Abstract
Infected cell protein 22 (ICP22) is posttranslationally phosphorylated by the viral kinases encoded by US3 and UL13 and nucleotidylylated by casein kinase II. In rabbit and rodent cells and in primary human fibroblasts infected with mutants from which the α22 gene encoding ICP22 had been deleted, a subset of late (γ2) gene products exemplified by UL38 and US11 proteins are expressed at a reduced level, as measured by the accumulation of both mRNA and protein. The same phenotype was observed in cells infected with mutants lacking the UL13 gene. The focus of this report is on three serine- and threonine-rich domains of ICP22. Two of these domains are homologs located between residues 38 to 66 and 300 to 328. The third domain is near the carboxyl terminus and contains the sequence T374SS. The results were as follows. (i) Alanine substitutions in the amino-terminal homolog precluded the posttranslational processing of ICP22 in rabbit skin cells and in Vero cells but had no effect on the accumulation of either US11 or UL38 protein. (ii) Alanine substitutions in the carboxyl-terminal homolog had no effect on posttranslational processing of ICP22 accumulating in Vero cells but precluded full processing of ICP22 accumulating in rabbit skin cells. The effect on accumulation of UL38 and US11 proteins was insignificant in Vero cells and minimal in rabbit skin cells. (iii) Substitutions of alanine for the threonine and serines in the third domain precluded full processing of ICP22 and caused a reduction of accumulation of US11 and UL38 proteins. These results indicate the following. (i) The posttranslational processing of ICP22 is sensitive to mutations within the domains of ICP22 tested and is cell-type dependent. (ii) Posttranslational processing of ICP22 is not required for accumulation of UL38 and US11 proteins to the same level as that seen in cells infected with the wild-type virus. (iii) The T374SS sequence shared by ICP22 and the US1.5 proteins is essential for the accumulation of a subset of γ2 proteins exemplified by US11 and UL38 and is the first step in mapping of the sequences necessary for optimal accumulation of US11 and UL38 proteins.
Herpes simplex virus 1 (HSV-1) encodes six transcriptional units whose expression does not require prior synthesis of viral proteins but is enhanced by a transcriptional factor, VP16 or α-TIF, carried into the cell by the infecting virus. The six transcripts encode five infected cell proteins (ICPs), designated ICP0, ICP4, ICP22, ICP27, and ICP47, and a protein designated US1.5. All six proteins perform multiple regulatory functions that affect the expression or accumulation of viral and cellular proteins in the course of viral replication. This report concerns ICP22. The background relevant to this report is as follows.
(i) The domain of the α22 gene contains two transcriptional units, each with its own promoter. The α22 mRNA initiates upstream from the open reading frame (ORF) and is spliced; the first exon is in its 5′ noncoding domain (6, 17, 21). The protein product, ICP22, contains 420 amino acids. The sequences encoding the second mRNA are contained in the coding domain of the α22 gene (3). This mRNA directs the synthesis of US1.5 protein containing 250 amino acids beginning with Met171 of ICP22 and is colinear with the remainder of ICP22 (A. P. W. Poon, W. O. Ogle, and B. Roizman, unpublished data). This protein, designated US1.5, is also expressed with α gene kinetics.
(ii) ICP22 is also nucleotidylylated by casein kinase II (8, 9) and phosphorylated largely by the protein kinase encoded by UL13 and to a lesser extent by protein kinase encoded by US3 (12, 14).
