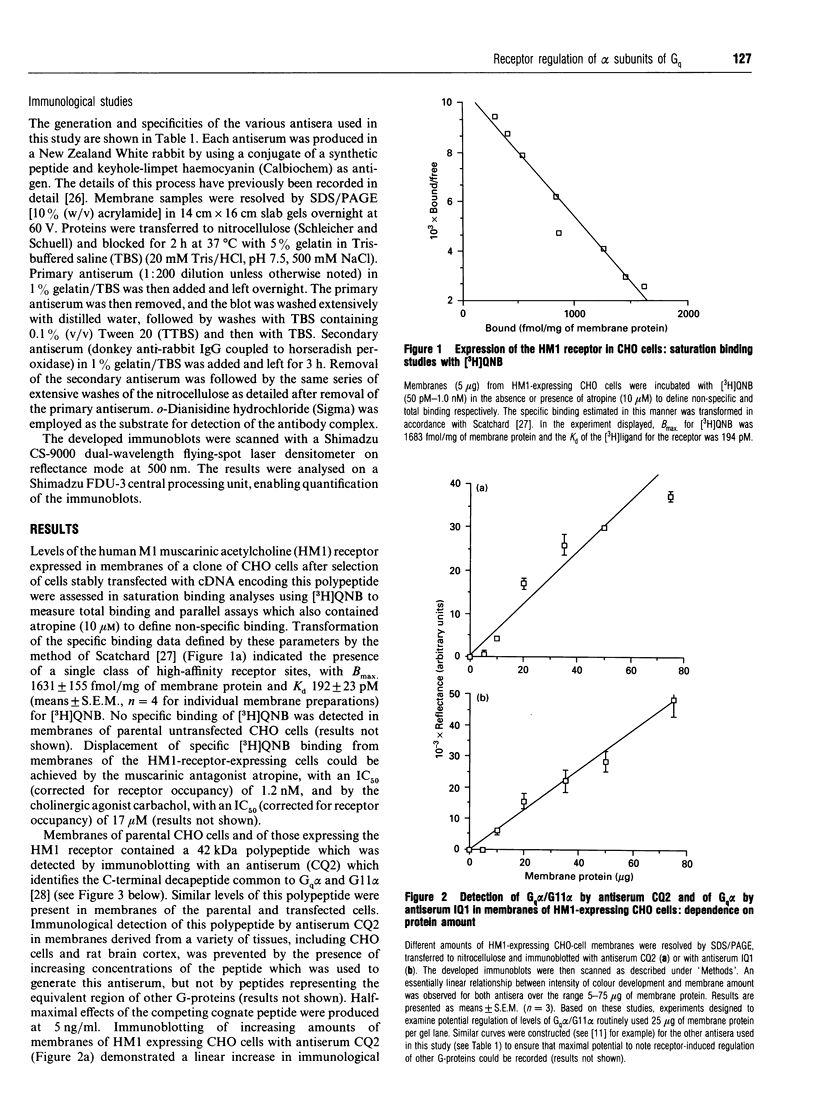
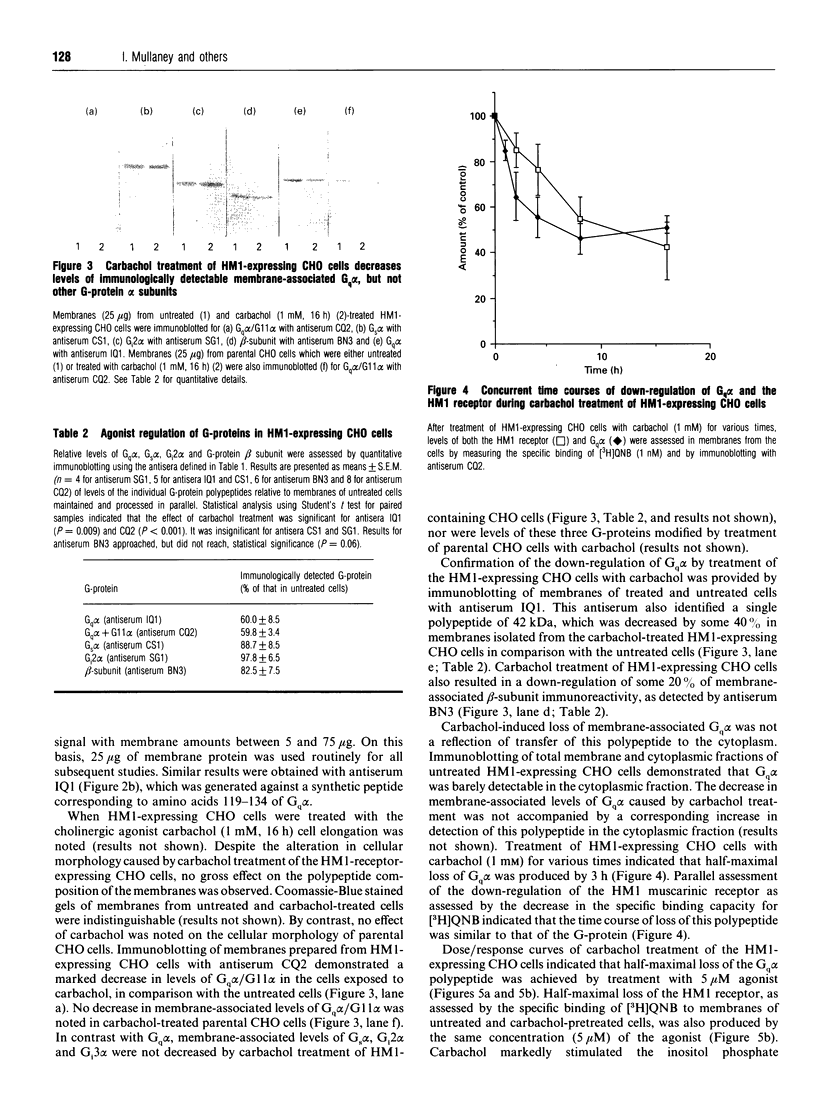
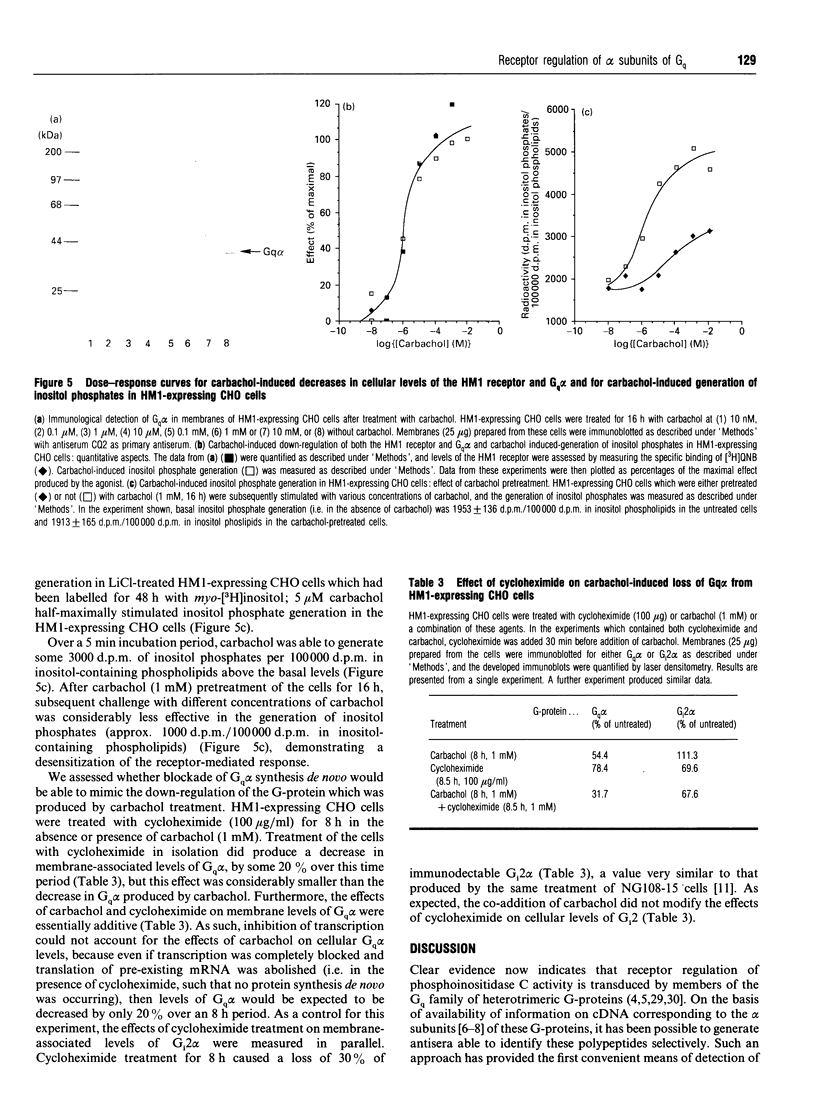
Abstract
CHO cells stably transfected with cDNA encoding the human M1 muscarinic acetylcholine (HM1) receptor were treated with the cholinergic agonist carbachol at various concentrations for differing times. Levels of the HM1 receptor and of a range of G-proteins were subsequently measured. Carbachol treatment of the transfected cells caused a substantial down-regulation of cellular levels of the alpha subunit of Gq (Gq alpha), but did not significantly alter cellular levels of the alpha subunits of Gs or Gi2. A small decrease in levels of G-protein beta-subunit was also produced. Parallel assessment of agonist-induced down-regulation of the HM1 receptor demonstrated that it was lost in concert with the G-protein. Similar concentrations of carbachol (5 microM) were required to produce half-maximal stimulation of inositol phosphate generation and loss of each of the HM1 receptor and Gq alpha, and half-maximal losses of both receptor and Gq alpha were produced by 3 h of treatment with 1 mM-carbachol. By contrast, treatment of the non-transfected parental CHO cells, which do not express detectable levels of the receptor, with carbachol had no effect on cellular Gq alpha levels. Concurrent treatment of the HM1-expressing CHO cells with carbachol and cycloheximide indicated that suppression of protein synthesis de novo did not mimic the effect of carbachol, and hence even complete inhibition of transcription of the Gq alpha gene and/or translation of pre-existing Gq alpha mRNA could not account for the agonist-induced effect. We have previously noted that cellular levels of both Gs alpha [McKenzie and Milligan (1990) J. Biol. Chem. 265, 17084-17093] and the alpha subunits of the pertussis-toxin-sensitive G-proteins Gi1, Gi2 and Gi3 [Green, Johnson and Milligan (1990) J. Biol. Chem. 265, 5206-5210] can be regulated in certain cell systems by agonist activation of receptors expected to interact with these G-proteins. These results demonstrate that the same is true of Gq alpha and suggest that agonist-induced co-ordinate loss of receptors and associated G-proteins may be a more common feature than has been appreciated to date.