Abstract
Background
The renin-angiotensin system has been identified as a potential therapeutic target for posttraumatic stress disorder, although its mechanisms are not well understood. Brain angiotensin type 2 receptors (AT2Rs) are a subtype of angiotensin II receptors located in stress and anxiety-related regions, including the medial prefrontal cortex (mPFC), but their function and mechanism in the mPFC remain unexplored. Therefore, we used a combination of imaging, cre/lox, and behavioral methods to investigate mPFC-AT2R–expressing neurons in fear and stess related behavior.
Methods
To characterize mPFC-AT2R–expressing neurons in the mPFC, AT2R-Cre/tdTomato male and female mice were used for immunohistochemistry. mPFC brain sections were stained with glutamatergic or interneuron markers, and density of AT2R+ cells and colocalization with each marker were quantified. To assess fear-related behaviors in AT2R-flox mice, we selectively deleted AT2R from mPFC neurons using a Cre-expressing adeno-associated virus. Mice then underwent Pavlovian auditory fear conditioning, elevated plus maze, and open field testing.
Results
Immunohistochemistry results revealed that AT2R was densely expressed throughout the mPFC and primarily expressed in somatostatin interneurons in a sex-dependent manner. Following fear conditioning, mPFC-AT2R Cre-lox deletion impaired extinction and increased exploratory behavior in female but not male mice, while locomotion was unaltered by mPFC-AT2R deletion in both sexes.
Conclusions
These results identify mPFC-AT2R+ neurons as a novel subgroup of somatostatin interneurons and reveal their role in regulating fear learning in a sex-dependent manner, potentially offering insights into novel therapeutic targets for posttraumatic stress disorder.
Keywords: Angiotensin II, AT2R, Fear extinction, Interneuron, PTSD, Prefrontal cortex
Plain Language Summary
Posttraumatic stress disorder (PTSD) is a significant predictor of cardiovascular disease (CVD), although the underlying mechanisms are poorly understood. The brain renin-angiotensin system (RAS) is important for cardiovascular and emotional stress regulation and may better help understand the link between PTSD and CVD risk. Our research reveals that the brain angiotensin II type 2 receptor (AT2R) subtype is located on specific somatostatin (SOM+) interneurons in the medial prefrontal cortex (mPFC) and plays a role in fear memory extinction, particularly in females. These findings reveal a role for the mPFC-AT2R in fear-based learning and memory, offering potential insights into the mechanisms underlying the PTSD-CVD association and therapeutic strategies.
Plain Language Summary
Posttraumatic stress disorder (PTSD) is a significant predictor of cardiovascular disease (CVD), although the underlying mechanisms are poorly understood. The brain renin-angiotensin system (RAS) is important for cardiovascular and emotional stress regulation and may better help understand the link between PTSD and CVD risk. Our research reveals that the brain angiotensin II type 2 receptor (AT2R) subtype is located on specific somatostatin (SOM+) interneurons in the medial prefrontal cortex (mPFC) and plays a role in fear memory extinction, particularly in females. These findings reveal a role for the mPFC-AT2R in fear-based learning and memory, offering potential insights into the mechanisms underlying the PTSD-CVD association and therapeutic strategies.
Posttraumatic stress disorder (PTSD) is a strong predictor of cardiovascular disease, the primary cause of death in both men and women in the United States. Nevertheless, the specific biological, behavioral, and causal mechanisms that explain the co-occurrence of PTSD and cardiovascular disease, particularly with notable differences between sex, remain unclear (1, 2, 3, 4). Given its involvement in maintaining cardiovascular homeostasis and emotional stress, the renin-angiotensin system (RAS) has emerged as a potentially significant bridge between these conditions (5, 6, 7, 8, 9). Angiotensin II serves as the main functional peptide of the RAS, and its effects are facilitated by binding to its primary receptor subtypes, the angiotensin type 1 receptor (AT1R) and type 2 receptor (AT2R) (10). These receptors are expressed throughout the brain, with more extensive research focused on their presence in regions like the hypothalamus and brainstem. However, AT1R and AT2R are also expressed in corticolimbic brain structures, such as the amygdala and medial prefrontal cortex (mPFC), which are crucial regions involved in conditioned fear memory and extinction learning (11, 12, 13, 14, 15). Our previous studies have demonstrated AT2R expression in the central amygdala and function in conditioned fear expression (11), while others have reported increased expression in the mPFC (12). However, the functional role of AT2R-expressing mPFC neurons in conditioned fear learning and memory remains largely unknown.
Brain AT2R has been studied in the context of cardiovascular (13,16,17) and stress (11,18, 19, 20) regulation. A Gi protein–coupled receptor, brain AT2R has been shown to decrease the firing rate and neuronal activity of both excitatory and inhibitory neurons in multiple brain regions (21). This inhibitory effect influences physiological outcomes of stress, showing both neuroprotective and hypotensive properties (14,22), as well as behavioral outcomes of stress, because activation of central amygdala AT2Rs decreases fear expression while whole-brain knockout increases anxiety in mice (11,19). Interestingly, while most of these studies have examined these effects in male mice, AT2R is an X-linked gene upregulated by estrogen signaling (10,23,24), and some studies have shown that AT2R knockout causes cognitive deficits only in female mice (25), indicating a potentially sex-specific function. Although AT2R is highly expressed in the mPFC (12,15), a region integral to both behavioral and physiological outcomes of fear, no studies reported to date have characterized AT2R-mPFC cell types and their potential sex-dependent role in conditioned fear learning and memory.
The mPFC is a robust top-down regulator of learning and memory and acts via its complex network of microcircuits in which interneurons can regulate the firing of excitatory efferent projection neurons to downstream brain regions such as the amygdala, brainstem, and thalamus (26). Highlighting the clinical importance of this circuit, these regulatory projections are hypoactive in PTSD, and reestablishing their activity can rescue impaired extinction and disordered fear learning (27, 28, 29, 30, 31, 32). Clinical studies have demonstrated that, among other neuropeptidergic modulators (31,33), the RAS plays an important role in mPFC fear regulation; treatment with the AT1R antagonist losartan in humans facilitated fear extinction, while concurrent functional magnetic resonance imaging showed that losartan increased mPFC connectivity to the basolateral amygdala (34). Given the opposing actions of AT1R and AT2R, and some evidence that the beneficial actions of losartan are in part mediated via AT2R (18), further investigation into the potential role of mPFC-AT2R in the regulation of fear extinction is warranted.
To provide a greater understanding of mPFC-AT2R’s mechanism and role in the regulation of fear learning and memory, we therefore aimed to characterize the cellular expression and functional role of mPFC-AT2R in conditioned fear learning. By doing so, we provide a novel understanding of AT2R’s mechanism and the cells that it acts on, as well as its role in the regulation of fear learning and memory. By using male and female mice, we investigated how AT2R’s female-biased effects influence both neurobiology and fear-related behavior; this factor is especially important given that women are twice as likely to develop PTSD as men (4,35,36). Here, we identify a novel population of AT2R-expressing interneurons in the mPFC that affect fear learning and memory in a sex-dependent manner, establishing a potential therapeutic target for disordered fear learning in females.
Methods and Materials
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the George Washington University and followed National Institutes of Health guidelines. Male and female transgenic mice (8–10 weeks old) were used for the following studies and were housed in a temperature- and humidity-controlled room on a 12-hour light/dark cycle. Food and water were available ad libitum. All mouse lines are on a C57BL/6 background and are summarized in Table 1.
Table 1.
Experimental Mouse Models Used
| Abbreviation | Genetic Information | Received From |
|---|---|---|
| AT2R-flox | Agtr2loxP/y | University of Florida |
| AT2R-cre | B6.Cg-Agtr2em 1(cre)Adk/J | University of Florida |
| Ai14 | B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Jackson Laboratories 007908 |
| AT2R-cre/tdTomato | Breeding AT2R-cre with Ai14 |
AT2R, angiotensin type 2 receptor.
