ranges for treatment of WD, specific for drug and laboratory methodology.
Clinical trial number
Keywords: Exchangeable copper, bioavailable copper, free serum copper, protein speciation
Graphical abstract
Highlights:
-
•
NCC assessed by two novel methods (NCC-Sp and NCC-Ex) and UCE were examined in 76 patients with stable WD on D-PEN.
-
•
In WD, NCC-Sp using protein speciation and ICP-MS or NCC-Ex (exchangeable Cu) correlated but with systematic deviation.
-
•
Normal ranges for NCC-Sp (46-213 μg/L) and NCC-Ex (41-71 μg/L) differed from older methods (50-150 μg/L).
-
•
In WD, NCC-Sp or NCC-Ex were often below and UCE often outside the target ranges recommended in guidelines.
-
•
NCC-Sp or NCC-Ex below 50 μg/L was associated with tighter control of liver biochemistry but not copper deficiency.
Introductions
Wilson disease (WD) is a rare autosomal recessively inherited disease affecting copper metabolism. Mutations in the ATB7B gene lead to dysfunction of the ATP7B protein (OMIM: 606882; Cu (2+)-transporting, beta polypeptide) that regulates the homeostasis of copper via hepatic excretion of excess copper into bile, and is responsible for the biosynthetic incorporation of copper into ceruloplasmin (Cp). Accumulated copper causes organ damage primarily in the liver and brain.
WD is fatal if left untreated. Globally, the most commonly used drug is D-penicillamine (PEN) that was introduced in the 1960s.1 At that time the focus of medical treatment was on whole body copper balance.2 The initial treatment goal was to create a negative copper balance, and the goal of long-term maintenance therapy to secure a level of excretion that balanced with dietary intake, preventing further accumulation of copper. The treatment effect was evaluated by clinical assessment and monitoring of the 24-hour urinary excretion of copper (UCE). After the very high values of UCE typically observed during the initial (de-coppering) phase of treatment, a target UCE of 200-500 μg/24 h was expected in the maintenance phase of chelation therapy2,3 and continues to be recommended by consensus expert opinion.4,5
Under physiologic conditions, most of the circulating copper is irreversibly bound to Cp. A minor fraction of plasma copper loosely bound to albumin, amino acids, and peptides6 is bioavailable for use in physiological processes. This bioavailable fraction was named the non-ceruloplasmin-bound copper (NCC), and the elevated levels found in untreated or ineffectively treated patients is assumed to be responsible for pathologic organ copper loading. Thus, “normalization” of NCC was proposed as an additional aim of treatment.3
At that time, NCC was assessed by measurement of serum copper and Cp. Based on the assumption of 6 copper atoms per Cp molecule, NCC-Cal (μg/L) was assumed to equal total serum copper (μg/L) minus 3.14 times Cp (mg/L).7 The normal range in healthy persons of 50-150 μg/L (0.8-2.4 μmol/L) became the treatment target in WD3 and is still used in guidelines.4,5,8,9 As expected, NCC-Cal was reported to be high at the time of diagnosis and decline with treatment.10 The NCC-Cal methodology has since been questioned for several reasons. (A) Even though NCC-Cal was probably more accurate when Cp was measured by an enzymatic assay (as Cp dependents on copper for its oxidase activity) than the more commonly utilized immunological method (the latter cannot discriminate copper-containing holoceruloplasmin from its copper-free precursor peptide, apoceruloplasmin7), most centers found the enzymatic method too demanding to be feasible for routine use. A high inter-laboratory variance and up to 20% biologically impossible zero or negative NCC-Cal values were reported.7,11,12 (B) The assumption of 6 copper atoms per molecule of Cp may be wrong.[13], [14], [15] (C) NCC-Cal was unrelated to adherence to treatment.16 For these reasons, recent guidelines advise against the use of NCC-Cal.5
To overcome these challenges, El Balkhi et al.17 developed the exchangeable copper assay, NCC-Ex, which does not require measurement of Cp. The chelating agent EDTA is used to chelate copper from the NCC pool and after separation from Cp by ultrafiltration, NCC-Ex is assessed by measurement of copper in the filtrate. The published normal range of NCC-Ex is 41-71 μg/L (0.64-1.12 μmol/L) in healthy adults17 and slightly lower in children.18 There are some data to support the use of NCC-Ex as a biomarker to monitor treatment in WD: NCC-Ex is elevated at time of diagnosis of WD,19 more so in those with extrahepatic disease,20 and decreases during treatment.19,21 Maintenance of a lower NCC-Ex is associated with treatment adherence.19,22 Preliminary data suggest higher NCC-Ex in WD presenting with acute liver failure.23 Accordingly, in untreated LEC rats, a WD model, NCC-Ex increased in parallel with disease progression.24 However, recent reports suggest that NCC-Ex may be subject to both under- and overestimation of NCC by either formation of a high molecular EDTA-copper-protein complex that is trapped in the filter25 or removal of some Cp-bound copper by EDTA.26
When the recent Chelate trial27 planned to use NCC as a primary endpoint, regulatory authorities did not accept NCC-Cal or NCC-Ex for the reasons mentioned above. Consequently, a new method was developed and implemented.25,26 With this method, serum proteins are separated by anion exchange high-performance liquid chromatography (protein speciation) and the copper content in each peak is measured by inductively coupled plasma mass spectrometry (ICP-MS). “NCC by protein speciation”, NCC-Sp, is then calculated as total Serum-Cu x (1 – Cp Cu fraction).25
In the present study, we wanted to examine the distribution of NCC values measured by modern techniques and UCE in the “real world” clinical situation, where experienced clinicians judged the maintenance treatment dose of PEN optimal without the need for adjustments. With this goal in mind, we performed a secondary analysis of data from the Chelate trial27 to examine levels of NCC and UCE in patients with WD selected by experienced clinicians as clinically stable in their maintenance phase of therapy. On the first visit, while patients were still on their pre-study PEN medication, NCC-Sp, NCC-Ex, and UCE were assessed. These data provided an opportunity to examine levels of NCC-Sp, NCC-Ex, and UCE present in clinically determined stable patients receiving maintenance therapy with PEN and to assess how newer methodologies for NCC measurement may impact recommended therapeutic targets.
Patients and methods
Patients
This report is a secondary analysis of data from the Chelate trial27 (NCT03539952), a randomized, open-label, phase III, multi-center trial to determine whether trientine tetrahydrochloride was non-inferior to PEN for maintenance treatment of patients with stable WD on PEN. To be included in the study, patients were (A) aged 18-75 years, (B) had a diagnostic Leipzig score ≥4, (C) on treatment with PEN for at least 1 year, (D) with unchanged dose for at least 4 months, and (E) the treating physician judged the patient clinically stable with no foreseeable need to change dose or additional therapy (inclusion criteria 5-7).27 In addition, exclusion criteria ensured that the potential participant was not in the “de-coppering” phase, and did not have unstable liver or neurological disease, severe anemia, or renal impairment. It should be noted that specific values of NCC or UCE were not required to enter the study at Visit 1.