(iii) R325, a mutant lacking the carboxyl-terminal 220 amino acids, was characterized extensively both in cell culture and in animal systems (11). The mutant is highly attenuated in experimental animal systems (7, 19). It replicates to wild-type virus levels in Vero and HEp-2 cells but at a significantly lower level in rodent or rabbit cells or in primary human fibroblasts. In infected cells, the accumulation of a subset of γ2 proteins exemplified by the products of US11 and UL38 genes is significantly reduced (10, 14). In addition, the levels of ICP0 and its mRNA are also reduced (14). Moreover, the phenotype of the R325 deletion mutant is similar to that of a mutant lacking a functional UL13 gene (14). On the basis of analysis of a similar α22 deletion mutant, it has been concluded that ICP22 mediates an altered phosphorylation of RNA polymerase II (15, 16). One hypothesis arising from these studies is that accumulation of the proteins exemplified by US11 and UL38 requires a posttranslationally modified carboxyl-terminal domain that is shared by ICP22 and US1.5 proteins. Consistent with this view, the accumulation of US11 and of UL38 proteins in cells infected with a mutant expressing the US1.5 protein but not ICP22 is similar to that of wild-type parent virus (10). This mutant is highly attenuated in mice.
(iv) The sequences unique to ICP22 may perform functions different from those of sequences shared by ICP22 and US1.5 proteins inasmuch as insertions of a 20-codon linker at codon 200 or 240 had no apparent effect on the functions associated with ICP22 and US1.5 described above.
In this study, we focused on the sites required for the posttranslational processing of ICP22. An earlier study identified codons 147 to 171 and 402 to 405 as essential for the posttranslational processing of ICP22 (10). In the course of that work, it was noted that the amino-terminal domain of ICP22 contains a set of serine- and threonine-rich sequence (amino acids 38 to 66) that is repeated near the carboxyl terminus of ICP22 shared with the US1.5 protein (amino acids 300 to 328). Inasmuch as UL13, the kinase largely responsible for the posttranslational modifications of ICP22, prefers serine- and threonine-rich residues, it was of interest to determine the role of these sequences in the posttranslational modification of ICP22. We report two surprising results. First, mutation of any one of the two distantly located repeats abolished or grossly reduced posttranslational modification of ICP22. Second, posttranslational processing was cell-type dependent. We also noted that posttranslational modification of ICP22 was not essential for the accumulation of US11 and UL38 proteins.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were obtained from the American Type Culture Collection, and rabbit skin cells (RSC) were originally obtained from J. McClaren. Cells were maintained in Dulbecco's Eagle medium supplemented with 5% fetal bovine serum (RSC) or 5% newborn calf serum (Vero cells). HSV-1(F) is the prototype HSV-1 strain used in this laboratory (4). The construction of HSV-1 recombinant viruses R7041 (US3−), R7353 (UL13−/US3−), and R7356 (UL13−) was previously described (12–14).
Plasmids.
pRB5210 contains most of HSV-1(F) BamHI N sequence (10). pRB5252 contains the entire ICP22 ORF in vector pUC19. The DNA fragment containing the entire ICP22 ORF was generated by PCR using pRB5210 as the template and primers B1 (GGG GAA TTC CGG CCG ATG GCC GAC ATT TCC CCA GGC GCT) and B2 (CCG GGA TCC CGG CCG GAG AAA CGT GTC GCT GCA CGG ATA). B1 included in the final product restriction sites EcoRI and EagI (underlined) at the 5′ end of the ICP22 ORF, and B2 incorporated a BamHI site immediately following the EagI site (underlined) at the 3′ end of the ICP22 ORF. The PCR product was digested with EcoRI and BamHI, and the purified fragment was subcloned into the EcoRI-BamHI site of pUC19. pRB5252 was used as the template for mutagenesis within the ICP22 ORF.
pRB5212 was derived from pRB5210. In this plasmid, the ICP22 ORF sequence from the initiation methionine codon to the carboxyl-terminal stop codon was deleted, leaving only a unique EagI site (10). This enables recloning of mutant ICP22 sequences derived from pRB5252 back into pRB5212 to provide flanking sequences required for recombination in isolation of ICP22 mutant viruses.