Immunohistochemistry
Immunohistochemistry (IHC) was used to validate injection location of AT2R-flox mice as well as to characterize AT2R-expressing mPFC neurons in AT2R-cre/tdTomato reporter mice. Mice were anesthetized with urethane (275 mg/mL; Thermo Fisher Scientific) and perfused with 4% paraformaldehyde (Electron Microscope Science). Brains were removed and postfixed overnight in the same solution and then transferred to 30% sucrose for 2 days for dehydration. Brains were then embedded in optimal cutting temperature compound (Thermo Fisher Scientific) and stored overnight at −80 °C. A cryostat (CryoStar NX 50; Thermo Fisher Scientific) was used to section brains into 30-μM free-floating serial brain sections. A total of 18 to 20 serial sections from each brain were divided equally for costaining by antibody, and 3 to 5 sections per brain were stained per antibody. Sections were washed in phosphate-buffered saline for 15 minutes and blocked with 5% normal donkey serum, 5% bovine serum albumin, and 0.3% Triton-X-100 in phosphate-buffered saline for 1 hour at room temperature. Primary antibodies (Table 2) were added to the solution and brain sections incubated for 24 hours at 4 °C. Sections were then rinsed (3 × 15 minutes) in phosphate-buffered saline and incubated in corresponding secondary antibodies (Thermo Fisher Scientific) for 2 hours at room temperature. Following a final series of rinses (3 × 15 minutes), sections were mounted on Superfrost Plus slides (Thermo Fisher Scientific) and air dried before being cover slopped with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific). After staining, sections were imaged using 25× water immersion objective on a Zeiss spinning disk confocal microscope (Carl Zeiss). Colocalization was quantified by blinded researchers using Zeiss Microscope Software ZEN 2 (Carl Zeiss). AT2R/tdTomato+ cell bodies were identified, counted, and marked, and then colocalization with each marker was assessed by switching microscope channels and identifying and counting each stained cell body and whether it was also marked as AT2R+.
Table 2.
Antibodies Used for Immunohistochemistry
| Antibody | Manufacturer (Catalog #) | Species | Dilution |
|---|---|---|---|
| Anti-GFP | Abcam (ab13970) | Goat | 1:2000 |
| Anti-TBR1 | Abcam (ab31940) | Rabbit | 1:500 |
| Anti-Calretinin | Abcam (ab702) | Rabbit | 1:100 |
| Anti-Calbindin | Abcam (ab75524) | Mouse | 1:1000 |
| Anti-nNos | Immunostar (24287) | Rabbit | 1:2000 |
| Anti-Parvalbumin | Abcam (ab11427) | Rabbit | 1:2000 |
| Anti-Somatostatin | Immunostar (20067) | Rabbit | 1:2000 |
Animal Stereotaxic Surgery
Ketamine (82.5 mg/kg) and xylazine (12.5 mg/kg) anesthesia were intraperitoneally injected. Cre or GFP (green fluorescent protein)–expressing AAV (adeno-associated virus) (GFP pAAV.CMV.PI.EGFP.WPRE.bGH, Addgene 105530; Cre pENN.AAV.CMVs.PI.Cre.rBG, Addgene 105537) were bilaterally injected into the mPFC of AT2R-flox mice at 2.5 mm caudal, ±0.3 mm lateral to bregma, and 2.1 mm below the skull surface with an UltraMicroPump III and microprocessor controller (World Precision Instruments); 400 nL was injected bilaterally at a rate of 100 nL/min. Following surgery, the mice were group housed in their home cages for a 3-week postoperative period.
Messenger RNA Extraction and Reverse Transcriptase–Quantitative Polymerase Chain Reaction
Mice were sacrificed, and brains were collected and flash frozen. The mPFC was collected using a 1-mm diameter brain tissue punch. Total RNA was extracted from the punches using Trizol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions, and 1 μg of RNA was reverse transcribed to complementary DNA using qScript cDNA Supermix (QuantBiol). Gene expression changes for 18S and AT2R were detected using TaqMan primers (18S [Mm04277571_s1], AT2R [Mm1341373_m1]; Thermo Fisher Scientific) and the Applied Biosystems ViiA Real-Time PCR Systems by relative quantitative reverse transcriptase–polymerase chain reaction, and expression fold change was calculated using the ΔΔCq calculation method, normalizing to loading housekeeping gene 18S control.
Cue-Dependent Pavlovian Fear Conditioning and Extinction
Pavlovian fear conditioning, as previously described by our laboratory (7,9,11), was used to determine the effects of mPFC-AT2R deletion on conditioned fear behavior by pairing an auditory cue with a light foot shock. Mice were habituated to the fear conditioning chamber for 2 days (20 minutes, 40 minutes) before aversive associative conditioning on experimental day 3, during which mice received 5 conditioned stimulus (CS)/unconditioned stimulus pairings using a 30-second auditory cue (6 kHz, 75 dB, CS) coterminating with a foot shock (0.5 seconds, 0.6 mA, unconditioned stimulus) at an intertrial interval of 5 minutes. Mice were placed in a novel context and underwent extinction training 24 hours later (experimental day 4), consisting of a 5-minute pre-CS period followed by 30 CS presentations (30 seconds each, 30-second intertrial interval). This protocol was repeated on experimental day 5 in the same context as extinction training to test retention of fear extinction. Seven days after fear conditioning (experimental day 10), mice were placed in the novel context again, and the CS was presented 40 times. The mice were recorded, and freezing was calculated using FreezeFrame version 3.0 software (Actimetrics).
Generalized Anxiety Measures
Open field (OF) and elevated plus maze (EPM) tests were used as described previously by our laboratory (11) to determine whether deletion of mPFC-AT2Rs affected locomotion or anxiety-like behavior. These tests were performed 1 week after fear conditioning and extinction testing. In the OF test, mice were placed in the center of the OF arena (35 × 35 × 35 cm; opaque plexiglass) and allowed to freely explore for 30 minutes. Locomotion, speed, and position in the apparatus were recorded and analyzed using Anymaze software (ANY-Maze). In the EPM test, mice were placed in the center of the apparatus facing the same closed arm and allowed to freely explore for 5 minutes. The total arm entries, open arm entries, and percentage of time spent in the open arms were analyzed using Anymaze software (ANY-Maze).
Data Presentation and Statistical Analysis
All data analyses were completed using GraphPad Prism version 9.0 software (GraphPad) and checked for outliers using the ROUT outlier test (Q = 1%) as well as for variance and normality. In all data reported, p < .05 was considered statistically significant. Mean differences between groups were compared using unpaired t tests and 2-way repeated-measures analysis of variance with Holm-Šídák post hoc analyses. To assure rigor, reproducibility, and minimal use of animals, power analyses were conducted for behavioral studies to determine a sample size providing 80% power and significance at a level of 5%.