After baseline evaluation, patients entered a 12-week run-in period to further ensure they met all inclusion criteria prior to entering the randomized treatment phase (weeks 12-48).27 In the present study we report only data from Day 1, the first visit in the screening period when patients were still on their pre-study dose of PEN. Of 77 patients selected for the trial by site investigators, one withdrew consent before blood sampling and we thus report data on 76 who had blood samples taken on Day 1. NCC-Ex was analyzed during the study, while NCC-Sp was batch analyzed in most cases after study completion.
Healthy volunteers
To establish normal ranges for the NCC-Sp assay, samples from 75 adult healthy volunteers were provided by a commercial biobank BioIVT, (Burgess Hill, United Kingdom). Samples were analyzed in two batches. To ensure ethnic diversity, Batch 1 included 18 Black, one Caucasian, and 31 Hispanic patients, and Batch 2 included one Black, 22 Caucasian, one Mixed, and one Asian/British patient(s). Demographic data are provided in Table 1.
Table 1.
Demographic data and parameters of copper metabolism.
| Healthy volunteers | Patients with WD | |
|---|---|---|
| N | 75 | 76 |
| Sex ratio, male/female | 38/37 | 39/37 |
| Age (mean±SD) | 38.9±12.4 | 42.9±14.6 |
| Height (mean±SD) | 169.7±9.6 | 174.0±9.9 |
| Weight (mean±SD) | 80.0±18.1 | 74.7±16.3 |
| Race, Caucasian/Asian/Black/Hispanic/other/unknown | 23/1/19/31/1/0 | 58/3/0/0/4/11 |
| Presentation | ||
| Hepatic | 18 | |
| Neurologic | 39 | |
| Psychiatric | 5 | |
| Asymptomatic | 12 | |
| Unknown | 2 | |
| Time since diagnosis (years) |
21.3±14.3 |
|
| Copper metabolism | ||
| Ceruloplasmin (mg/L)∗ | Normal range: 150-350 mg/L | 83.2±49.7 |
| Urinary copper excretion (μg/24h) | Normal range: 15-60 | 586±384 |
| Total S-copper (μg/L) | ||
| Mean±SD | 1,092±264 | 298±255 |
| Median; range | 1,030; 665-1,960 | 206; 20-913 |
| Ceruloplasmin bound copper (μg/L)∗∗ | 976±290 | 244±251 |
| NCC-Sp (μg/L) | ||
| Mean±SD | 116±62 | 56.6±26.2 |
| Median; range | 86; 40-310 | 57; 17-149 |
| Normal range | 46-213 | |
| NCC-Ex μg/L | See note∗∗∗ | |
| Mean | 57.2 | 57.9±24.7 |
| Median; range | 57.8; 36-71 | 54.5; 13.1-124 |
| Normal range | 41-71 μg/L | |
NCC, non-ceruloplasmin-bound copper; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; WD, Wilson disease.
Ceruloplasmin was below the detection limit of the assay (52 mg/L) in 43 out of 76 patients. In these 43 patients, 52 mg/L was used to for calculation of mean±SD.
Provided by the NCC-Sp assay.25
From ref 17 including 44 healthy persons (29 females, 15 males) with mean age 41.6 years.
Laboratory analyses
Handling of samples: Detailed instructions for the collection, processing, storage and shipment of blood and urine samples were provided in a specific Laboratory Manual. Blood samples were collected in trace element-free tubes, and immediately centrifuged at 3,000 rpm for 10 min. Serum was separated and frozen at –80 °C for storage and transport. 24-hour urine was collected in copper-free containers. After measurement of urine volume, samples of urine were transferred into trace element-free tubes and frozen for storage and subsequent shipment.
Total serum copper was measured by ICP-MS after microwave digestion by Quotient (Alnwick, Northumberland, United Kingdom).
Ceruloplasmin was measured by QPS, (Groningen, The Netherlands) by the enzymatic methodology. A ceruloplasmin oxidase activity of 1 mU/ml corresponded to 3.03 mg/L ceruloplasmin. The assay range is 101-303 mg/L and the lower limit of quantification (LLOQ) 52 mg/L. QPS does not provide a reference interval and we used 150-350 mg/L adapted from.28
NCC-Ex was assessed according to the original report.17,29 Samples were analyzed centrally at QPS (Groningen, The Netherlands). In brief, after thawing, serum was diluted 1:1 with EDTA 3 g/L and incubated precisely 1 h before ultrafiltration using a Amicon® Ultra4® centrifugal filter device with a 30-kD cut-off Ultracel® regenerated cellulose membrane. Copper concentration in the ultra-filtrate (NCC-Ex) was measured by Inductively Coupled Plasma Optical Emission Spectrometry.17 In 5 of 76 patients, NCC-Ex analysis at Visit 1 failed for different reasons. These analyses were not repeated, so values were available from 71.
NCC-Sp was assessed by Quotient (Alnwick, Northumberland, United Kingdom), NE66 2DH) as described previously.25 Speciation of serum proteins was achieved by high-performance liquid chromatography separation using a strong anion-exchange fast protein liquid chromatography MonoQ™ column. The copper concentration in each peak was assessed by ICP-MS, including the void, the albumin and the Cp peaks. The copper signal in the Cp peak area relative to the sum in all peaks was assumed to be the Cp-bound fraction of total serum copper. NCC-Sp was calculated as total Cu∗(1-fraction of Cp-bound copper). The decision to replace NCC-Ex with NCC-Sp as a primary endpoint for the Chelate study was made during the study and most were batch analyzed post-study. Values of NCC-Sp at Visit 1 were available from 69 out of 76 patients; 65 had paired samples of both NCC-Ex and NCC-Sp.
NCC-Cal was not part of the Chelate trial protocol but could be calculated from total serum copper and ceruloplasmin where available. When ceruloplasmin was above the LLOQ, NCC-Cal was calculated as total Cu (μg/L) - 3.14∗Ceruloplasmin (mg/L).30
UCE: 24-hour urine was collected on current treatment. Urine copper concentration was analyzed by mass spectrometry at QPS (Groningen, The Netherlands). Copper-free containers and test tubes were used.
Statistical analysis
Values are reported as mean±1 SD unless otherwise stated. Associations were analyzed by linear regression. Group comparisons were analyzed by unpaired Student’s t test when normally distributed and by the Mann-Whitney U test (two group comparison) or Kruskal-Wallis test (three group comparison) when not. p <0.05 was considered statistically significant.
