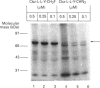
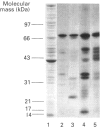
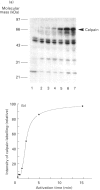
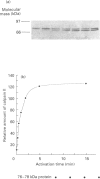
Abstract
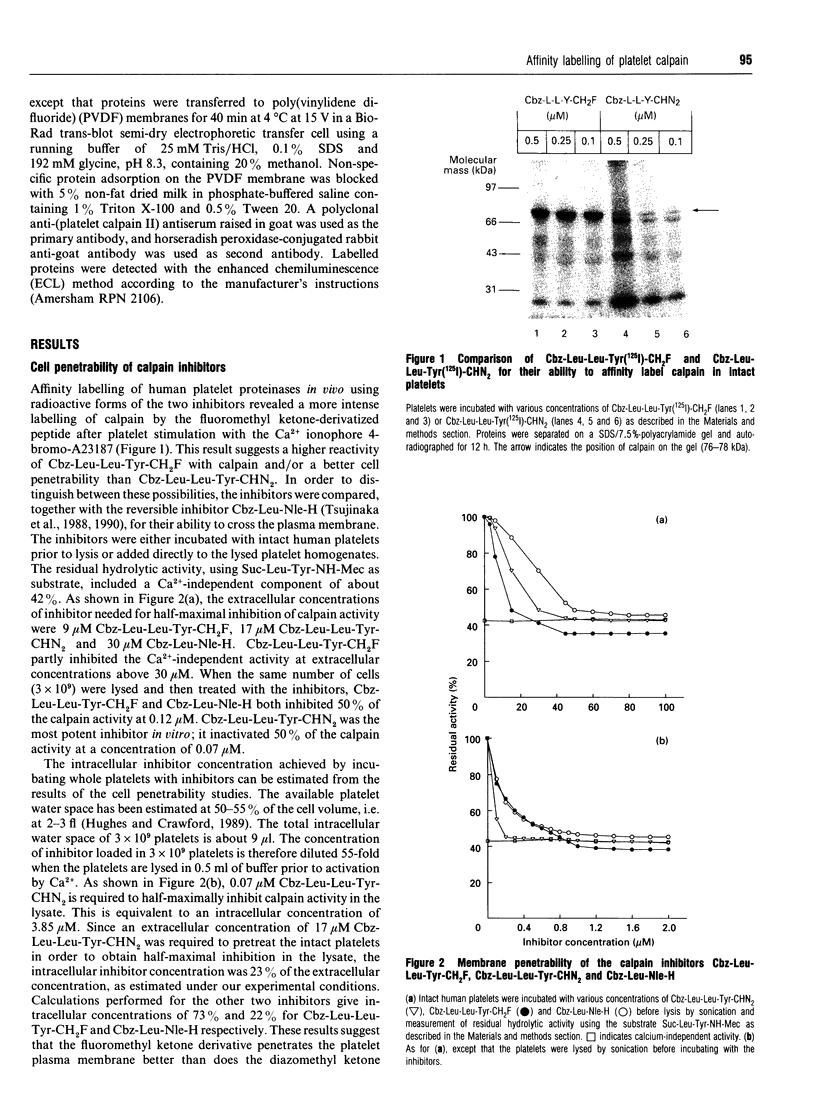
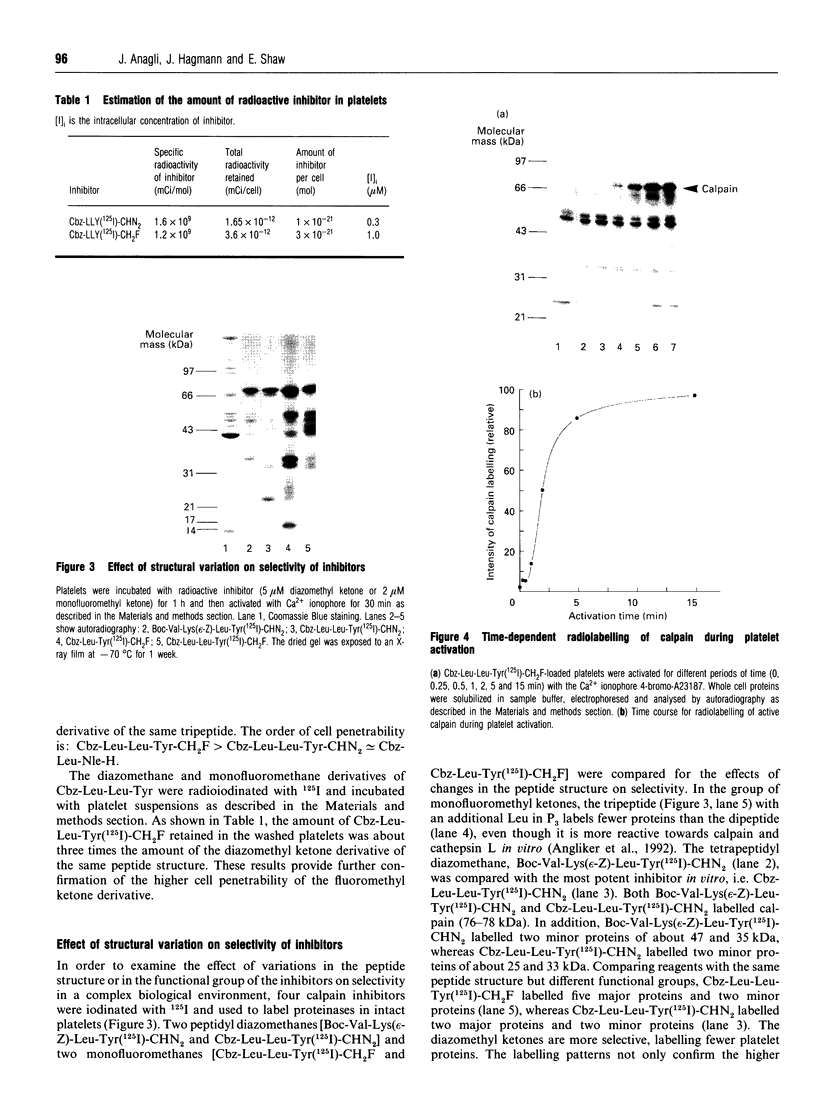
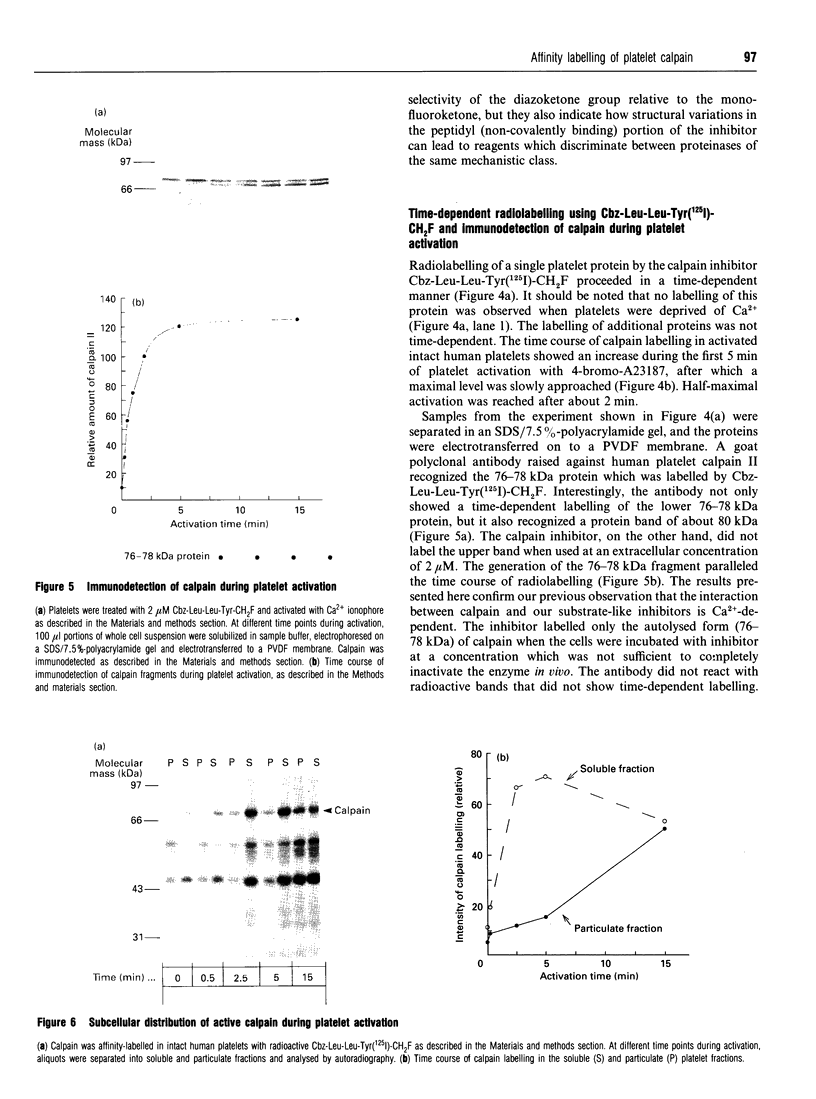
Two irreversible calpain inhibitors, benzyloxycarbonyl (Cbz)-Leu-Leu-Tyr-Ch2F and Cbz-Leu-Leu-Tyr-CHN2, were shown earlier [Anagli, Hagmann and Shaw (1991) Biochem. J. 274, 497-502] to penetrate intact platelets and to inactivate calpain. This permitted an evaluation of certain functions attributed to this proteinase. For example, in platelets pretreated with these inhibitors, talin and actin-binding protein were protected from subsequent degradation when the Ca2+ level was raised. On the other hand, additional properties of stimulated platelets attributed to calpain remained unaffected by this treatment, and such hypotheses may be dismissed. Radioiodinated inhibitors permitted confirmation of the labelling of calpain by the procedures used. Although Cbz-Leu-Leu-Tyr-CHN2 is more effective in vitro than the corresponding fluoromethyl ketone, we now show that the latter penetrates more readily. These two inhibitors, and two additional ones, t-butyloxycarbonyl-Val-Lys(Cbz)-Leu-Tyr- CHN2 and Cbz-Leu-Tyr-CH2F, have been radioiodinated to permit a comparison of their intracellular labelling patterns in activated platelets. Calpain is the major target of all four inhibitors. Although they are closely related peptide structures, variations with respect to the labelling of additional proteins were observed. These were minor in the case of the peptidyl diazomethyl ketones, but were major in the case of the fluoromethyl ketones. However, in contrast to calpain, this labelling was neither time-dependent nor Ca(2+)-dependent. Radiolabelling and cellular fractionation studies were used to localize active calpain during platelet activation. Calpain appears to be activated in the cytosol and translocated to the membrane or cytoskeletal sites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagli J., Hagmann J., Shaw E. Investigation of the role of calpain as a stimulus-response mediator in human platelets using new synthetic inhibitors. Biochem J. 1991 Mar 1;274(Pt 2):497–502. doi: 10.1042/bj2740497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelastro M. R., Mehdi S., Burkhart J. P., Peet N. P., Bey P. Alpha-diketone and alpha-keto ester derivatives of N-protected amino acids and peptides as novel inhibitors of cysteine and serine proteinases. J Med Chem. 1990 Jan;33(1):11–13. doi: 10.1021/jm00163a002. [DOI] [PubMed] [Google Scholar]