Results
AT2R-cre/tdTomato+ Cells Are Densely Expressed Throughout the mPFC of Males and Females
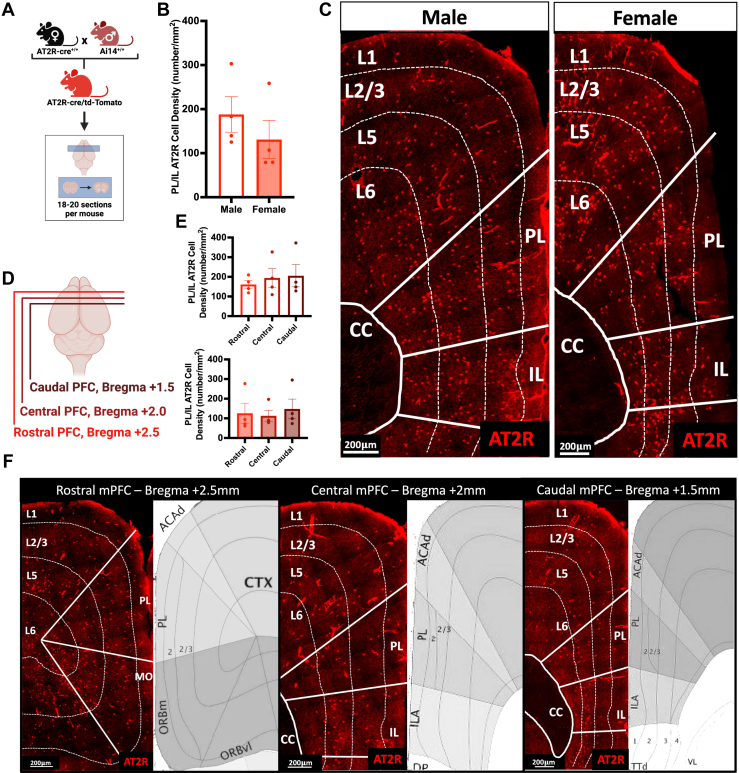
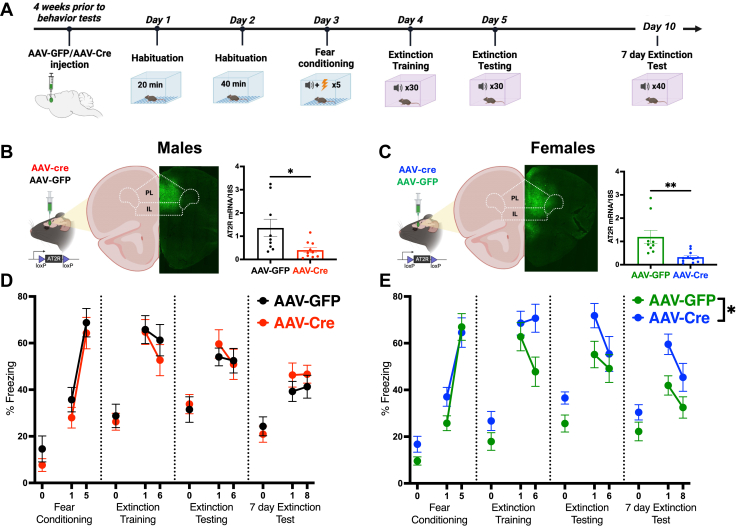
Using an AT2R-Cre mouse crossbred with a tdTomato reporter mouse (Figure 1A), AT2R-tdTomato+ expression in the mPFC of male and female mice was analyzed by IHC. Males and females had similar expression of AT2R density in the prelimbic (PL) and infralimbic (IL) regions of the mPFC (males: 187.8 ± 40.3 cells/mm2, females: 131.0 ± 43.0 cells/mm2, p = .37) (Figure 1B) throughout cortical layers L2/3–L6 (Figure 1C). This density did not change across the rostral-caudal depth of the mPFC (Figure 1D) in males or females (Figure 1E, F). This dense and nonspecific expression of AT2R+ cells through the mPFC indicates that they may contribute to mPFC top-down regulation of fear learning (27,29, 30, 31,37).
Figure 1.
AT2R/tdTomato+ mPFC expression in male and female mice. (A) Generation of the AT2R-cre/tdTomato reporter mouse and experimental approach to test mPFC-AT2R distribution with immunohistochemistry. (B) AT2R+ cells (red) are equally expressed in the PL and IL regions of the mPFC of male (n = 4) and female (n = 4) mice (t6 = 0.96, p = .37). (C) Representative coronal sections showing laminar distribution of AT2R+ neurons in L2/3, L5, and L6 of the mPFC of male and female mice. (D) Approach for assessing distribution of AT2R+ neurons through the depth of the mPFC. (E) Rostral/caudal depth of the mPFC had no impact on the distribution of AT2R+ neurons in male (rostral mPFC: 160.9 ± 20.0 cells/mm2, central mPFC: 194.3 ± 43.4 cells/mm2, caudal mPFC: 205.4 ± 56.5 cells/mm2, F2,9 = 0.36, p = .71) or female (rostral mPFC: 125.7 ± 50.9 cells/mm2, central mPFC: 112.8 ± 28.0 cells/mm2, caudal mPFC: 147.8 ± 50.1 cells/mm2, F2,9 = 0.16, p = .85) brains. (F) Representative coronal sections showing laminar distribution of AT2R+ neurons throughout the rostral/caudal mPFC compared with reference images from the Allen Mouse Brain Atlas (mouse.brain-map.org) at the same slice positions as the confocal images. Scale bar = 200 μm. ACAd, dorsal anterior cingulate area; AT2R, angiotensin II type 2 receptor; CC, corpus callosum; CTX, cortex; IL, infralimbic cortex; ILA, infralimbic area; L, layer; MO, motor area; mPFC, medial PFC; ORBm, medial orbital area; ORBvl, ventrolateral orbital area; PFC, prefrontal cortex; PL, prelimbic cortex; TTd, dorsal taenia tecta; VL, ventrolateral area.
Distribution of AT2R-Expressing Neurons Across mPFC Cell Types
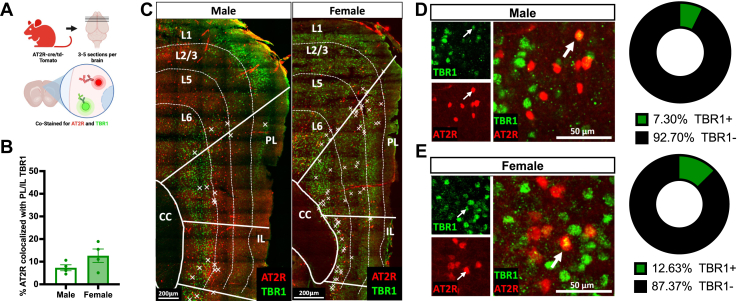
Although the mPFC is primarily populated by glutamatergic projection neurons (26), AT2R-tdTomato+ costaining with TBR1, a marker for excitatory neurons (38, 39, 40) (Figure 2A), revealed sparse colocalization in the mPFC of both males and females (n = 4/group, males: 7.3% ± 1.3%, females: 12.6% ± 3.0%, p = .15) (Figure 2B–E). This low percentage of costaining indicates that mPFC-AT2R may be acting at a local or microcircuit (29,41, 42, 43) level rather than acting directly on mPFC projection neurons.
Figure 2.
AT2R/tdTomato+ expression on excitatory mPFC neurons. (A) Experimental approach for costaining of AT2R-tdTomato+ cells (red) with TBR1 neurons (green) in the mPFC (n = 4/group). (B) Sex does not significantly affect AT2R colocalization with TBR1 in the PL and IL regions of the mPFC (male: 7.3% ± 1.3% colocalized, female: 12.6% ± 3.0% colocalized, t6 = 1.63, p = .15). (C) Representative coronal sections from male (left) and female (right) mPFC showing laminar distribution of AT2R/TBR1 colocalizations. Colocalization is indicated with X. (D, E) Representative coronal sections from the male (D) and female (E) mPFC. AT2R-tdTomato and TBR1 signals are colocalized (indicated with arrows). Right: percentage of AT2R+ neurons colocalized with TBR1 in the mPFC of male (D) and female (E) mice. Scale bar = 200 μm. AT2R, angiotensin II type 2 receptor; CC, corpus callosum; IL, infralimbic cortex; L, layer; mPFC, medial prefrontal cortex; PL, prelimbic cortex; TBR1, T-box brain transcription factor 1.
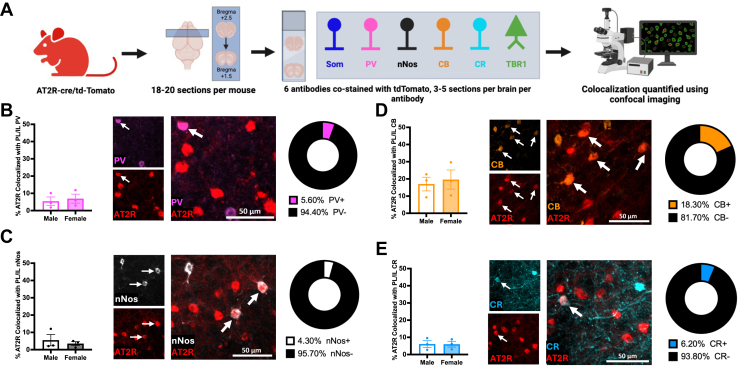
To further characterize AT2R+ cells in the mPFC, IHC was used for costaining analysis of mPFC sections taken from male and female AT2R-cre/tdTomato mice (n = 3/group) (Figure 3A). Colocalization was examined between AT2R-tdTomato+ cells and 5 interneuron cell-type markers: somatostatin (SOM) interneurons, parvalbumin (PV) interneurons, neuronal nitric oxide synthase (nNos) interneurons, calbindin (CB) interneurons, and calretinin (CR) interneurons. Sex did not impact AT2R+ cell distribution on PV interneurons (Figure 3B), nNos interneurons (Figure 3C), CB interneurons (Figure 3D), or CR interneurons (Figure 3E). Across males and females, there was minimal colocalization through the mPFC of AT2R-tdTomato+ cells with these 4 interneuron markers (PV 5.60%, nNos 4.30%, CB 18.30%, CR 6.20%) (Figure 3B–E).