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adie E. J., Mullaney I., McKenzie F. R., Milligan G. Concurrent down-regulation of IP prostanoid receptors and the alpha-subunit of the stimulatory guanine-nucleotide-binding protein (Gs) during prolonged exposure of neuroblastoma x glioma cells to prostanoid agonists. Quantification and functional implications. Biochem J. 1992 Jul 15;285(Pt 2):529–536. doi: 10.1042/bj2850529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda T. T., 3rd, Steele D. A., Slepak V. Z., Simon M. I. G alpha 16, a G protein alpha subunit specifically expressed in hematopoietic cells. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5587–5591. doi: 10.1073/pnas.88.13.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Ross E. M. Catecholamine-stimulated GTPase cycle. Multiple sites of regulation by beta-adrenergic receptor and Mg2+ studied in reconstituted receptor-Gs vesicles. J Biol Chem. 1986 Feb 5;261(4):1656–1664. [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Campbell P. T., Hnatowich M., O'Dowd B. F., Caron M. G., Lefkowitz R. J., Hausdorff W. P. Mutations of the human beta 2-adrenergic receptor that impair coupling to Gs interfere with receptor down-regulation but not sequestration. Mol Pharmacol. 1991 Feb;39(2):192–198. [PubMed] [Google Scholar]
- Chang F. H., Bourne H. R. Dexamethasone increases adenylyl cyclase activity and expression of the alpha-subunit of Gs in GH3 cells. Endocrinology. 1987 Nov;121(5):1711–1715. doi: 10.1210/endo-121-5-1711. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Caron M. G., Lefkowitz R. J. From ligand binding to gene expression: new insights into the regulation of G-protein-coupled receptors. Trends Biochem Sci. 1992 Jan;17(1):37–39. doi: 10.1016/0968-0004(92)90425-9. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Green A., Johnson J. L., Milligan G. Down-regulation of Gi sub-types by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1990 Mar 25;265(9):5206–5210. [PubMed] [Google Scholar]
- Green A., Milligan G., Dobias S. B. Gi down-regulation as a mechanism for heterologous desensitization in adipocytes. J Biol Chem. 1992 Feb 15;267(5):3223–3229. [PubMed] [Google Scholar]
- Gutowski S., Smrcka A., Nowak L., Wu D. G., Simon M., Sternweis P. C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991 Oct 25;266(30):20519–20524. [PubMed] [Google Scholar]