- Angliker H., Anagli J., Shaw E. Inactivation of calpain by peptidyl fluoromethyl ketones. J Med Chem. 1992 Jan 24;35(2):216–220. doi: 10.1021/jm00080a003. [DOI] [PubMed] [Google Scholar]
- Angliker H., Wikström P., Rauber P., Stone S., Shaw E. Synthesis and properties of peptidyl derivatives of arginylfluoromethanes. Biochem J. 1988 Dec 1;256(2):481–486. doi: 10.1042/bj2560481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A., Kessler M., Lee K., Lynch G. Calpain inhibitors improve the recovery of synaptic transmission from hypoxia in hippocampal slices. Brain Res. 1990 Nov 5;532(1-2):63–68. doi: 10.1016/0006-8993(90)91742-y. [DOI] [PubMed] [Google Scholar]
- Ariyoshi H., Shiba E., Kambayashi J., Sakon M., Tsujinaka T., Uemura Y., Mori T. Characteristics of various synthetic peptide calpain inhibitors and their application for the analysis of platelet reaction. Biochem Int. 1991 Apr;23(6):1019–1033. [PubMed] [Google Scholar]
- Banik N. L., Hogan E. L., Hsu C. Y. The multimolecular cascade of spinal cord injury. Studies on prostanoids, calcium, and proteinases. Neurochem Pathol. 1987 Aug;7(1):57–77. doi: 10.1007/BF02834292. [DOI] [PubMed] [Google Scholar]
- Cottin P., Poussard S., Desmazes J. P., Georgescauld D., Ducastaing A. Free calcium and calpain I activity. Biochim Biophys Acta. 1991 Aug 30;1079(2):139–145. doi: 10.1016/0167-4838(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Crawford C., Mason R. W., Wikstrom P., Shaw E. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem J. 1988 Aug 1;253(3):751–758. doi: 10.1042/bj2530751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino G. N., Huff C. A., Croall D. E. Autoproteolysis of the small subunit of calcium-dependent protease II activates and regulates protease activity. J Biol Chem. 1986 Sep 15;261(26):12047–12052. [PubMed] [Google Scholar]