Plasmids pRB5314 and pRB5316 contain mutant ICP22 sequence with threonine codon 300 replaced by an alanine codon and serine codon 301 replaced by a glycine codon. In pRB5314, mutations were introduced by site-directed mutagenesis (see below) using pRB5252 as the template. The two complementary oligonucleotides B3 (TCT CAG CGC GGC AGG CGA TGA TGA GAT CTC) and B4 (GAG ATC TCA TCA TCG CCT GCC GCG CTG AGA) which were used as primers for PCR also incorporated in the final product a diagnostic BglII site (underlined) that alters a single base, causing a silent mutation. The entire ICP22 ORF in pRB5314 was sequenced, and replacement of threonine codon 300 by an alanine codon and serine codon 301 by a glycine codon was verified. To construct pRB5316, pRB5314 was digested with EagI to excise the entire ICP22 ORF. Purified fragment containing mutant ICP22 ORF was cloned into the unique EagI site of pRB5212. The resultant plasmid pRB5316 was used for isolation of recombinant virus R7827.
Plasmids pRB5292 and pRB5295 contain multiple mutations in the ICP22 ORF, resulting in replacements of nine threonine/serine codons by alanine/glycine codons. In pRB5292, threonine codons 300, 309, and 319, and serine codons 306, 316, 322, 324, and 326 were all mutated to alanine codons, while serine codon 301 was replaced by a glycine codon. The entire ICP22 ORF in pRB5292 was sequenced, and the presence of the above mutations was verified. To construct plasmid pRB5295, pRB5292 was digested with EagI and mutant ICP22 sequence purified and subcloned into the EagI site of pRB5212. The resultant plasmid pRB5295 was used for isolation of recombinant virus R7837.
Plasmids pRB5409 and pRB5410 contain mutant ICP22 sequence with serine codons 38, 39, 41, and 45 and threonine codon 47 replaced by alanine codons. To construct plasmid pRB5409, a 143-bp EcoRI-StyI fragment containing the mutations was generated by PCR from pRB5252 using primers B1 and B5 (TC GAC CTC AGA CTC CAA GGC TGC ATC GGC TTC TAC CTC AGC CTC CGC TGC GAG GGG GCG GGA AGG GCG CT). B5 incorporated changes of serine codons 38, 39, 41, and 45 and threonine codon 47 to alanine codons. This PCR product was digested with EcoRI and StyI, and the purified fragment was subcloned into a purified fragment of pRB5252 which had been digested with EcoRI and StyI to remove the corresponding segment containing wild-type S38, S39, S41, S45, and T47 sequences. The entire ICP22 ORF in pRB5409 was sequenced, and substitution of the indicated serine and threonine codons by alanine codons was verified. To construct plasmid pRB5410, pRB5409 was digested with EagI to excise the entire ICP22 ORF. A purified fragment containing mutant ICP22 sequence was cloned into the EagI site of plasmid pRB5212. The subsequent plasmid pRB5410 was used for isolation of recombinant virus R7851.
Plasmid pRB5411 contains mutant ICP22 sequence with threonine codon 374 and serine codons 375 and 376 all replaced by alanine codons. In pRB5411, mutations were introduced by site-directed mutagenesis using pRB5210 as the template. The two complementary oligonucleotides B6 (GCG GTC GTG GCC GAT GCG GCC GCC GTG GAA CGC CCG GGC) and B7 (GCC CGG GCG TTC CAC GGC GGC CGC ATC GGC CAC GAC CGC) which were used as primers for PCR also incorporated a diagnostic NotI site (underlined) in the final product. The entire ICP22 ORF in pRB5411 was sequenced, and replacement of the indicated serine and threonine codons by alanine codons was verified. Plasmid pRB5411 was used for isolation of recombinant virus R7855.
Site-directed mutagenesis.