Results
In 75 self-reported healthy persons, the median total plasma copper was 1,030 μg/L (IQR 911-1,210 μg/L and 2.5%-97.5% range of 717-1,731 μg/L) (Table 1). There was no effect of gender (p = 0.55 by Mann-Whitney U test) but slightly lower values in Caucasian patients (p = 0.002 by Kruskal-Wallis test) (Table S1). NCC-Sp range was 40-310 μg/L with a median of 86 μg/L (IQR 63-171 μg/L) (Table 1). The distribution of NCC-Sp in these healthy persons was bimodal (Fig. S1). NCC-Sp was higher in males than females and higher in Caucasian patients than Black and Hispanic patients (Table S1). The normal range of NCC-Sp (46-213 μg/L) was taken as the range from the 2.5% to 97.5% percentiles. NCC-Ex was not measured in this population, since a normal range of 41-71 μg/L (5%-95% percentiles) in adults was previously published.17 For comparison 5-95% percentiles for NCC-Sp were 50-209 μg/L.
The patients with WD had on average been treated for ≈21 years before entering the study and the majority presented with neurologic disease. Baseline characteristics are provided in Table 1.
NCC-Sp (mean±SD: 56.6±26.2 μg/L) was available in 69 out of 76 patients and was within the generally recommended target range of 50-150 μg/L in 42 (61%) patients, while 27 (39%) were below, with 19 (28%) even below 40 μg/L, the lowest value measured in healthy controls (Fig. 1). None were above 150 μg/L. NCC-Sp was below the normal range of 46-213 μg/L in 25 (36%) and within in the remaining 44 (64%).
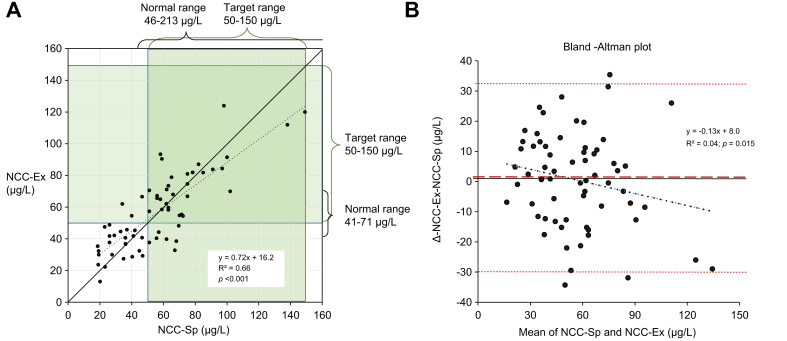
Fig. 1.
NCC-Sp vs. NCC-Ex in 69 patients with clinically stable WD on PEN.
(A) The regression line is dotted and the line of identity solid. On both axes the normal ranges are depicted and the recommended target range during maintenance treatment with PEN (50-150 μg/L) marked in shaded green. NCC-Sp was below the target range in 39% and below the lowest value in healthy volunteers (40 μg/L) in 28%. NCC-Sp and NCC-Ex correlated positively (r2 = 0.66, p <0.001). (B) Bland-Altman plot. The regression line is black dotted. The mean difference (NCC-Ex minus NCC-Sp) was 0.49 μmol/L (red broken line) and close to zero. The confidence limits (-30.5 μg/L; +31.5 μg/L) are depicted in red dotted lines. The slope of the line of regression (-0.13) differed from zero (p = 0.015), indicating a systematic deviation. NCC, non-ceruloplasmin-bound copper; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; PEN, D-penicillamine; WD, Wilson disease.
NCC-Ex (mean±SD: 57.9±24.7 μg/L) was available in 71 patients and was within the 50-150 μg/L range in 38 (54%) and below in 33 (46%). Compared to the normal range for NCC-Ex of 41-71 μg/L, 29 (41%) were within that range, while 22 (31%) were below and 20 (28%) above (Fig. 1). Thus, with both methodologies, a substantial fraction of patients had values below the current target ranges.
NCC-Sp and NCC-Ex correlated positively (r2=0.66; p = 0.001) in 65 out of 76 patients where both were measured (Fig. 1A). The mean difference between the assays (0.49 μg/L) was close to zero but with a considerable scatter as seen in the Bland-Altman plot (Fig. 1B). The Bland-Altman regression line with a negative slope illustrated a systematic deviation with the tendency that NCC-Ex was higher than NCC-Sp when values were below 60 μg/L, and lower above this cut-off.
NCC-Cal was not part of the study protocol but could be calculated from total serum copper and ceruloplasmin in 33 patients with measurable ceruloplasmin (above the LLOQ of 52 mg/L). NCC-Cal was (mean±SD) 156±72 mg/L. Values were generally ≈2 times higher than NCC-Sp or NCC-Ex (Fig. S3). NCC-Cal was within the 50-150 μg/L target in 14 (42.5%), below in 3 (9%) and above in 16 (48.5%) (Fig. S3). NCC-Cal did not correlate with UCE (Fig. S3).
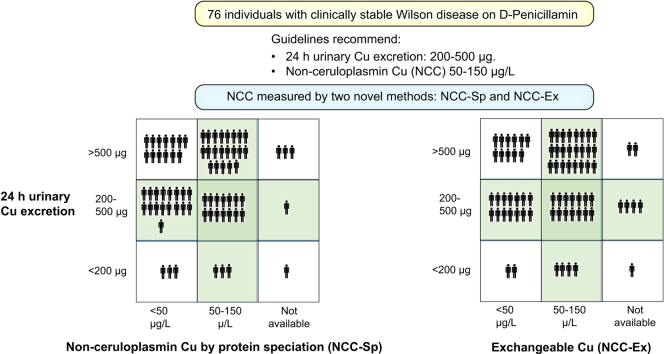
UCE was available in 76 patients. Current guidelines recommend a target range of 200-500 μg/24 h during maintenance treatment with PEN.4,5,8,9 UCE was within that range in 32 (42%), above in 37 (49%), and below in 7 (9%) (Fig. 2).
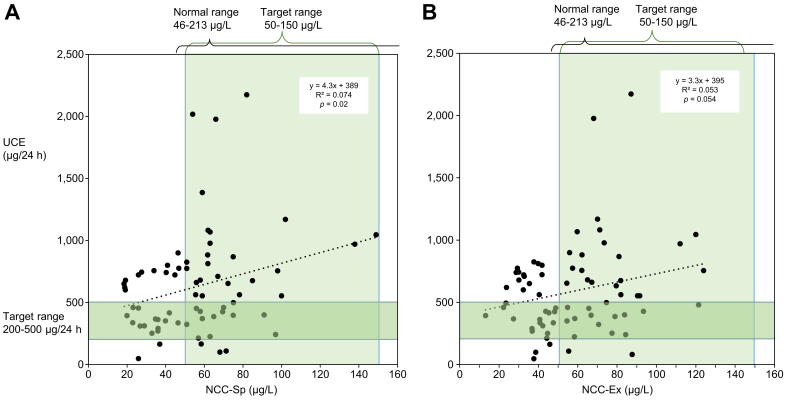
Fig. 2.