- Fox J. E., Austin C. D., Reynolds C. C., Steffen P. K. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J Biol Chem. 1991 Jul 15;266(20):13289–13295. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Austin C. D. The role of calpain in stimulus-response coupling: evidence that calpain mediates agonist-induced expression of procoagulant activity in platelets. Blood. 1990 Dec 15;76(12):2510–2519. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- Gopalakrishna R., Barsky S. H. Hydrophobic association of calpains with subcellular organelles. Compartmentalization of calpains and the endogenous inhibitor calpastatin in tissues. J Biol Chem. 1986 Oct 25;261(30):13936–13942. [PubMed] [Google Scholar]
- Hirai S., Kawasaki H., Yaniv M., Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991 Aug 5;287(1-2):57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- Hughes K., Crawford N. Reversible electropermeabilisation of human and rat blood platelets: evaluation of morphological and functional integrity 'in vitro' and 'in vivo'. Biochim Biophys Acta. 1989 Jun 6;981(2):277–287. doi: 10.1016/0005-2736(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Inomata M., Saito Y., Kon K., Kawashima S. Binding sites for calcium-activated neutral protease on erythrocyte membranes are not membrane phospholipids. Biochem Biophys Res Commun. 1990 Sep 14;171(2):625–632. doi: 10.1016/0006-291x(90)91192-u. [DOI] [PubMed] [Google Scholar]
- Johnson G. V., Litersky J. M., Jope R. S. Degradation of microtubule-associated protein 2 and brain spectrin by calpain: a comparative study. J Neurochem. 1991 May;56(5):1630–1638. doi: 10.1111/j.1471-4159.1991.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Johnson P. Calpains (intracellular calcium-activated cysteine proteinases): structure-activity relationships and involvement in normal and abnormal cellular metabolism. Int J Biochem. 1990;22(8):811–822. doi: 10.1016/0020-711x(90)90284-a. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y., Tsujinaka T., Sakon M., Kambayashi J., Ohshiro T., Murachi T., Mori T. Elucidation of calpain dependent phosphorylation of myosin light chain in human platelets. Biochem Int. 1987 Nov;15(5):935–944. [PubMed] [Google Scholar]
- Kuboki M., Ishii H., Kazama M. Characterization of calpain I-binding proteins in human erythrocyte plasma membrane. J Biochem. 1990 May;107(5):776–780. doi: 10.1093/oxfordjournals.jbchem.a123124. [DOI] [PubMed] [Google Scholar]