Figure 3.
Characterization of AT2R/tdTomato+ in mPFC cell types. (A) Experimental approach to test mPFC AT2R+ cell type in the mPFC of male and female mice (n = 3/group). (B–E) Representative sections show that sex has no effect on AT2R (red) colocalization with (B) PV (pink) interneurons (male: 5.5% ± 2.5% colocalized, female: 6.9% ± 2.6% colocalized, t4 = 0.41, p = .70), (C) nNos (white) interneurons (male: 5.5% ± 3.3% colocalized, female: 3.5% ± 1.2% colocalized, t4 = 0.58, p = .59), (D) CB (orange) interneurons (male: 17.0% ± 4.0% colocalized, female: 19.6% ± 5.6% colocalized, t4 = 0.38, p = .72), or (E) CR (teal) interneurons (male: 6.1% ± 1.9% colocalized, female: 6.0% ± 1.7% colocalized, t4 = 0.03, p = .98). Colocalization indicated with arrows. Scale bar = 50 μm. AT2R, angiotensin II type 2 receptor; CB, calbindin; CR, calretinin; IL, infralimbic cortex; mPFC, medial prefrontal cortex; nNos, neuronal nitric oxide synthase; PL, prelimbic cortex; PV, parvalbumin; Som, somatostatin.
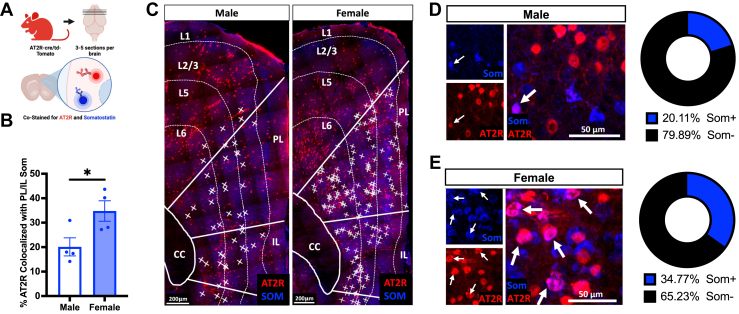
Increased SOM+ mPFC-AT2R–Expressing Cells in Female Mice
Colocalization analysis between AT2R and somatostatin in mPFC brain sections from male and female AT2R-cre/tdTomato mice (n = 4/group) (Figure 4A) revealed that somatostatin was the primary AT2R-expressing cell type in females and males and was more densely colocalized in the PL/IL of females than males (males: 20.1% ± 3.7%, females: 34.8% ± 4.2%, p = .03) (Figure 4B–E). In the PL of both males and females, somatostatin colocalization was distributed throughout L2/3–L6, while in the IL, colocalization was biased toward L5 and L6 (Figure 4C). Because SOM interneurons in the mPFC have a known regulatory role in fear memory and extinction (44, 45, 46, 47) and are activated by stress in a sex-dependent manner (48), we sought to examine the functional role of these AT2R-expressing neurons in fear extinction in male and female AT2R-flox mice.
Figure 4.
AT2R/tdTomato+ expression on mPFC SOM interneurons in female mice. (A) Experimental approach for costaining of AT2R-tdTomato+ cells (red) with SOM neurons (blue) in the mPFC (n = 4/group). (B) Females have significantly greater AT2R/SOM colocalizations in the PL and IL regions of the mPFC (males: 20.1% ± 3.7% colocalized, females: 34.8% ± 4.2% colocalized, t6 = 2.62, p = .03). (C) Representative coronal sections from male (left) and female (right) mPFC showing laminar distribution of AT2R/SOM colocalizations. Colocalizations are indicated with X. (D, E) Left: representative coronal sections from the male (D) and female (E) mPFC. AT2R-tdTomato and somatostatin signals are colocalized (indicated with arrows). Right: percentage of AT2R+ neurons colocalized with SOM in the mPFC of male (D) and female (E) mice. Scale bar = 200 μm. ∗p < .05. AT2R, angiotensin II type 2 receptor; CC, corpus callosum; IL, infralimbic cortex; L, layer; mPFC, medial prefrontal cortex; PL, prelimbic cortex; SOM/Som, somatostatin.
Cre/LoxP mPFC-AT2R Deletion Inhibits Fear Extinction in Female but Not Male Mice
To assess the role of mPFC-AT2R in fear learning, AT2Rflox/flox male and female mice received either Cre-expressing AAV or GFP-expressing AAV into the mPFC to selectively delete AT2R. Four weeks postinjection, AT2R gene expression was significantly decreased in AAV-Cre injected mice compared with the AAV-GFP control group, confirming site-specific AT2R deletion in the mPFC (males: p = .02; females: p < .01) (Figure 5B, C). Once mPFC-AT2R deletion was confirmed, we used Pavlovian fear conditioning (5 CS-unconditioned stimulus pairings) and extinction (3 days, 30 CS per day) to determine the impact on fear behavior (Figure 5A). In males, AT2R deletion had no impact on fear conditioning or freezing to the CS during extinction testing (p = .76) (Figure 5D).
Figure 5.
AT2R cre/lox mPFC deletion delays extinction learning in females but not males. (A) Experimental protocol for the auditory cue-dependent fear extinction test. (B) Left: strategy for injection of AAV-cre (red) or AAV-GFP (black) into the mPFC of male AT2R-flox mice and representative injection image in the mPFC. Right: injection of AAV-cre into the mPFC successfully decreased expression of AT2R in males (GFP: 1.35 ± 0.38 fold change, Cre: 0.40 ± 0.11 fold change, t17 = 2.54, p = .02). (C) Left: strategy for injection of AAV-cre (blue) or AAV-GFP (green) into the mPFC of female AT2R-flox mice and representative injection image in the mPFC. Right: injection of AAV-cre into the mPFC decreased expression of AT2R in females (GFP: 1.20 ± 0.28 fold change, Cre: 0.32 ± 0.07 fold change, t18 = 3.28, p < .01). (D) AT2R deletion from the male mPFC does not impact fear acquisition or extinction learning across 3 testing days in males (F1,17 = 0.09, p = .76). (E) In females, AT2R deletion from the mPFC significantly inhibits extinction learning (F1,20 = 5.35, p = .03). n = 9–12/group. Blocks represent groupings of 5 conditioned stimuli exposures; for example, block 1 represents conditioned stimuli 1–5, while block 6 represents conditioned stimuli 25–30. ∗p < .05, ∗∗p < .01. AAV, adeno-associated virus; AT2R, angiotensin II type 2 receptor; GFP, green fluorescent protein; IL, infralimbic cortex; mPFC, medial prefrontal cortex; mRNA, messenger RNA; PL, prelimbic cortex.
In females, however, mPFC-AT2R deletion significantly inhibited extinction learning across 3 days (p = .03) (extinction training, extinction testing, and a 7-day test of long-term extinction retention). Cre-injected females displayed higher freezing across all days of extinction testing. Together, these results show that mPFC-AT2R deletion significantly delays both short- and long-term extinction learning in female, but not male, AT2R-flox mice.