NCC-Sp and NCC-Ex vs. UCE in patients with stable WD on PEN.
(A) NCC-Sp and vs. UCE (n = 69) and (B) NCC-Ex vs. UCE (n = 71). All patients were clinically stable on maintenance dose of PEN. The generally recommended target ranges during maintenance treatment for UCE (200-500 μg/24 h) and NCC (50-150 μg/L) are marked as green shaded areas and normal ranges for the assays on top of the graphs. With NCC-Sp and UCE only 14/69 patients (20%) were within both ranges. The same was true for 14/71 (19%) with NCC-Ex and UCE. NCC-Sp positively correlated with UCE (p = 0.02) while NCC-Ex tended to do so (p = 0.054). NCC, non-ceruloplasmin-bound copper; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; PEN, D-penicillamine; UCE, 24-hour urinary copper excretion; WD, Wilson disease.
UCE weakly but statistically significantly correlated to NCC-Sp (r2=0.07, p = 0.02) and tended towards the same with NCC-Ex (r2= 0.053, p = 0.054) (Fig. 2). The distribution of UCE vs. NCC-Sp or NCC-Ex according to guideline recommendations are provided in Table 2. Among 69 patients where both NCC-Sp and UCE were available, only 14 (20%) were within recommended ranges for both NCC-Sp and UCE (Fig. 2A). The same percent were found within the recommended ranges for NCC-Ex and UCE, 14/71 (19%) (Table 2).
Table 2.
Relation between UCE and NCC-SP or NCC-Ex in 76 patients with stable WD.
| NCC-Ex (μg/L) |
NCC-Sp (μg/L) |
|||||
|---|---|---|---|---|---|---|
| <50 |
≥50 |
n.a. |
<50 |
≥50 |
n.a. |
|
| N | 33 | 38 | 5 | 27 | 42 | 7 |
| UCE (μg/24h) | ||||||
| <200 | 3 | 3 | 1 | 2 | 4 | 1 |
| 200-500 | 17 | 14 | 1 | 14 | 14 | 4 |
| >500 | 13 | 21 | 3 | 11 | 24 | 2 |
NCC, non-ceruloplasmin-bound copper; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; UCE, 24-hour urinary copper excretion; WD, Wilson disease.
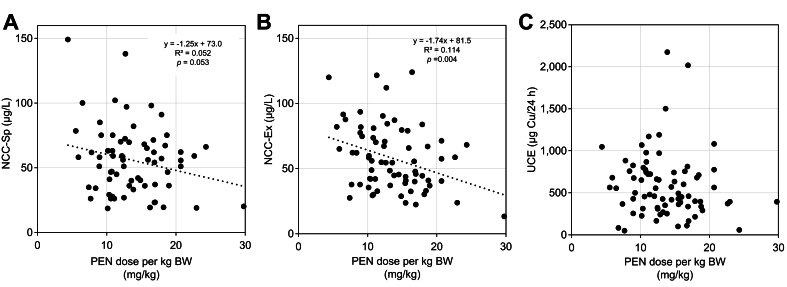
We also examined the relationship of the dose of PEN in mg/kg to NCC-Sp, NCC-Ex and UCE, respectively (Fig. 3). In these clinically stable patients with an average of ≈21 years of treatment, the doses in mg/kg varied considerably from the recommended 10-15 mg/kg (Table 3). Higher doses were associated with lower NCC-Sp (r2 = 0.052, p = 0.053), and lower NCC-Ex (r2=0.114, p = 0.004), while no association was seen with UCE (r2 = 0.01, p = 0.34) (Fig. 3).
Fig. 3.
Relation between daily dose of PEN in mg/kg BW and measures of copper metabolism in patients with WD who were stable on PEN.
(A) Dose and NCC-SP (n = 69). (B) Dose and NCC-Ex (n = 71). (C) Dose and UCE (n = 76). PEN dose in mg/kg tended to be negatively correlated with NCC-SP (p = 0.053) and NCC-Ex (p = 0.004). PEN dose did not correlate with UCE. BW, body weight; NCC, non-ceruloplasmin-bound copper; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; PEN, D-penicillamine; UCE, 24-hour urinary copper excretion; WD, Wilson disease.
Table 3.
Doses of PEN in mg/kg/day in 76 patients with WD.
| Dose (mg/kg) | n (N = 76) |
|---|---|
| 4-7.5 | 6 |
| 7.5-10 | 8 |
| 10-12.5 | 21 |
| 12.5-15 | 13 |
| 15-17.5 | 14 |
| 17.5-20 | 7 |
| 20-22.5 | 3 |
| 22.5-25 | 3 |
| 25-30 | 1 |
The mean±SD dose was 983±292 mg/day.
PEN, D-penicillamine; WD, Wilson disease.
To test the hypotheses that NCC-Sp, NCC-Ex and UCE were related to the clinical course of disease, we examined their relation to liver biochemistry (Table 4). With both NCC-Sp and NCC-Ex, patients with values below the 50-150 μg target range had significantly lower aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyltransferase levels and higher albumin levels than patients with values within the target range. Thus, either NCC-Sp or NCC-Ex below the recommended target range was associated with better control of liver biochemistry. A similar relationship was not observed with UCE (Table 4).
Table 4.
Hepatic and hematological values related to NCC-Sp, NCC-Ex and UCE within or outside recommended ranges.