Mutagenesis of targeted sequences was achieved by PCR using two complementary oligonucleotides as primers. These complementary sequences contain the desired mutations accompanied by a diagnostic endonuclease cleavage site. The PCR mixture contained 10 to 50 ng of template DNA, 125 to 250 ng of primers, and Pfu polymerase (Stratagene) in a final volume of 50 μl. The cycling parameters were as follows: 1 cycle at 95°C for 30 s; then 20 cycles of 95°C for 30 s, 55°C for 1 min, and 68°C for variable time periods depending on the size of the template (2 min per kb of template; e.g., 10 min for pRB5252 or 16 min for pRB5210). The final product was treated with phenol-chloroform before ligation; ligated DNA was digested with DpnI at 37°C for 1 h and then transformed into Escherichia coli JM109. Mutant plasmids were diagnosed by the presence of an endonuclease cleavage site introduced by the complementary primers.
Construction of recombinant viruses.
DNA fragments containing mutant ICP22 sequences inserted into pRB5212 were used to rescue ICP22 deletion virus R7802 (10) in isolation of recombinant viruses. RSC (25-cm2 cultures) were cotransfected with DNA of deletion virus R7802 and mutant plasmid (pRB5316, pRB5295, pRB5410, or pRB5411) and were harvested at 100% cytopathic effect. Dilutions of transfection cultures were plated on Vero cells to obtain isolated plaques. A single plaque was subjected to four rounds of purification on Vero cells and then amplified on Vero cells.
The recombinant viruses isolated in this study and their corresponding serine/threonine substitutions in the ICP22 amino acid sequence are listed in Table 1. The entire ICP22 ORF of all viruses had been sequenced to verify the presence of no mutations other than those of targeted serine/threonine residues.
TABLE 1.
Alignment of native and substituted amino acid sequences of ICP22 in wild-type and recombinant virusesa
 |
Preparation of cell lysates, electrophoretic separation of proteins, and immunoblotting.
Replicate cultures of Vero cells or RSC in 25-cm2 flasks were either mock infected or infected at a multiplicity of infection (MOI) of 5 PFU of virus per cell and maintained at 37°C in medium 199V (medium 199 supplemented with 1% calf serum). Cells were harvested 18 h after infection, washed three times with phosphate-buffered saline, and then solubilized in 200 μl of disruption buffer (50 mM Tris-HCl [pH 7], 2% sodium dodecyl sulfate, 710 mM β-mercaptoethanol, 3% sucrose). After 50-μl aliquots of lysates were boiled for 5 min, solubilized proteins were subjected to electrophoresis in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, reacted with a primary antibody followed by appropriate secondary antibody conjugated to alkaline phosphatase (Bio-Rad), and visualized according to the manufacturer's instructions.
Antibodies.
A mouse monoclonal antibody to US11 and rabbit polyclonal antibodies R77 (against the amino-terminal region of ICP22) and W1 (against UL38) were described previously (1, 5, 18, 20).
RESULTS
Construction of recombinant viruses.
Materials and Methods describes the construction of a series of plasmids containing mutated domains of the HSV-1 α22 gene. Figure 1 describes the construction of recombinant viruses carrying substitutions of wild-type α22 gene with mutated sequences cloned in the plasmids. In this series of experiments, we took advantage of the observation published earlier that the recombinant virus R7802 lacking the entire α22 ORF replicates but does not form plaques in RSC (10). Cotransfection of R7802 recombinant virus DNA with the mutated α22 ORF results in at least partial rescue. The plaques formed by the progeny of transfection were invariably the desired recombinants. In all instances, the sequence of the α22 gene of the recombinant virus was confirmed by sequencing (data not shown). The native and mutated sequences of the amino-terminal and carboxyl-terminal homologs are shown in Table 1.
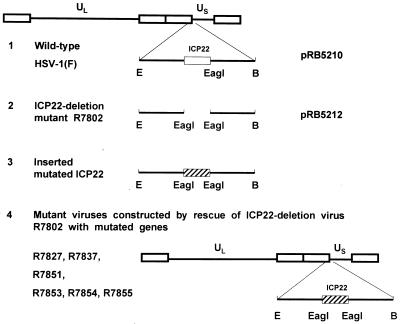
FIG. 1.