- Hadcock J. R., Ros M., Watkins D. C., Malbon C. C. Cross-regulation between G-protein-mediated pathways. Stimulation of adenylyl cyclase increases expression of the inhibitory G-protein, Gi alpha 2. J Biol Chem. 1990 Sep 5;265(25):14784–14790. [PubMed] [Google Scholar]
- Kelly E., Keen M., Nobbs P., MacDermot J. Segregation of discrete GS alpha-mediated responses that accompany homologous or heterologous desensitization in two related somatic hybrids. Br J Pharmacol. 1990 Feb;99(2):309–316. doi: 10.1111/j.1476-5381.1990.tb14700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. A., Feldman A. M., Robishaw J. D., Ladenson P. W., Ahn T. G., Moroney J. F., Smallwood P. M. Influence of thyroid hormone status on expression of genes encoding G protein subunits in the rat heart. J Biol Chem. 1990 Feb 25;265(6):3553–3560. [PubMed] [Google Scholar]
- McClue S. J., Selzer E., Freissmuth M., Milligan G. Gi3 does not contribute to the inhibition of adenylate cyclase when stimulation of an alpha 2-adrenergic receptor causes activation of both Gi2 and Gi3. Biochem J. 1992 Jun 1;284(Pt 2):565–568. doi: 10.1042/bj2840565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Delta-opioid-receptor-mediated inhibition of adenylate cyclase is transduced specifically by the guanine-nucleotide-binding protein Gi2. Biochem J. 1990 Apr 15;267(2):391–398. doi: 10.1042/bj2670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Prostaglandin E1-mediated, cyclic AMP-independent, down-regulation of Gs alpha in neuroblastoma x glioma hybrid cells. J Biol Chem. 1990 Oct 5;265(28):17084–17093. [PubMed] [Google Scholar]
- Milligan G. Foetal-calf serum stimulates a pertussis-toxin-sensitive high-affinity GTPase activity in rat glioma C6 BU1 cells. Biochem J. 1987 Jul 15;245(2):501–505. doi: 10.1042/bj2450501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Green A. Agonist control of G-protein levels. Trends Pharmacol Sci. 1991 Jun;12(6):207–209. doi: 10.1016/0165-6147(91)90551-3. [DOI] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Milligan G. Multiple heterotrimeric guanine nucleotide binding proteins: roles in the determination of cellular signalling specificity. Biochem Soc Trans. 1992 Feb;20(1):135–140. doi: 10.1042/bst0200135. [DOI] [PubMed] [Google Scholar]