| Discrimination value | NCC-Sp (μg/L) |
NCC-Ex (μg/L) |
UCE (μg/24h) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <50 |
≥50 |
<50 |
≥50 |
<200 |
200-500 |
>500 |
||||
| Mean±SD | Mean±SD | p value | Mean±SD | Mean±SD | p value | Mean±SD | Mean±SD | Mean±SD | p value | |
| N | 25 | 39 | 33 | 32 | 7 | 32 | 37 | |||
| Albumin (g/L) | 44.8±2.7 | 43.1±3.5 | 0.028 | 44.5±3.0 | 42.6±3.5 | 0.014 | 44.3±2.3 | 44.1±3.0 | 43.0±3.7 | n.s. |
| AST (U/L) | 21.3±6.7 | 30.0±10.9 | 0.0003 | 22.0±6.9 | 28.8±11.4 | 0.003 | 24.6±7.0 | 26.0±8.9 | 26.1±11.6 | n.s. |
| ALT (U/L) | 26.0±16.3 | 41.0±22.5 | 0.006 | 25.3±15.2 | 39.3±23.8 | 0.004 | 32.1±18.5 | 33.3±22.8 | 34.9±22.8 | n.s. |
| GGT (U/L) | 26.3±13.2 | 54.5±87.9 | 0.037 | 25.2±12.2 | 63.1±88.9 | 0.013 | 37.2±38.1 | 31.9±22.9 | 54.8±87.8 | n.s. |
| Bilirubin (μmol/L) | 13.3±5.6 | 17.1±8.1 | 0.031 | 16.6±10.2 | 15.6±7.9 | n.s. | 13.4±6.6 | 15.8±10.4 | 16.2±7.8 | n.s. |
| LDH (U/L) | 159.4±26.6 | 172.2±35.4 | n.s. | 164.6±30.1 | 168.3±35.0 | n.s. | 159.6±29.8 | 174.4±33.9 | 164.0±28.1 | n.s. |
| Protein (g/L) | 72.2±4.1 | 71.5±5.0 | n.s. | 72.0±4.2 | 70.8±5.4 | n.s. | 72.5±4.1 | 72.2±5.5 | 70.6±4.5 | n.s. |
| PT (sec) | 13.7±0.6 | 14.3±2.2 | n.s. | 13.8±0.7 | 14.3±2.1 | n.s. | 14.2±2.6 | 14.1±2.3 | 14.1±0.8 | n.s. |
| Hb (g/dl) | 14.39±1.07 | 14.09±1.35 | n.s. | 14.33±1.13 | 14.23±1.23 | n.s. | 14.32±1.13 | 14.29±1.12 | 14.12±1.31 | n.s. |
| Reticulocytes (%) | 1.38±0.47 | 1.44±0.52 | n.s. | 1.39±0.51 | 1.42±0.53 | n.s. | 1.48±0.41 | 1.37±0.51 | 1.39±0.53 | n.s. |
| Leukocytes (109/L) | 5.60±1.65 | 5.27±1.78 | n.s. | 5.45±1.63 | 5.31±1.72 | n.s. | 5.49±1.38 | 5.21±1.40 | 5.41±1.89 | n.s. |
| Lymphocytes (109/L) | 1.75±0.55 | 1.57±0.54 | n.s. | 1.59±0.50 | 1.61±0.57 | n.s. | 1.68±0.46 | 1.60±0.45 | 1.56±0.57 | n.s. |
| Monocytes (109/L) | 0.30±0.11 | 0.32±0.16 | n.s. | 0.31±0.10 | 0.34±0.16 | n.s. | 0.30±0.10 | 0.30±0.09 | 0.35±0.17 | n.s. |
| Neutrophils (109/L) | 3.37±1.32 | 3.20±1.35 | n.s. | 3.36±1.27 | 3.18±1.31 | n.s. | 3.33±1.02 | 3.11±1.03 | 3.34±1.48 | n.s. |
| Basophils (109/L) | 0.047±0.032 | 0.045±0.021 | n.s. | 0.048±0.029 | 0.041±0.022 | n.s. | 0.05±0.02 | 0.05±0.02 | 0.04±0.03 | n.s. |
| Platelets (109/L) | 214±65 | 187±68 | n.s. | 196±66 | 192±69 | n.s. | 223±58 | 202±58 | 183±73 | n.s. |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; Hb, hemoglobin; LDH, lactate dehydrogenase; NCC, non-ceruloplasmin-bound copper; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; PT, prothrombin time; UCE, 24-hour urinary copper excretion. p-values were calculated by unpaired t-test.
A value of NCC <50 μg/L and UCE >500 μg/24 h has been taken to indicate possible overtreatment5,8 but no patient in this cohort complained of symptoms of copper deficiency, such as progressive gait instability, muscle weakness, or ascending paresthesia, which are neurological symptoms related to spinal cord demyelination (i.e. progressive posterior cord syndrome) or fatigue.[31], [32], [33] In addition, there were no hematological disturbances (low white blood cell count, sideroblastic anemia or progressing thrombocytopenia) in patients with NCC-Sp or NCC-Ex below 50 μg/L as would be expected with copper deficiency (Table 4).
Discussion
In this study, we examined for the first time how novel assays for measuring non-ceruloplasmin bound copper (NCC-Sp and NCC-Ex) and UCE corresponded to recommendations from current international guidelines in patients with WD who were in the maintenance phase of PEN treatment. To do so, we conducted a secondary analysis of data from the Chelate trial.27 The patients were judged clinically stable by their treating physician without a foreseeable need for dose change. We used data collected at the first visit when all the patients were still on their maintenance dose of PEN before the experimental treatment. Their average length of treatment was 21 years. NCC-Sp and NCC-Ex and UCE were evaluated with respect to the target ranges recommended in current guidelines, originally proposed in the 1980s.3 The range of 50-150 μg/L for NCC was chosen because that was the range of NCC-Cal in normal individuals. The clinical validation is sparse.[34], [35], [36], [37] NCC-Cal is now being abandoned as explained in the introduction but the target range of 50-150 μg/L – based on this method – is still used owing to the absence of additional data.5,8,9 NCC-Ex avoided some of the pitfalls of the NCC-Cal method and there is some degree of clinical validation to support its use.[19], [20], [21], [22], [23], [24],38 However, detection of possible bias in NCC-Ex led to the development of NCC-Sp.25,26 The primary observation in our analysis was that even in our highly selected group of patients, a large fraction were below the 50-150 μg/L range: 39% for NCC-Sp and 46% for NCC-Ex values, respectively. These “real world” data suggest a recalibration of the range for NCC using novel technologies for treated patients with WD is necessary.
NCC-Ex and NCC-Sp varied around the line of identity with considerable scatter and a trend of overestimation by NCC-Ex in the lower range and underestimation by NCC-Ex in the middle range (Fig. 1). This suggests a systematic difference between these two assays. The published normal range of NCC-Ex is 41-71 μg/L (0.64-1.12 μmol/L) in adults17 and slightly lower in children,18 which are notably lower than with NCC-Sp (46-213 μg/L) and NCC-Cal (50-150 μg/L). To understand these differences better, it would have been useful to compare NCC-Sp and NCC-Ex in the same cohort of healthy controls, but that was beyond our capacity. The reported effects of age and sex on NCC-Ex are minimal17 and will likely not explain discrepancies between normal ranges for NCC-Sp and NCC-Ex. As further discussed below, the healthy cohort in the our study displayed an ethnic diversity that was not reported in the NCC-Ex healthy cohort.17
The NCC-Sp method was developed and validated according to current standards.25 In our control population of 75 healthy persons, NCC-Sp had a bimodal distribution (Fig. S1) with a 2.5-97.5 percentile range of 46-213 μg/L, taken as the normal range for the present study. A bimodal distribution was not reported for NCC-Ex17 but may have been overlooked with only 44 participants. This should be further explored because both NCC-Sp and NCC-Ex presented with skewed but monomodal distributions in the WD patient population (Fig. S2). In a recent re-examination of a large dataset, total serum copper was higher in females than males, and lower in Caucasian and Hispanic patients than in Black patients.39 In our much smaller sample, a gender difference was not detected but a similar effect of ethnicity was observed for total Cu (Table S1). A similar evaluation of any measure of NCC does not exist. In the initial evaluation of NCC-Ex,17 with 29 females and 15 males, no gender difference was detected and ethnicity was not reported. In our sample, NCC-Sp was higher in males than females and we observed a possible effect of ethnicity on NCC-Sp, as values were higher in Caucasian patients than in Black and Hispanic patients (Table S1). However, our sample size is too small to draw firm conclusions, and further studies are warranted.