Schematic representation of the construction of recombinant viruses. 1, fragment of HSV-1(F) BamHI N sequence in plasmid pRB5210 showing the wild-type ICP22 ORF (open rectangle); 2, deletion of ICP22 ORF from the initiation methionine codon to the carboxyl-terminal stop codon in the deletion virus R7802 and in plasmid pRB5212, leaving only a single EagI site; 3, mutant ICP22 sequence (hatched rectangle) excised from mutated pRB5252 by digestion with EagI and inserted into the unique EagI site in pRB5212; 4, resultant plasmid containing the inserted mutated ICP22 ORF, used to rescue ICP22 deletion virus R7802 for isolation of recombinant viruses R7827, R7837, R7851, and R7855. R7853, R7854, and R7855 were isolated from the same transfection stock. The mutated amino acid sequences present in these recombinant viruses are shown in Table 1. Abbreviations: B, BamHI; E, EcoRI.
The posttranslational processing of ICP22 carrying mutations in the carboxyl-terminal homolog of ICP22 is host cell dependent.
In this series of experiments, replicate cultures of Vero cells (Fig. 2, lanes 1 to 6) or RSC (lanes 7 to 12) were exposed to 5 PFU of HSV-1(F), R7041 (US3−), R7356 (UL13−), R7353 (US3−/UL13−), R7827, or R7837 per cell. The cells were harvested at 18 h after infection, solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with a monoclonal antibody to US11 or polyclonal antibodies to ICP22 and UL38 as described in Materials and Methods. The results were as follows (Fig. 2).
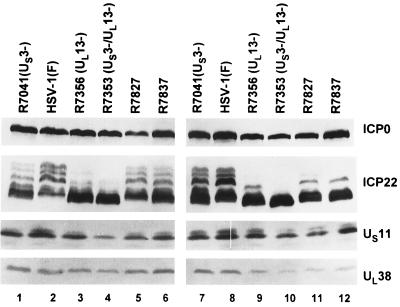
FIG. 2.
Photograph of immunoblots of electrophoretically separated proteins from Vero cells and RSC infected with HSV-1(F), R7041 (US3−), R7356 (UL13−), R7353 (US3−/UL13−), and recombinant viruses R7827 and R7837. Replicate cultures of Vero cells (lanes 1 to 6) or RSC (lanes 7 to 12) were infected with viruses at an MOI of 5 and harvested at 18 h after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with a monoclonal antibody to US11 or polyclonal antibodies to ICP22 and UL38 as described in Materials and Methods.
(i) The ICP22 in either Vero cells or RSC infected with R7353 (US3−/UL13−) migrated the fastest and exhibited no slow-migrating forms. In both cell lines, ICP22 encoded by R7356 (UL13−) (lanes 3 and 9) exhibited in addition a single slow-migrating form, the accumulation of which appears to be linked to the presence of the US3 protein kinase. The accumulation of the fast-migrating form, on the other hand, was associated with the absence of UL13 protein kinase, as previously reported (14).
(ii) In Vero cells, the isoforms of ICP22 encoded by R7827 (lane 5) were similar to those of wild-type virus (lane 2), whereas the accumulations of isoforms of ICP22 of R7837 (lane 6) were similar to those of R7041 (US3−) (lane 1). In RSC, the isoforms of ICP22 of R7827 and R7837 (lanes 11 and 12) were similar to those accumulating in cells infected with R7356 (UL13−) (lane 9).
(iii) In the experiment shown in Fig. 2, Vero cells infected with R7353 (US3−/UL13−) exhibited a slight decrease in the accumulation of UL38 and US11 proteins. Cells infected with the mutant viruses R7827 and R7837 accumulated the same or larger amounts of both proteins, suggesting that these mutations did not have an adverse effect on the accumulation of these proteins. RSC infected with R7356, R7353, R7827, and R7837 exhibited a decreased accumulation of UL38 protein.