- Milligan G., Saggerson E. D. Concurrent up-regulation of guanine-nucleotide-binding proteins Gi1 alpha, Gi2 alpha and Gi3 alpha in adipocytes of hypothyroid rats. Biochem J. 1990 Sep 15;270(3):765–769. doi: 10.1042/bj2700765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. M., Mullaney I., Godfrey P. P., Arkinstall S. J., Wakelam M. J., Milligan G. Widespread distribution of Gq alpha/G11 alpha detected immunologically by an antipeptide antiserum directed against the predicted C-terminal decapeptide. FEBS Lett. 1991 Aug 5;287(1-2):171–174. doi: 10.1016/0014-5793(91)80043-3. [DOI] [PubMed] [Google Scholar]
- Plevin R., Palmer S., Gardner S. D., Wakelam M. J. Regulation of bombesin-stimulated inositol 1,4,5-trisphosphate generation in Swiss 3T3 fibroblasts by a guanine-nucleotide-binding protein. Biochem J. 1990 Jun 15;268(3):605–610. doi: 10.1042/bj2680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransnäs L. A., Svoboda P., Jasper J. R., Insel P. A. Stimulation of beta-adrenergic receptors of S49 lymphoma cells redistributes the alpha subunit of the stimulatory G protein between cytosol and membranes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7900–7903. doi: 10.1073/pnas.86.20.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M. H. Pharmacology and second messenger interactions of cloned muscarinic receptors. Biochem Pharmacol. 1991 Oct 9;42(9):1645–1653. doi: 10.1016/0006-2952(91)90498-t. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shenker A., Goldsmith P., Unson C. G., Spiegel A. M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991 May 15;266(14):9309–9313. [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Simon M. I. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann M., Wilkie T. M., Simon M. I. Diversity of the G-protein family: sequences from five additional alpha subunits in the mouse. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7407–7409. doi: 10.1073/pnas.86.19.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Thomas R. F., Holt B. D., Schwinn D. A., Liggett S. B. Long-term agonist exposure induces upregulation of beta 3-adrenergic receptor expression via multiple cAMP response elements. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4490–4494. doi: 10.1073/pnas.89.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]