NCC-Sp was lower (56.61±26.2 μg/L) in patients with WD on maintenance treatment with PEN than in healthy persons (116±62 μg/L; p <0.001 by unpaired t-test). Indeed, 28% of patients were even below 40 μg/L, the lowest value in healthy volunteers, interestingly without evidence for copper deficiency in these study patients. A similar situation was observed with NCC-Ex (Fig. 1). Validated target ranges adapted to the phase of the disease for NCC-Ex have not yet been published, but lower than normal values are often seen during maintenance therapy, without established signs of copper deficiency (Aurelia Poujois, Personal communication). Accordingly, in a study of 36 children with WD treated for a median of 6 years with either PEN (83%) or trientine (17%),21 the median (IQR) of NCC-Ex was 24 (14-40) μg/L, which is low relative to the normal range for NCC-Ex but was not associated with signs of copper deficiency.
Calculation of NCC-Cal was only possible in 33 patients with Cp above LLOQ. In these patients, NCC-Cal was considerably higher than with newer methods (Fig. S3). Thus, the NCC-Cal/NCC-Sp ratio was 2.9±1.2 and NCC-Cal/NCC-Ex 2.5±0.9. Our findings are in line with a report of NCC-Cal/NCC-Ex ratios of 2.8 in patients with hepatic and 3.4 in those with neurologic WD.40 We are not able to explain this discrepancy but note that the calculation depends heavily on the Cp concentration, molecular weight and Cu/Cp ratio, where small systematic errors will lead to larger relative errors in the NCC-Cal estimate. NCC-Cal did not correlate to 24 h UCE. These observations add to the concerns about using this methodology.
Our data enabled comparison of UCE results to NCC-Sp and NCC-Ex (Fig. 2). UCE was outside the 200-500 μg/24 h range in 58%. Indeed, only 20% fulfilled both criteria of the NCC and UCE target ranges (Table 2). In the absence of chelator treatment, UCE has been regarded to reflect NCC in the bloodstream4,36 and thus the amount of excess copper in the body.36 During treatment with PEN, the interpretation of UCE is further complicated by the cupriuretic effect of the drug, and that is pragmatically considered in the recommendation of a target range of 200-500 μg/24 h. If patients had been overtreated for extended periods, UCE would be expected to decline over time and reach low values; however, shorter periods of overtreatment may increase UCE above target ranges. Elevated UCE may also be seen when non-adherent patients remember to take their medication during UCE collection. In accordance, the visit-to-visit variability of UCE is known to be very large and single values are not easy to use for clinical decisions.41
Our findings reflected this complexity. UCE only weakly correlated with NCC-Sp (Fig. 2) and not to PEN dose in mg/kg (Fig. 3). A large fraction of patients (49%) had UCE above the 200-500 μg/24 h target range. Similar findings were reported in 79 patients with WD on PEN for more than 60 months, where 50% had UCE above 600 μg/24 h,41 and in 22 compliant patients treated with PEN for a mean of 12 years and UCE (mean±SD) of 653.95±354.19 μg/24 h.16 In the latter study, UCE did not differ between adherent and non-adherent patients. The origin of the 200-500 μg/24 h target range has been difficult to track. It is assumed to neutralize the pathological copper balance in patients with WD, and that may not always be correct if patients do not follow a low copper diet. In our cross-sectional study we only note that there was no correlation between UCE and dose of penicillamine, ALT, or AST (Fig. 3 and Table 4). Given these difficulties with interpretation of UCE, our data illustrates why experienced clinicians may not mechanically use these measures to drive dosage decisions in patients with WD, but rather choose to prioritize clinical symptoms over laboratory values. To overcome uncertainty with the use of UCE and simplify interpretation, some centers use assessment of UCE after 48 h off PEN therapy,16,41 however our data do not allow for evaluation of this alternative.
Our dataset reflects standard daily (real world) practice, raising a question of why the observed NCC-Sp and NCC-Ex in patients with stable WD on PEN were lower than recommended in guidelines and values observed in healthy controls? We suggest two possible hypotheses; first, the treatment was actually optimal, and treatment target ranges for NCC-Sp and NCC-Ex may in fact be lower than the normal ranges for healthy individuals. Secondly, a large proportion of patients with WD below-target NCC-Sp values might be overtreated, and their dosage of PEN could have been reduced without losing clinical efficacy. In the present cross-sectional study, we can only note that biochemical or clinical signs of overtreatment were not detected. Given the associations of lower NCC-Sp and NCC-Ex with lower values of ALT and AST and higher albumin there may be a protective value of keeping NCC low. The level of NCC-Sp below which there is a risk of copper deficiency is still unknown. Further clinical validation in longitudinal studies should aim at determining optimal target ranges for NCC in patients with WD in different phases of specific treatments and taking different methodologies into account.
In conclusion, we examined 76 patients with WD who were judged by expert clinicians to be clinically stable on a given dose of PEN after years of treatment. Nevertheless, only 20% had both NCC-Sp and UCE within currently recommended target ranges. The tendency was that UCE was above and NCC-Sp below current recommendations, as would be expected with overtreatment, but no signs of copper deficiency were detected in these patients. Our data raise the question of whether the therapeutic target ranges for NCC-Sp and NCC-Ex should be lower than the normal range in healthy persons in order to provide maximal organ protection in treated patients. In addition, as normal ranges differ by assay, the targeted ranges should be assay specific as well as treatment specific. The observed range of UCE values raise questions about interpretation of values during treatment and the potential benefit of assessment off therapy. Thus, our study provides the first data on the ranges of NCC-Sp for healthy individuals and PEN-treated patients with WD and highlights the need for longitudinal studies to develop and validate methodologic-specific targets for NCC in different phases of treatment of the disorder.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cp, ceruloplasmin; ICP-MS, inductively coupled plasma mass spectroscopy; NCC, non-ceruloplasmin-bound copper; NCC-Cal, NCC calculated from total Cu and ceruloplasmin; NCC-Ex, exchangeable copper; NCC-Sp, NCC determined from total copper protein speciation assay; PEN, D-penicillamine; UCE, 24-hour urinary copper excretion; WD, Wilson disease.