The key conclusion to be derived from these results is that UL13-mediated posttranslational processing of ICP22 carrying alanine substitutions in the carboxyl-terminal homolog is cell-type dependent.
The posttranslational processing of ICP22 carrying mutations in the amino-terminal homolog is cell-type independent and has no effect on the accumulation of γ2 proteins.
The experiments described below were performed essentially as described above except that the recombinant virus R7851 carrying alanine substitutions for S38, S39, S41, S45, and T47 in ICP22 was used instead of R7827 and R7837. We again observed that the fast-migrating form of ICP22 was absent from cells infected with viruses carrying US3 and UL13 protein kinases (Fig. 3, compare lanes 2 and 8 with lanes 1, 3, 4, 7, 9, and 10). The key findings shown in Fig. 3 are as follows.
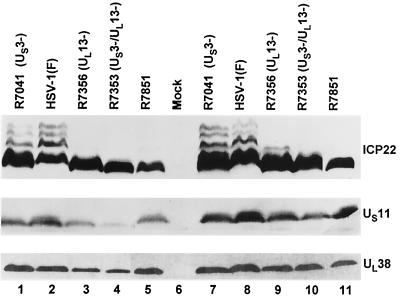
FIG. 3.
Photograph of immunoblots of electrophoretically separated proteins from Vero cells and RSC infected with HSV-1(F), R7041 (US3−), R7356 (UL13−), R7353 (US3−/UL13−), and recombinant virus R7851. Replicate cultures of Vero cells (lanes 1 to 5) or RSC (lanes 6 to 11) were either mock infected or infected with viruses at an MOI of 5 and harvested at 18 h after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with a monoclonal antibody to US11 or polyclonal antibodies to ICP22 and UL38 as described in Materials and Methods.
(i) In both Vero cells and RSC infected with R7851, the isoforms of ICP22 accumulating were of the fast-migrating type comparable to those accumulating in R7353 (UL13−/US3−)-infected cells.
(ii) The levels of accumulation of UL38 and US11 proteins in R7851-infected cells could not be differentiated from those of cells infected with the wild-type virus.
The key conclusions to be derived from these results are that serine-threonine mutations in the amino-terminal homolog preclude processing of ICP22 mediated by either UL13 or US3 protein kinase and the processing of ICP22 is not required for expression of the subset of γ2 proteins exemplified by UL38 and US11 proteins.
The posttranslational processing of ICP22 carrying alanine substitutions for T374, S375, and S376 of ICP22 is host cell independent and affects the accumulation of γ2 proteins.
The experiments described below were essentially the same as detailed above except that the test virus was R7855, a recombinant virus which carries alanine substitutions in T374, S375, and S376 of ICP22. The amino acid sequence of ICP22 was verified by sequencing. The key findings shown in Fig. 4 are as follows.
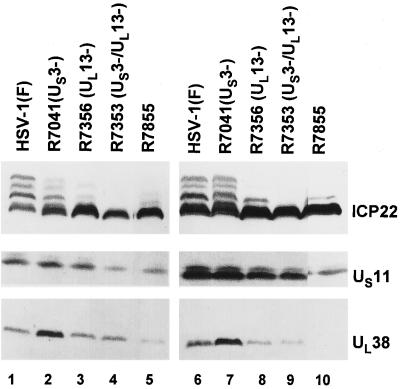
FIG. 4.
Photograph of immunoblots of electrophoretically separated proteins from Vero cells and RSC infected with HSV-1(F), R7041 (US3−), R7356 (UL13−), R7353 (US3−/UL13−), and recombinant virus R7855. Replicate cultures of Vero cells (lanes 1 to 5) or RSC (lanes 6 to 10) were infected with the virus at an MOI of 5 and harvested at 18 h after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with a monoclonal antibody to US11 or polyclonal antibodies to ICP22 and UL38 as described in Materials and Methods. All exposures for a given cell line were identical. Lane 4 was in a different position of the same gel. To maintain order in the presentation of data, the lane was cut and moved to its present location.