- Kuroda T., Mikawa K., Mishima H., Kishimoto A. H1 histone stimulates limited proteolysis of protein kinase C subspecies by calpain II. J Biochem. 1991 Sep;110(3):364–368. doi: 10.1093/oxfordjournals.jbchem.a123587. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch G., Baudry M. Brain spectrin, calpain and long-term changes in synaptic efficacy. Brain Res Bull. 1987 Jun;18(6):809–815. doi: 10.1016/0361-9230(87)90220-6. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi S., Angelastro M. R., Wiseman J. S., Bey P. Inhibition of the proteolysis of rat erythrocyte membrane proteins by a synthetic inhibitor of calpain. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1117–1123. doi: 10.1016/s0006-291x(88)80989-6. [DOI] [PubMed] [Google Scholar]
- Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem Sci. 1991 Apr;16(4):150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Calcium-dependent proteases: an enzyme system active at cellular membranes? FASEB J. 1987 Aug;1(2):110–115. doi: 10.1096/fasebj.1.2.2886390. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980 Jan 1;109(1):129–133. doi: 10.1016/0014-5793(80)81326-3. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Proteolysis of nuclear proteins by mu-calpain and m-calpain. J Biol Chem. 1991 Jul 25;266(21):13920–13924. [PubMed] [Google Scholar]
- Michetti M., Viotti P. L., Melloni E., Pontremoli S. Mechanism of action of the calpain activator protein in rat skeletal muscle. Eur J Biochem. 1991 Dec 18;202(3):1177–1180. doi: 10.1111/j.1432-1033.1991.tb16487.x. [DOI] [PubMed] [Google Scholar]
- Murachi T. Intracellular regulatory system involving calpain and calpastatin. Biochem Int. 1989 Feb;18(2):263–294. [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E. Extralysosomal protein degradation. Annu Rev Biochem. 1986;55:455–481. doi: 10.1146/annurev.bi.55.070186.002323. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Viotti P. L., Michetti M., Salamino F., Horecker B. L. Identification of two calpastatin forms in rat skeletal muscle and their susceptibility to digestion by homologous calpains. Arch Biochem Biophys. 1991 Aug 1;288(2):646–652. doi: 10.1016/0003-9861(91)90247-g. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kishi M., Saito M., Tanaka T., Higuchi N., Kominami E., Katunuma N., Murachi T. Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib. 1990;3(3):195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Commun. 1968 Sep 6;32(5):898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Schollmeyer J. E. Calpain II involvement in mitosis. Science. 1988 May 13;240(4854):911–913. doi: 10.1126/science.2834825. [DOI] [PubMed] [Google Scholar]
- Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- Shimohama S., Suenaga T., Araki W., Yamaoaka Y., Shimizu K., Kimura J. Presence of calpain II immunoreactivity in senile plaques in Alzheimer's disease. Brain Res. 1991 Aug 30;558(1):105–108. doi: 10.1016/0006-8993(91)90722-8. [DOI] [PubMed] [Google Scholar]
- Siman R., Noszek J. C., Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989 May;9(5):1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalla M., Kuo T. H., Wiener J. Calcium-activated protease in hamster cardiomyopathy. Muscle Nerve. 1987 Jan;10(1):54–59. doi: 10.1002/mus.880100111. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imajoh S., Emori Y., Kawasaki H., Minami Y., Ohno S. Regulation of activity of calcium activated neutral protease. Adv Enzyme Regul. 1988;27:153–169. doi: 10.1016/0065-2571(88)90015-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujinaka T., Ariyoshi H., Uemura Y., Sakon M., Kambayashi J., Mori T. Potential participation of calpain in platelet activation studied by use of a cell penetrating calpain inhibitor (calpeptin). Life Sci. 1990;46(15):1059–1066. doi: 10.1016/0024-3205(90)90414-m. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T., Kajiwara Y., Kambayashi J., Sakon M., Higuchi N., Tanaka T., Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin). Biochem Biophys Res Commun. 1988 Jun 30;153(3):1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- Wang K. K. Developing selective inhibitors of calpain. Trends Pharmacol Sci. 1990 Apr;11(4):139–142. doi: 10.1016/0165-6147(90)90060-L. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- White G. C., 2nd Calcium-dependent proteins in platelets: response of calcium-activated protease in normal and thrombasthenic platelets to aggregating agents. Biochim Biophys Acta. 1980 Aug 1;631(1):130–138. doi: 10.1016/0304-4165(80)90061-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman U. J., Schlaepfer W. W. Two-stage autolysis of the catalytic subunit initiates activation of calpain I. Biochim Biophys Acta. 1991 Jun 24;1078(2):192–198. doi: 10.1016/0167-4838(91)99009-h. [DOI] [PubMed] [Google Scholar]