Financial support
The paper is a secondary analysis of data collected during the Chelate trial (NCT03539952) and the sponsor of the trial (Orphalan, ASA France) generously provided these data. Peter Ott and Thomas Sandahl were supported by The Memorial Foundation of Manufacturer Vilhelm Pedersen & Wife.
Conflicts of interest
PO received consulting fee from Orphalan, institutional research grant from Alexion, consulting fee from Ultragenyx, Vivet, and MEXBRAIN. TS received received consulting fee from Orphalan, institutional research grant from Alexion, consulting fee from Ultragenyx, advisory activities for Vivet. The Department of Hepatology and Gastroenterology has received an unrestricted research grant from Orphalan. AA received institutional research grants from Orphalan, Alexion, Univer, and the Wilson Disease Association;consulting fees from Alexion and Orphalan; speaker’s fee from Orphalan; travel assistance to meetings from Orphalan and Univer. Advisory board at Alexion, Univar, and Orphalan. DC received consulting fees, travel reimbursements and honoraria for advisory activities and speaker assignments, from Orphalan. EC-B received consulting fees and honoraria for advisory activities from Alexion, Orphalan, Univar, travel assistance to meetings from Orphalan and Univar. AC received research (institutional) grants from Orphalan, Alexion, Public Health Research Institute in Canada; consulting fees from Wilson Therapeutics, Alexion, Vivet Therapeutics, and Orphalan; speaker’s fees from Ever Pharma; and travel assistance to meetings from Orphalan. GD received lecture fees, honoraria for advisory activities and travel support from AbbVie, Advanz, Alexion, Falk Foundation, Gilead, Intercept, Novartis, Orphalan and Univar. JM received consultancy fees from Orphalan and Alexion and honorarium from Shionogi. AP received institutional grants from Orphalan, Addmedica, Alexion; consulting fees and honoraria for advisory activities from Alexion, Orphalan, Vivet therapeutics, Univar. KHW received research (institutional) grants from Orphalan; consulting fees from Orphalan, Univar, Pfizer, Alexion, and Vivet Therapeutics, Desitin, Ultragenyx; speaker’s fees from Falk, AbbVie, Alexion, and Orphalan; travel assistance to meetings from Alexion and Univar. OK is employed by Orphalan. MLS received research grants from Orphalan, Alexion, Vivet therapeutics and the Wilson Disease Association, and honoraria and advisory activities for Arbomed and DepYmed.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Peter Ott, Michael Schilsky, Thomas Sandahl and Omar Kamlin conceived and planned the study to be accepted by all authors. Peter Ott and Thomas Sandahl provided initial data analysis and draft manuscript in interaction with Michael Schilsky and Omar Kamlin. All authors participated in generating the presented data and critically participated in further revisions of the paper.
Data availability statement
See Chelate study statement (Lancet Gastroenterol Hepatol 2022 Vol. 7 Issue 12 Pages 1092-1102).
Acknowledgements
The Chelate Study was sponsored by Orphalan. The two first authors were supported by The Memorial Foundation of Manufacturer Vilhelm Pedersen & Wife.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101115.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Walshe J.M. Treatment of Wilson's disease with penicillamine. Lancet. 1960;1:188–192. doi: 10.1016/s0140-6736(60)90109-4. [DOI] [PubMed] [Google Scholar]
- 2.Scheinberg I.H., Sternlieb I. The long term management of hepatolenticular degeneration (Wilson's disease) Am J Med. 1960;29:316–333. doi: 10.1016/0002-9343(60)90028-0. [DOI] [PubMed] [Google Scholar]
- 3.Scheinberg I.H., Sternlieb I. Vol. 23. WB Saunders; Philadelphia: 1984. (Wilson's disease. Major problems in Internal Medicine). [Google Scholar]
- 4.EASL EASL clinical practice guidelines: wilson's disease. J Hepatol. 2012;56:671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Schilsky M.L., Roberts E.A., Bronstein J.M., et al. A multidisciplinary approach to the diagnosis and management of wilson disease:executive summary of the 2022 practice guidance on wilson disease from the American association for the study of liver diseases. Hepatology. 2022 Dec 7 doi: 10.1002/hep.32801. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Scheinberg I.H., Sternlieb I. Copper metabolism. Pharmacol Rev. 1960;12:355–381. [PubMed] [Google Scholar]
- 7.Walshe J.M. Wilson's disease: the importance of measuring serum caeruloplasmin non-immunologically. Ann Clin Biochem. 2003;40:115–121. doi: 10.1258/000456303763046021. [DOI] [PubMed] [Google Scholar]
- 8.Nagral A., Sarma M.S., Matthai J., et al. Wilson's disease: clinical practice guidelines of the Indian national association for study of the liver, the Indian society of pediatric Gastroenterology, Hepatology and nutrition, and the movement disorders society of India. J Clin Exp Hepatol. 2019;9:74–98. doi: 10.1016/j.jceh.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shribman S., Marjot T., Sharif A., et al. Investigation and management of Wilson's disease: a practical guide from the British Association for the Study of the Liver. Lancet Gastroenterol Hepatol. 2022;7:560–575. doi: 10.1016/S2468-1253(22)00004-8. [DOI] [PubMed] [Google Scholar]
- 10.Walshe J.M. Copper chelation in patients with Wilson's disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q J Med. 1973;42:441–452. [PubMed] [Google Scholar]
- 11.Duncan A., Yacoubian C., Beetham R., et al. The role of calculated non-caeruloplasmin-bound copper in Wilson's disease. Ann Clin Biochem. 2017;54:649–654. doi: 10.1177/0004563216676843. [DOI] [PubMed] [Google Scholar]
- 12.Twomey P.J., Viljoen A., House I.M., et al. Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin Chem. 2005;51:1558–1559. doi: 10.1373/clinchem.2005.052688. [DOI] [PubMed] [Google Scholar]
- 13.Holmberg CGL C.B. Investigations in serum copper. II, isolation of the copper containing protein, and a description of some of its properties. Acta Chemica Scand. 1948;2:7. [Google Scholar]
- 14.Nakagawa O. Purification and properties of crystalline human ceruloplasmin. Int J Pept Protein Res. 1972;4:385–394. doi: 10.1111/j.1399-3011.1972.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindley F.P. Wiley Online Library; 2011. Ceruloplasmin. Encyclopedia of inorganic and bioorganic chemistry. [Google Scholar]