(i) ICP22 was only partially posttranslationally processed in both Vero cells (lanes 1 to 5) and RSC (lanes 6 to 10) infected with the recombinant virus.
(ii) The accumulations of US11 and UL38 proteins were reduced in both cell lines but especially so in RSC infected with the mutant (compare lane 5 with lane 10).
We conclude from these results that changes at amino acids 374, 375, and 376 also affect the processing of ICP22 by viral protein kinases.
DISCUSSION
ICP22 is posttranslationally processed largely by the UL13 protein kinase and to a lesser extent by the US3 protein kinase as well as by cellular enzymes. Earlier studies have also shown that the phenotype of deletions within the coding sequence of ICP22 closely paralleled that of a mutant lacking UL13 (12, 14). A central and still unresolved question is whether UL13 is required in order to phosphorylate ICP22 or whether both are required independently. The obvious operational solution to this question is to map the site of phosphorylation of ICP22 by UL13 and determine whether mutagenesis of that site such as to preclude posttranslational processing would alter the phenotype of ICP22. This and a preceding report from this laboratory (10) attempted to define the requirements for the posttranslational modification of ICP22. The salient feature of the results presented in this report are as follows. (i) Posttranslational processing mediated by UL13 protein kinase was abolished by mutations introduced into ICP22 at sites distant from each other. This observation indicates that amino acid substitution is not an unambiguous method for mapping the sites of posttranslational modifications. (ii) Posttranslational processing of ICP22 carrying mutations in the carboxyl-terminal repeat sequence was cell-type dependent. (iii) Alanine substitutions in the amino-terminal homolog precluded posttranslational processing but had no effect on the accumulation of late proteins exemplified by UL38 and US11. The significance of these results is as follows.
(i) Results presented in an earlier report showed that deletion of the carboxyl-terminal 18 amino acids but not the carboxyl-terminal 15 amino acids precluded posttranslational processing of ICP22 (10). The prospect that the site of binding of UL13 or the site of phosphorylation of ICP22 was identified was dimmed by the observation that mutations elsewhere, and particularly at residues 147 to 170 and 380 to 396, also precluded phosphorylation. In this work we have extended these studies to substitutions of serines/threonines with alanines in amino acids 38 to 47, 300 to 328, and 374 to 376. The effect of the serine/threonine mutations in amino acids 38 to 47 are of particular interest because in an earlier report it was shown that the US1.5 protein was fully posttranslationally processed in cells infected with a mutant unable to express ICP22 (10). Since the amino-terminal substitutions are in a domain not shared with the US1.5 protein, it follows that the determinants of phosphorylation of ICP22 reside at many sites along the protein. Such an effect could be due to one of two possibilities: either each mutation exerts a global effect on the conformation of the protein to a degree such that UL13 is unable to phosphorylate ICP22, or the substrate of UL13 is a multimeric structure and the mutations preclude its formation.
(ii) Alanine substitutions in the carboxyl-terminal homolog had no effect on the processing of ICP22 in Vero cells but precluded full processing in RSC. This observation presents an interesting paradox. In this laboratory, the expression of specific genes is tested in several cell lines and the cell line expressing the highest amounts of the viral protein is used for further studies of the products of the specific gene. The notion that cell lines may differ with respect to the level of viral gene expression is implicit in the observation that viral promoters contain cellular response elements whose activators may vary from one cell line to the next. It would not be expected that the modification of a viral protein by a viral enzyme would be cell-line dependent unless the interaction were dependent on the presence of a cellular protein. For example, if UL13 kinase substrate were ICP22 complexed with a cellular protein, the phosphorylation of ICP22 could be cell-line dependent if in specific cell lines this complex would not form. It is conceivable, for example, that an RSC partner forms a less stable complex with ICP22 than does the corresponding primate cell partner. Such a protein has been previously described (2). Mutations in ICP22 could further destabilize the complex or render it unrecognizable by the UL13 protein kinase.