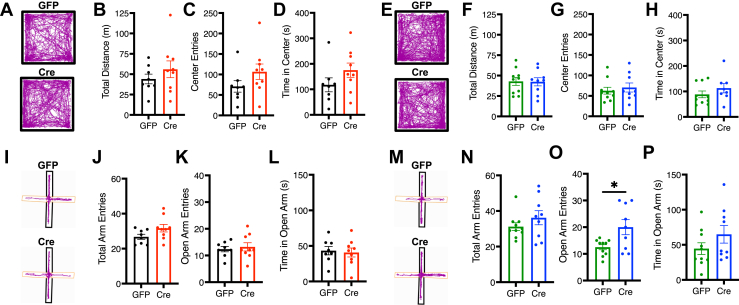
Effects of mPFC-AT2R Deletion on Locomotion, Approach/Avoidance, and Exploration Behaviors
To determine whether the difference in fear extinction observed with AT2R-mPFC deletion may be a result of effects on anxiety, locomotion, or a deficit in exploratory behavior, male and female GFP- and Cre-injected AT2R-flox mice underwent OF (Figure 6A, E) and EPM (Figure 6I, M) testing. mPFC-AT2R deletion did not affect the total distance traveled in the OF in males (GFP 44.11 ± 5.92 m, Cre 56.00 ± 10.29 m, p = .35) (Figure 6B) or females (GFP 42.86 ± 4.85 m, Cre 42.39 ± 4.97 m, p = .95) (Figure 6F) or the total arm entries in the EPM in males (GFP 26.75 ± 1.45, Cre 31.67 ± 2.17, p = .09) (Figure 6J) or females (GFP 31.20 ± 2.18, Cre 36.22 ± 3.96, p = .27) (Figure 6N). While mPFC-AT2R deletion did not impact anxiety-like measures in the OF in males (center entries: GFP 70.75 ± 13.99, Cre 106.6 ± 18.87, p = .16; time in center: GFP 117.60 ± 27.33 seconds, Cre 175.20 ± 27.85 seconds, p = .16) (Figure 6C, D) or females (center entries: GFP 62.60 ± 8.40, Cre 70.44 ± 11.06, p = .58; time in center: GFP 88.38 ± 13.22 seconds, Cre 113.00 ± 18.67 seconds, p = .29) (Figure 6G, H), nor approach, avoidance, or exploratory behavior in the EPM in males (open arm entries: GFP 12.38 ± 1.02, Cre 13.22 ± 1.54, p = .66; time in open arm: GFP 43.25 ± 5.88 seconds, Cre 40.73 ± 6.27 seconds, p = .78) (Figure 6K, L), mPFC-AT2R deletion did increase some measures of exploratory behavior in the EPM in females (open arm entries: GFP 12.50 ± 0.96, Cre 20.00 ± 2.81, p = .02; time in open arm: GFP 44.54 ± 8.37 seconds, Cre 64.99 ± 12.62 seconds, p = .19) (Figure 6K, L). Taken together, these results demonstrate that AT2R deletion from the mPFC did not affect locomotion in males or females but may have increased some aspects of exploratory behavior in females.
Figure 6.
Effects of AT2R cre/lox medial prefrontal cortex deletion on behavioral assays of locomotion and exploration in males and females. (A, E) Representative locomotor traces of GFP- and Cre-injected males (AAV-GFP: black; AAV-Cre: red) and females (AAV-GFP: green; AAV-Cre: blue) in the open field test. (A–H) AT2R deletion has no impact on locomotion [(B)t15 = 0.97, p = .35; (F)t17 = 0.07, p = .95, total distance traveled] or generalized anxiety [(C)t15 = 1.49, p = .16; (G)t17 = 0.57, p = .58, center entries; (D)t15 = 1.47, p = .16; (H)t17 = 1.11, p = .29, time in center] in males or females. (I, M) Representative locomotor traces of GFP- and Cre-injected males and females in the elevated plus maze test. (I–P) AT2R deletion has no impact on exploratory behavior [(J) total arm entries, t15 = 1.84, p = .09] or generalized anxiety in males [(K) open arm entries, t15 = 0.45, p = .66; (L) time in open arm, t15 = 0.29, p = .78]. Medial prefrontal cortex AT2R deletion increases some aspects of exploratory behavior in females [(N) total arm entries, t17 = 1.14, p = .27; (O) open arm entries, t17 = 2.64, p = .02; (P) open arm time, t17 = 1.38, p = .19]. n = 8–10/group. ∗p < .05. AT2R, angiotensin II type 2 receptor; GFP, green fluorescent protein.
Discussion
The current results identified mPFC-AT2R+ interneurons and their role in the extinction of conditioned fear in a sex-dependent manner, supporting the significant ongoing research into the neurobiology of PTSD that is directed toward enhancing translational preclinical models with the goal of developing more effective therapies (49,50). This is especially important when investigating sex differences in PTSD, given the lack of therapies developed using female animals (36,51). Many studies are focused on the neuronal circuitry of PTSD in therapy development because pharmacologically targeting the circuits involved in fear extinction, for example, is critical for further understanding and developing exposure-based therapies, one of the most widely used treatment strategies for PTSD (28, 52, 53, 54). Further understanding sex-based differences in neuronal circuits may also provide insight into developing PTSD therapies that are effective in both males and females (35,36,51,55). Consistent with this, recent preclinical and clinical studies have identified the brain RAS as a potential drug target for extinction-based therapies and have begun investigating the neuronal mechanisms that it may work through (7,8,11,34,56).
Previous studies in mice have shown that brain AT2R can influence the neural control of cognition and fear memory as well as physiological and cardiovascular stress, potentially in a sex-dependent manner (11,13,19,20,23,25, 57,58). For example, pharmacological activation of AT2R in the central amygdala decreases the expression of a fear memory (11), while global knockout of AT2R results in anxiety-like behavior and cognitive deficits, particularly in females (19,25,57). In the hypothalamus, activation of AT2R has been shown to attenuate experimental hypertension by decreasing the firing of pressor neurons, a protective effect that, similar to behavioral findings, is also more pronounced in females (23,58).
Studies involving AT1R can also inform the functional role of AT2R because AT1R and AT2R serve opposing physiological roles. Preclinical studies from our lab and others have shown that systemic AT1R blockade with losartan and central amygdala–specific AT1R deletion enhance extinction learning (7,59,60). In clinical studies, losartan improves extinction, decreases PTSD symptom severity, and increases top-down control of the mPFC over the amygdala (34,61). Because evidence shows that losartan upregulates AT2R, this indicates that the benefit of AT1R blockade in both humans and mice may be via an AT2R-dependent mechanism (62,63).
Although the AT2R receptor is expressed in several regions of the brain that can influence these functions directly, such as the hypothalamus, brainstem, amygdala, and hippocampus (12), the current study focused on the mPFC, a critical area for fear or threat learning in both humans and mice (27,28). While immunostaining studies have shown that AT2R is highly expressed in the rodent mPFC (12,15), no research reported to date has investigated the function of mPFC-AT2R or how its activity affects top-down regulation of fear learning. Here, we identified a novel population of mPFC-AT2R-expressing SOM interneurons in the mPFC and and their potential role in fear-related behaviors.
To first classify the cell types that express AT2R in the mPFC, we crossed AT2R-cre mice (58) with Ai14 tdTomato reporter mice. Consistent with previous immunostaining studies (12,15), we found a high density of AT2R-expressing cells throughout the PL and IL cortices of the mPFC (Figure 1), 2 subnuclei heavily involved in top-down regulation of fear and extinction (26,27,29,64). The mPFC is primarily (80%–90%) glutamatergic (26); however, IHC revealed that only 5% to 15% of AT2R-expressing cells in the mPFC were excitatory (Figure 2), indicating that their function may be through interneuron modulation. In the smaller (10%–20%) class of mPFC neurons, several overlapping and nonoverlapping interneuron types are vital to modulating the activity of excitatory projection neurons to regulate fear memory and extinction (26,41,43,44,46,48,65). However, these populations often have opposing or additive effects on mPFC output. For example, the 2 main interneuron populations, PV and SOM, are both necessary for fear memory processing (41,44); however, PV interneurons target the cell body of excitatory projecting cells to control neuronal output, while SOM interneurons target the apical dendrite to control inputs onto projecting cells (66). There is also a large degree of heterogeneity within interneuron subtypes, as illustrated by SOM interneurons that can be broadly classified into Martinotti cells, the primary subtype in L2/3, L5, and L6 that preferentially synapse onto excitatory projecting cells, or non-Martinotti cells, which are primarily in L4 and synapse onto PV interneurons (47,66, 67, 68). Therefore, it is essential to characterize which mPFC interneuron subtypes AT2R is expressed on to advance understanding of its functional role and mechanism of action.