- 16.Dziezyc K., Litwin T., Chabik G., et al. Measurement of urinary copper excretion after 48-h d-penicillamine cessation as a compliance assessment in Wilson's disease. Funct Neurol. 2015;30:264–268. doi: 10.11138/FNeur/2015.30.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Balkhi S., Poupon J., Trocello J.M., et al. Determination of ultrafiltrable and exchangeable copper in plasma: stability and reference values in healthy subjects. Anal Bioanal Chem. 2009;394:1477–1484. doi: 10.1007/s00216-009-2809-6. [DOI] [PubMed] [Google Scholar]
- 18.Yim J., Kwon S.B., Han J.S., et al. Total and exchangeable copper assay using inductively coupled plasma mass spectrometry and establishment of a pediatric reference interval. Arch Pathol Lab Med. 2021;145:877–882. doi: 10.5858/arpa.2020-0029-OA. [DOI] [PubMed] [Google Scholar]
- 19.Guillaud O., Brunet A.S., Mallet I., et al. Relative exchangeable copper: a valuable tool for the diagnosis of Wilson disease. Liver Int. 2018;38:350–357. doi: 10.1111/liv.13520. [DOI] [PubMed] [Google Scholar]
- 20.Poujois A., Trocello J.M., Djebrani-Oussedik N., et al. Exchangeable copper: a reflection of the neurological severity in Wilson's disease. Eur J Neurol. 2017;24:154–160. doi: 10.1111/ene.13171. [DOI] [PubMed] [Google Scholar]
- 21.Ngwanou D.H., Couchonnal E., Parant F., et al. Long-term urinary copper excretion and exchangeable copper in children with wilson disease under chelation therapy. J Pediatr Gastroenterol Nutr. 2022;75:e75–e80. doi: 10.1097/MPG.0000000000003531. [DOI] [PubMed] [Google Scholar]
- 22.Jacquelet E., Poujois A., Pheulpin M.C., et al. Adherence to treatment, a challenge even in treatable metabolic rare diseases: a cross sectional study of Wilson's disease. J Inherit Metab Dis. 2021;44:1481–1488. doi: 10.1002/jimd.12430. [DOI] [PubMed] [Google Scholar]
- 23.Sarma MSS J., Srivastava A., Yacha S.K., et al. Can we predict survival of wilson disease using exchangeable copper? (Abstract 48, AASLD 2021) Hepatology. 2021;74:1. [Google Scholar]
- 24.Schmitt F., Podevin G., Poupon J., et al. Evolution of exchangeable copper and relative exchangeable copper through the course of Wilson's disease in the Long Evans Cinnamon rat. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Castillo Busto M.E., Cuello-Nunez S., Ward-Deitrich C., et al. A fit-for-purpose copper speciation method for the determination of exchangeable copper relevant to Wilson's disease. Anal Bioanal Chem. 2021;414:13. doi: 10.1007/s00216-021-03517-y. [DOI] [PubMed] [Google Scholar]
- 26.Solovyev N., Ala A., Schilsky M., et al. Biomedical copper speciation in relation to Wilson's disease using strong anion exchange chromatography coupled to triple quadrupole inductively coupled plasma mass spectrometry. Anal Chim Acta. 2020;1098:27–36. doi: 10.1016/j.aca.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Schilsky M.L., Czlonkowska A., Zuin M., et al. Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2022;7:1092–1102. doi: 10.1016/S2468-1253(22)00270-9. [DOI] [PubMed] [Google Scholar]
- 28.Stepien K.M., Guy M. Caeruloplasmin oxidase activity: measurement in serum by use of o-dianisidine dihydrochloride on a microplate reader. Ann Clin Biochem. 2018;55:149–157. doi: 10.1177/0004563217695350. [DOI] [PubMed] [Google Scholar]
- 29.El Balkhi S., Trocello J.M., Poupon J., et al. Relative exchangeable copper: a new highly sensitive and highly specific biomarker for Wilson's disease diagnosis. Clin Chim Acta. 2011;412:2254–2260. doi: 10.1016/j.cca.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Schilsky M.L., Roberts E.A., Bronstein J.M., et al. A multidisciplinary approach to the diagnosis and management of wilson disease: executive summary of the 2022 practice guidance on wilson disease from the American association for the study of liver diseases. Hepatology. 2023;77:1428–1455. doi: 10.1002/hep.32805. [DOI] [PubMed] [Google Scholar]
- 31.Dzieżyc K., Litwin T., Sobańska A., et al. Symptomatic copper deficiency in three Wilson's disease patients treated with zinc sulphate. Neurol Neurochir Pol. 2014;48:214–218. doi: 10.1016/j.pjnns.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Lozano Herrero J., Muñoz Bertrán E., Ortega González I., et al. Myelopathy secondary to copper deficiency as a complication of treatment of Wilson's disease. Gastroenterol Hepatol. 2012;35:704–707. doi: 10.1016/j.gastrohep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Chevalier K., Obadia M.A., Nouzha Djebrani-Oussedik N., et al. Can Patients with Wilson's Disease Develop Copper Deficiency? Mov Disord Clin Pract. 2023 Jun 22;10(9):1306–1316. doi: 10.1002/mdc3.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer G.J., Askari F., Dick R.B., et al. Treatment of Wilson's disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res. 2009;154:70–77. doi: 10.1016/j.trsl.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Brewer G.J., Dick R.D., Johnson V.D., et al. Treatment of Wilson's disease with zinc: XV long-term follow-up studies. J Lab Clin Med. 1998;132:264–278. doi: 10.1016/s0022-2143(98)90039-7. [DOI] [PubMed] [Google Scholar]
- 36.Walshe J.M. Serum 'free' copper in Wilson disease. QJM. 2012;105:419–423. doi: 10.1093/qjmed/hcr229. [DOI] [PubMed] [Google Scholar]
- 37.Chanpong A., Dhawan A. Long-term urinary copper excretion on chelation therapy in children with wilson disease. J Pediatr Gastroenterol Nutr. 2021;72:210–215. doi: 10.1097/MPG.0000000000002982. [DOI] [PubMed] [Google Scholar]
- 38.Mariño Z., Molera-Busoms C., Badenas C., et al. Benefits of using exchangeable copper and the ratio of exchangeable copper in a real-world cohort of patients with Wilson disease. J Inherit Metab Dis. 2023;46:982–991. doi: 10.1002/jimd.12639. [DOI] [PubMed] [Google Scholar]
- 39.Andrew D., Gail R., Morag B., et al. Recommended reference intervals for copper and zinc in serum using the US National Health and Nutrition Examination surveys (NHANES) data. Clin Chim Acta. 2023;546 doi: 10.1016/j.cca.2023.117397. [DOI] [PubMed] [Google Scholar]
- 40.Shribman S., Heller C., Burrows M., et al. Plasma neurofilament light as a biomarker of neurological involvement in wilson's disease. Mov Disord. 2021;36:503–508. doi: 10.1002/mds.28333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeiffenberger J., Lohse C.M., Gotthardt D., et al. Long-term evaluation of urinary copper excretion and non-caeruloplasmin associated copper in Wilson disease patients under medical treatment. J Inherit Metab Dis. 2019;42:371–380. doi: 10.1002/jimd.12046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See Chelate study statement (Lancet Gastroenterol Hepatol 2022 Vol. 7 Issue 12 Pages 1092-1102).