(iii) This report presents evidence that posttranslational processing of ICP22 is not essential for optimal accumulation of the subset of γ2 proteins exemplified by UL38 and US11. This is consistent with the earlier report showing that the truncated isoform of ICP22, US1.5, is sufficient for expression of the subset of γ2 genes exemplified by UL38 and US11 (10). It is also noteworthy that of the three sequences targeted for mutagenesis, the two homologs belong to the broader set of sequences that are determinants of posttranslational processing of ICP22. The third sequence, shared by ICP22 and US1.5 proteins, affected both posttranslational processing and the accumulation of UL38 and US11 proteins. Since the carboxyl-terminal homolog and the third sequence targeted for mutagenesis are shared with US1.5 protein, we have in effect begun to delineate the domains of the US1.5 protein required for optimal accumulation of US11 and UL38 proteins.
ACKNOWLEDGMENTS
This study was aided by Public Health Service grants CA47451, CA71933, and CA78766 from the National Cancer Institute.
REFERENCES
- 1.Ackermann M, Sarmiento M, Roizman B. Application of antibody to synthetic peptides for characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J Virol. 1985;56:207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruni R, Fineschi B, Ogle W O, Roizman B. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J Virol. 1999;73:3810–3817. doi: 10.1128/jvi.73.5.3810-3817.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter K L, Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J Virol. 1996;70:172–178. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejercito P, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 5.Leopardi R, Ward P L, Ogle W O, Roizman B. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the UL13 protein kinase. J Virol. 1997;71:1133–1139. doi: 10.1128/jvi.71.2.1133-1139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackem S, Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of α genes. Proc Natl Acad Sci USA. 1980;77:7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meignier B, Longnecker R, Mavromara-Nazos P, Sears A E, Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell C, Blaho J A, Roizman B. Casein kinase II specifically nucleotidylylates in vitro the amino acid sequence of the protein encoded by the α22 gene of herpes simplex virus 1. Proc Natl Acad Sci USA. 1994;91:11864–11868. doi: 10.1073/pnas.91.25.11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell C, Blaho J A, McCormick L, Roizman B. The nucleotidylylation of herpes simplex virus 1 regulatory protein α22 by human casein kinase II. J Biol Chem. 1997;272:25394–25400. doi: 10.1074/jbc.272.40.25394. [DOI] [PubMed] [Google Scholar]
- 10.Ogle W O, Roizman B. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J Virol. 1999;73:4305–4315. doi: 10.1128/jvi.73.5.4305-4315.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 12.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice S A, Long M C, Lam V, Spencer C A. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice S A, Long M C, Lam V, Schaffer P A, Spencer C A. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rixon F J, Clements J B. Detailed structural analysis of two spliced HSV-1 immediate-early mRNAs. Nucleic Acids Res. 1982;10:2241–2256. doi: 10.1093/nar/10.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roller R J, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwyzer M, Wirth U V, Vogt B, Fraefel C. BICP22 of bovine herpesvirus 1 is encoded by a spliced 1.7 kb RNA which exhibits immediate early and late transcription kinetics. J Gen Virol. 1994;75:1703–1711. doi: 10.1099/0022-1317-75-7-1703. [DOI] [PubMed] [Google Scholar]
- 20.Ward P L, Ogle W O, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson R J, Sullivan M, vande Woude G F. Structures of two spliced herpes simplex virus type 1 immediate-early mRNAs which map at the junctions of the unique and reiterated regions of the virus DNA S component. J Virol. 1981;37:431–444. doi: 10.1128/jvi.37.1.431-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]