Although AT2R has been studied using both interneuron (13) and non-interneuron (11,58) populations, its functional role in the mPFC has not been examined. After determining sparse excitatory coexpression (Figure 2), we stained AT2R-cre/tdTomato brains for 5 interneuron markers (PV, SOM, nNos, CB, and CR) to elucidate its potential role in specific mPFC interneuron function. In both males and females, we found that AT2R primarily colocalized with SOM+-expressing interneurons, and females had greater coexpression with somatostatin than males across the PL and IL (Figure 4), indicating that AT2R may preferentially modulate SOM+ interneurons, a key population of interneurons previously identified to play a role in cognition (43,69,70) and fear learning (44, 45, 46) distributed in cortical layers L6 through L2/3 (26,47). The minimal levels of AT2R colocalization with other interneuron populations (Figure 3) may inform the type of somatostatin cell that AT2R is found on. For example, denser levels of AT2R/CB costaining combined with lower coexpression with CR and nNos interneurons indicates that AT2R is primarily found on excitatory cell-projecting Martinotti cells (47,68,71,72), although this conclusion is limited by the current study's lack of AT2R-SOM interneuron triple immunostaining. Additionally, the distribution of somatostatin colocalization in females in L5 and L2/3 (Figure 4) indicates that mPFC-AT2R may have a role in the top-down control of fear and reward processing (44,45). While our study did not include mPFC-AT2R electrophysiology, previous research has demonstrated that AT2R activation reduces spontaneous neural activity by enhancing potassium channel activity (15,21,22,58,73). Consequently, we suggest a working hypothesis that AT2R activation diminishes somatostatin inhibition onto excitatory projection neurons, thereby enhancing mPFC’s top-down regulation of fear. Our IHC findings (Figure 4) combined with the known sex-biased effects of brain AT2R (23,25) lead us to also hypothesize that AT2R would have a greater effect on fear memory in female mice.
Recent studies from our laboratory and others have demonstrated a role for AT2R in the expression of conditioned fear and behavioral stress (11,18). The current study used male and female AT2R-flox mice to further investigate the effects of selective mPFC-AT2R deletion on fear extinction learning using 3 extinction tests across 7 days to examine both short- and long-term extinction memory. Consistent with previous studies on AT2R sex-specific effects (23, 24, 25,74), mPFC-AT2R deletion significantly delayed short- and long-term extinction learning in females but had no effect on fear acquisition or extinction in males (Figure 5). Future studies will need to adopt a more targeted Cre/Lox approach, focusing on specific subnuclei within the mPFC, such as the IL and PL regions, which play distinct roles in regulating fear expression and extinction (75,76).
Interestingly, while mPFC-AT2R deletion did not impact locomotion or approach/avoidance behavior in males or females, increased exploratory behavior in females (Figure 6) was observed. These effects of AT2R deletion on extinction learning and exploration may suggest a functional role for AT2R-mPFC SOM+ interneurons and support previous studies showing mPFC SOM+ cells may encode fear memory (44, 45, 46) and regulate stress in a sex-dependent manner (48). Furthermore, increased somatostatin signaling, which we hypothesize occurs with AT2R deletion, increases exploratory behavior in mice (69).
The behavioral effects that we see with mPFC-AT2R deletion may also be dependent on a TrkB (tyrosine kinase receptor 2)/BDNF (brain-derived neurotrophic factor) signaling pathway because there is emerging evidence that cortical AT2R is dependent on coexpression with TrkB to influence learned fear (18,77,78). Future studies will directly interrogate the electrophysiological impact of AT2R activation or deletion on SOM interneuron activity and mPFC projections, as well as whether activation of AT2R-expressing mPFC interneurons can facilitate fear extinction.
Conclusions
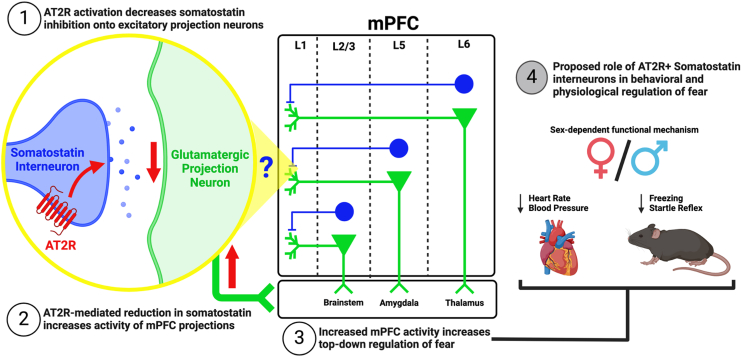
In summary, these results identify an AT2R-expressing SOM+ interneuron population in the mPFC, with increased expression in females compared to males. We also showed that AT2R deletion from the mPFC inhibited extinction learning in females only. Taken together, these results provide a basis for our working hypothesis that activation of mPFC-AT2R on SOM+ interneurons decreases inhibition onto excitatory projection neurons, thereby increasing mPFC modulation to its downstream brain targets involved in conditioned fear such as the amygdala, thalamus, and brainstem (Figure 7).
Figure 7.
Proposed mechanism for mPFC-AT2R+ interneurons role in conditioned fear. (1) Activation of mPFC-AT2R on somatostatin interneurons of the mPFC decreases the activity of these interneurons. (2) This decreases inhibition of glutamatergic projection neurons and increases their firing to downstream regions. (3) Because of preferential somatostatin inhibition on brainstem (L2/3), amygdala (L5), and thalamic (L6) projecting excitatory neurons, mPFC top-down regulation is increased to these regions [see (26)]. (4) Potential role of mPFC+AT2R interneurons in the physiology of conditioned fear. AT2R, angiotensin II type 2 receptor; L, layer; mPFC, medial prefrontal cortex.
Our study offers novel insights into the potential neurobiological mechanisms of mPFC-AT2R regulation of conditioned fear. However, several limitations prevent this study from directly linking the expression of mPFC-AT2Rs on SOM interneurons to their sex-specific function in fear extinction. Future studies are needed to improve our understanding of the mechanisms that underlie mPFC-AT2R sex-dependent fear learning, which may lead to improved therapeutics for the treatment of PTSD and comorbid cardiovascular disease risk.
Acknowledgments and Disclosures
This work and PJM were supported by Congressionally Directed Medical Research Programs (Grant No. PR210574).
We thank Drs. Eric G. Krause and Annette D. de Kloet for generously providing the AT2R-cre and AT2R-flox mouse lines. We would also like to thank Dr. Anastas Popratiloff, Dr. Cheryl Clarkson-Paredes, and the GW Nanofabrication and Imaging Center. Finally, we thank the animal care and veterinary staff at the George Washington University for maintaining the health and well-being of our research animals. Figures were created with BioRender.com.
A previous version of this article was published as a preprint on bioRxiv: https://www.biorxiv.org/content/10.1101/2023.11.21.568156v1
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2024.100340.
Supplementary Material
References
- 1.Lancaster C.L., Teeters J.B., Gros D.F., Back S.E. Posttraumatic stress disorder: Overview of evidence-based assessment and treatment. J Clin Med. 2016;5:105. doi: 10.3390/jcm5110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edmondson D., von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–329. doi: 10.1016/S2215-0366(16)30377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brudey C., Park J., Wiaderkiewicz J., Kobayashi I., Mellman T.A., Marvar P.J. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2015;309:R315–R321. doi: 10.1152/ajpregu.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimerling R., Allen M.C., Duncan L.E. Chromosomes to social contexts: Sex and gender differences in PTSD. Curr Psychiatry Rep. 2018;20:114. doi: 10.1007/s11920-018-0981-0. [DOI] [PubMed] [Google Scholar]
- 5.Seligowski A.V., Duffy L.A., Merker J.B., Michopoulos V., Gillespie C.F., Marvar P.J., et al. The renin-angiotensin system in PTSD: A replication and extension. Neuropsychopharmacology. 2021;46:750–755. doi: 10.1038/s41386-020-00923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury N.M., Marvar P.J., Gillespie C.F., Wingo A., Schwartz A., Bradley B., et al. The renin-angiotensin pathway in posttraumatic stress disorder: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marvar P.J., Goodman J., Fuchs S., Choi D.C., Banerjee S., Ressler K.J. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry. 2014;75:864–872. doi: 10.1016/j.biopsych.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swiercz A., Iyer L., Yu Z., Edwards A., Prashant N.M., Nguyen B.N., et al. Evaluation of an angiotensin Type 1 receptor blocker on the reconsolidation of fear memory. Biol Psychiatry. 2020;87:S171–S172. doi: 10.1038/s41398-020-01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swiercz A.P., Seligowski A.V., Park J., Marvar P.J. Extinction of fear memory attenuates conditioned cardiovascular fear reactivity. Front Behav Neurosci. 2018;12:276. doi: 10.3389/fnbeh.2018.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson L., Eldahshan W., Fagan S.C., Ergul A. Within the brain: The renin angiotensin system. Int J Mol Sci. 2018;19:876. doi: 10.3390/ijms19030876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Z., Swiercz A.P., Moshfegh C.M., Hopkins L., Wiaderkiewicz J., Speth R.C., et al. Angiotensin II Type 2 receptor–expressing neurons in the central amygdala influence fear-related behavior. Biol Psychiatry. 2019;86:899–909. doi: 10.1016/j.biopsych.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet A.D., Wang L., Ludin J.A., Smith J.A., Pioquinto D.J., Hiller H., et al. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2016;221:891–912. doi: 10.1007/s00429-014-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kloet A.D., Pitra S., Wang L., Hiller H., Pioquinto D.J., Smith J.A., et al. Angiotensin Type-2 receptors influence the activity of vasopressin neurons in the paraventricular nucleus of the hypothalamus in male mice. Endocrinology. 2016;157:3167–3180. doi: 10.1210/en.2016-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammed M., Sumners C., Sheng W., Harden S.W., Frazier C.J., Johnson D., et al. Angiotensin AT2 receptors in the solitary tract nucleus lower blood pressure via inhibition of GABA signaling. FASEB J. 2020;34:1–11. [Google Scholar]
- 15.Sumners C., Alleyne A., Rodríguez V., Pioquinto D.J., Ludin J.A., Kar S., et al. Brain angiotensin type-1 and type-2 receptors: Cellular locations under normal and hypertensive conditions. Hypertens Res. 2020;43:281–295. doi: 10.1038/s41440-019-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kloet A.D., Steckelings U.M., Sumners C. Protective angiotensin Type 2 receptors in the brain and hypertension. Curr Hypertens Rep. 2017;19:46. doi: 10.1007/s11906-017-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faria-Costa G., Leite-Moreira A., Henriques-Coelho T. Cardiovascular effects of the angiotensin type 2 receptor. Rev Port Cardiol. 2014;33:439–449. doi: 10.1016/j.repc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Diniz C.R.A.F., Casarotto P.C., Fred S.M., Biojone C., Castrén E., Joca S.R.L. Antidepressant-like effect of losartan involves TRKB transactivation from angiotensin receptor type 2 (AGTR2) and recruitment of FYN. Neuropharmacology. 2018;135:163–171. doi: 10.1016/j.neuropharm.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Okuyama S., Sakagawa T., Chaki S., Imagawa Y., Ichiki T., Inagami T. Anxiety-like behavior in mice lacking the angiotensin II type-2 receptor. Brain Res. 1999;821:150–159. doi: 10.1016/s0006-8993(99)01098-7. [DOI] [PubMed] [Google Scholar]
- 20.Kerr D.S., Bevilaqua L.R.M., Bonini J.S., Rossato J.I., Köhler C.A., Medina J.H., et al. Angiotensin II blocks memory consolidation through an AT2 receptor-dependent mechanism. Psychopharmacol (Berl) 2005;179:529–535. doi: 10.1007/s00213-004-2074-5. [DOI] [PubMed] [Google Scholar]
- 21.Gao L., Zucker I.H. AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol. 2011;11:124–130. doi: 10.1016/j.coph.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong H.G., Marshall K.C. Angiotensin II modulation of glutamate excitation of locus coeruleus neurons. Neurosci Lett. 1990;118:261–264. doi: 10.1016/0304-3940(90)90642-m. [DOI] [PubMed] [Google Scholar]
- 23.Barsha G., Walton S.L., Kwok E., Denton K.M. In: Sex Differences in Cardiovascular Physiology and Pathophysiology. LaMarca B., Alexander B.T., editors. Elsevier; Amsterdam: 2019. Sex differences in the role of the angiotensin type 2 receptor in the regulation of blood pressure; pp. 73–103. [Google Scholar]
- 24.Armando I., Jezova M., Juorio A.V., Terrón J.A., Falcón-Neri A., Semino-Mora C., et al. Estrogen upregulates renal angiotensin II AT(2) receptors. Am J Physiol Renal Physiol. 2002;283:F934–F943. doi: 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- 25.Sakata A., Mogi M., Iwanami J., Tsukuda K., Min L.-J., Fujita T., et al. Sex-different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res. 2009;1300:14–23. doi: 10.1016/j.brainres.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 26.Anastasiades P.G., Carter A.G. Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci. 2021;44:550–563. doi: 10.1016/j.tins.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnsten A.F.T. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnsten A.F.T., Raskind M.A., Taylor F.B., Connor D.F. The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89–99. doi: 10.1016/j.ynstr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courtin J., Bienvenu T.C.M., Einarsson E.Ö., Herry C. Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience. 2013;240:219–242. doi: 10.1016/j.neuroscience.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Gilmartin M.R., Balderston N.L., Helmstetter F.J. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014;37:455–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giustino T.F., Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fucich E.A., Paredes D., Saunders M.O., Morilak D.A. Activity in the ventral medial prefrontal cortex is necessary for the therapeutic effects of extinction in rats. J Neurosci. 2018;38:1408–1417. doi: 10.1523/JNEUROSCI.0635-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giustino T.F., Maren S. Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci. 2018;12:43. doi: 10.3389/fnbeh.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F., Geng Y., Xin F., Li J., Feng P., Liu C., et al. Human extinction learning is accelerated by an angiotensin antagonist via ventromedial prefrontal cortex and its connections with basolateral amygdala. Biol Psychiatry. 2019;86:910–920. doi: 10.1016/j.biopsych.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Hiscox L.V., Sharp T.H., Olff M., Seedat S., Halligan S.L. Sex-based contributors to and consequences of post-traumatic stress disorder. Curr Psychiatry Rep. 2023;25:233–245. doi: 10.1007/s11920-023-01421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pooley A.E., Benjamin R.C., Sreedhar S., Eagle A.L., Robison A.J., Mazei-Robison M.S., et al. Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol Sex Differ. 2018;9:31. doi: 10.1186/s13293-018-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen Institute for Brain Science Allen Reference Atlas-Mouse Brain. 2004. atlas.brain-map.org Available at:
- 38.Hevner R.F., Shi L., Justice N., Hsueh Y.-P., Sheng M., Smiga S., et al. Tbr1 regulates differentiation of the preplate and Layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 39.Dwyer N.D., O’Leary D.D.M. Tbr1 conducts the orchestration of early cortical development. Neuron. 2001;29:309–311. doi: 10.1016/s0896-6273(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 40.Bedogni F., Hodge R.D., Elsen G.E., Nelson B.R., Daza R.A.M., Beyer R.P., et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binette A.N., Liu J., Bayer H., Crayton K.L., Melissari L., Sweck S.O., Maren S. Parvalbumin-positive interneurons in the medial prefrontal cortex regulate stress-induced fear extinction impairments in male and female rats. J Neurosci. 2023;43:4162–4173. doi: 10.1523/JNEUROSCI.1442-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung D.W., Wills Z.P., Fish K.N., Lewis D.A. Developmental pruning of excitatory synaptic inputs to parvalbumin interneurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2017;114:E629–E637. doi: 10.1073/pnas.1610077114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S.-S., Mack N.R., Shu Y., Gao W.-J. Prefrontal GABAergic interneurons gate long-range afferents to regulate prefrontal cortex-associated complex behaviors. Front Neural Circuits. 2021;15 doi: 10.3389/fncir.2021.716408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummings K.A., Clem R.L. Prefrontal somatostatin interneurons encode fear memory. Nat Neurosci. 2020;23:61–74. doi: 10.1038/s41593-019-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cummings K.A., Bayshtok S., Dong T.N., Kenny P.J., Clem R.L. Control of fear by discrete prefrontal GABAergic populations encoding valence-specific information. Neuron. 2022;110:3036–3052.e5. doi: 10.1016/j.neuron.2022.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koppensteiner P., Von Itter R., Melani R., Galvin C., Lee F.S., Ninan I. Diminished fear extinction in adolescents is associated with an altered somatostatin interneuron–mediated inhibition in the infralimbic cortex. Biol Psychiatry. 2019;86:682–692. doi: 10.1016/j.biopsych.2019.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riedemann T. Diversity and function of somatostatin-expressing interneurons in the cerebral cortex. Int J Mol Sci. 2019;20:2952. doi: 10.3390/ijms20122952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girgenti M.J., Wohleb E.S., Mehta S., Ghosal S., Fogaca M.V., Duman R.S. Prefrontal cortex interneurons display dynamic sex-specific stress-induced transcriptomes. Transl Psychiatry. 2019;9:292. doi: 10.1038/s41398-019-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andero R., Ressler K.J. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cain C.K. In: Milad M.R., Norrholm S.D., editors. Vol. 64. Springer; Berlin, Heidelberg: 2023. Beyond fear, extinction, and freezing: Strategies for improving the translational value of animal conditioning research; pp. 19–57. (Fear Extinction. Current Topics in Behavioral Neurosciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shansky R.M. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenster R.J., Lebois L.A.M., Ressler K.J., Suh J. Brain circuit dysfunction in post-traumatic stress disorder: From mouse to man. Nat Rev Neurosci. 2018;19:535–551. doi: 10.1038/s41583-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dulka B.N., Bagatelas E.D., Bress K.S., Grizzell J.A., Cannon M.K., Whitten C.J., Cooper M.A. Chemogenetic activation of an infralimbic cortex to basolateral amygdala projection promotes resistance to acute social defeat stress. Sci Rep. 2020;10:6884. doi: 10.1038/s41598-020-63879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singewald N., Schmuckermair C., Whittle N., Holmes A., Ressler K.J. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day H.L.L., Stevenson C.W. The neurobiological basis of sex differences in learned fear and its inhibition. Eur J Neurosci. 2020;52:2466–2486. doi: 10.1111/ejn.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazaroni T.L.N., Bastos C.P., Moraes M.F.D., Santos R.S., Pereira G.S. Angiotensin-(1-7)/Mas axis modulates fear memory and extinction in mice. Neurobiol Learn Mem. 2016;127:27–33. doi: 10.1016/j.nlm.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Jing F., Mogi M., Sakata A., Iwanami J., Tsukuda K., Ohshima K., et al. Direct stimulation of angiotensin II Type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab. 2012;32:248–255. doi: 10.1038/jcbfm.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohammed M., Johnson D.N., Wang L.A., Harden S.W., Sheng W., Spector E.A., et al. Targeting angiotensin type-2 receptors located on pressor neurons in the nucleus of the solitary tract to relieve hypertension in mice. Cardiovasc Res. 2022;118:883–896. doi: 10.1093/cvr/cvab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Z., Kisner A., Bhatt A., Polter A.M., Marvar P.J. Central amygdala angiotensin type 1 receptor (Agtr1) expressing neurons contribute to fear extinction. Neuropharmacology. 2023;229 doi: 10.1016/j.neuropharm.2023.109460. [DOI] [PubMed] [Google Scholar]
- 60.Stout D.M., Risbrough V.B. Angiotensin II signaling and fear extinction: Translational evidence and novel receptor targets. Biol Psychiatry. 2019;86:874–876. doi: 10.1016/j.biopsych.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 61.Stein M.B., Jain S., Simon N.M., West J.C., Marvar P.J., Bui E., et al. Randomized, placebo-controlled trial of the angiotensin receptor antagonist losartan for posttraumatic stress disorder. Biol Psychiatry. 2021;90:473–481. doi: 10.1016/j.biopsych.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Wang D., Hu S., Zhu J., Yuan J., Wu J., Zhou A., et al. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J Cell Mol Med. 2013;17:1577–1587. doi: 10.1111/jcmm.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He D.-H., Lin J.-X., Zhang L.-M., Xu C.-S., Xie Q. Early treatment with losartan effectively ameliorates hypertension and improves vascular remodeling and function in a prehypertensive rat model. Life Sci. 2017;173:20–27. doi: 10.1016/j.lfs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Capuzzo G., Floresco S.B. Prelimbic and infralimbic prefrontal regulation of active and inhibitory avoidance and reward-seeking. J Neurosci. 2020;40:4773–4787. doi: 10.1523/JNEUROSCI.0414-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodward E.M., Coutellier L. Age- and sex-specific effects of stress on parvalbumin interneurons in preclinical models: Relevance to sex differences in clinical neuropsychiatric and neurodevelopmental disorders. Neurosci Biobehav Rev. 2021;131:1228–1242. doi: 10.1016/j.neubiorev.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shepherd G.M., Grillner S., editors. Handbook of Brain Microcircuits. 2nd ed. Oxford University Press; New York, NY: 2018. [Google Scholar]
- 67.Liguz-Lecznar M., Dobrzanski G., Kossut M. Somatostatin and somatostatin-containing interneurons-From plasticity to pathology. Biomolecules. 2022;12:312. doi: 10.3390/biom12020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nigro M.J., Hashikawa-Yamasaki Y., Rudy B. Diversity and connectivity of Layer 5 somatostatin-expressing interneurons in the mouse barrel cortex. J Neurosci. 2018;38:1622–1633. doi: 10.1523/JNEUROSCI.2415-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brockway D.F., Griffith K.R., Aloimonos C.M., Clarity T.T., Moyer J.B., Smith G.C., et al. Somatostatin peptide signaling dampens cortical circuits and promotes exploratory behavior. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kupferschmidt D.A., Cummings K.A., Joffe M.E., MacAskill A., Malik R., Sánchez-Bellot C., et al. Prefrontal interneurons: Populations, pathways, and plasticity supporting typical and disordered cognition in rodent models. J Neurosci. 2022;42:8468–8476. doi: 10.1523/JNEUROSCI.1136-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yavorska I., Wehr M. Somatostatin-expressing inhibitory interneurons in cortical circuits. Front Neural Circuits. 2016;10:76. doi: 10.3389/fncir.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKlveen J.M., Moloney R.D., Scheimann J.R., Myers B., Herman J.P. “Braking” the prefrontal cortex: The role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry. 2019;86:669–681. doi: 10.1016/j.biopsych.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 73.Steckelings U.M., de Kloet A., Sumners C. Centrally mediated cardiovascular actions of the angiotensin II type 2 receptor. Trends Endocrinol Metab. 2017;28:684–693. doi: 10.1016/j.tem.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sullivan J.C. Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1220–R1226. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- 75.Sierra-Mercado D., Padilla-Coreano N., Quirk G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bloodgood D.W., Sugam J.A., Holmes A., Kash T.L. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl Psychiatry. 2018;8:60. doi: 10.1038/s41398-018-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laukkanen L., Diniz C.R.A.F., Foulquier S., Prickaerts J., Castrén E., Casarotto P.C. Facilitation of TRKB activation by the angiotensin II receptor type-2 (AT2R) agonist C21. Pharmaceuticals (Basel) 2021;14:773. doi: 10.3390/ph14080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goel R., Bhat S.A., Hanif K., Nath C., Shukla R. Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-κB-mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol. 2018;55:1725–1739. doi: 10.1007/s12035-017-0450-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.