Abstract
Aim: This study assessed real-world treatment in patients with metastatic urothelial carcinoma (mUC) in Germany. Materials & methods: Patients diagnosed with mUC from 2015 to 2019 were identified in two claims databases: AOK PLUS and GWQ. Results: 3226 patients with mUC were analyzed; 1286 (39.9%) received systemic treatment within 12 months of diagnosis (platinum-based chemotherapy: 64.2%). Factors associated with receiving treatment were: younger age, male sex, less comorbidity and recent diagnosis. In AOK PLUS and GWQ populations, unadjusted median overall survival (interquartile range) from diagnosis in treated patients was 13.7 (6.8–32.9) and 13.8 (7.1–41.7) months, and in untreated patients was 3.0 (1.2–10.8) and 3.6 (1.2–18.8) months, respectively. Conclusion: A significant proportion of patients with mUC in Germany receive no systemic treatment.
Keywords: : first-line treatment, metastatic urothelial carcinoma, overall survival, progression-free survival, real-world treatment pattern, systemic treatment, treatment discontinuation
Plain language summary
What is this article about?
This article reports the results from a study in Germany between 2015 and 2019 that investigated how advanced bladder cancer that has spread to other organs was treated and how long people lived after diagnosis. The study looked at systemic therapies, which means treatments that affect the entire body.
What were the results?
Only 40% of people diagnosed with advanced bladder cancer received systemic treatment within the first 12 months. Of those who did receive systemic treatment, the majority received combination therapy that included a chemotherapy drug containing platinum (64%). Systemic treatment was more likely to be given to people who were younger, less sick, male, or more recently diagnosed. After 12 months, 56% of treated people were still alive, compared with 26% of people without treatment. On average, people who received systemic treatment lived for about 14 months, while people without systemic treatment lived for only 3 to 4 months.
What do the results of the study mean?
Many people with advanced bladder cancer in Germany do not receive systemic treatment. People who receive treatment are likely to live longer than those who do not receive treatment.
Plain language summary
Summary points.
This study sought to explore real-world treatment rates, treatment patterns, and outcomes in patients with metastatic urothelial carcinoma (mUC) in Germany.
Patients in Germany with an incident mUC diagnosis from 2015 to 2019 were identified in two claims databases (AOK PLUS and GWQ) covering ≈8 million patients.
Of 3226 patients with mUC identified, 1892 (58.6%) did not receive systemic treatment, 1286 (39.9%) received systemic treatment within the first 12 months after diagnosis and 48 (1.5%) received systemic treatment ≥12 months after diagnosis.
Among treated patients, 825 (64.2%) received platinum-based chemotherapy, 322 (25.0%) received non-platinum chemotherapy, and 139 (10.8%) received immunotherapy.
Factors associated with a higher likelihood of receiving systemic treatment within 12 months included younger age, lower comorbidity score, more recent diagnosis and male sex.
The probability of survival at 12 months after diagnosis in the treated and untreated cohorts was 56.1 and 26.1%.
The probability of survival at 12 months after diagnosis for patients receiving platinum-based chemotherapy, non-platinum chemotherapy, or immunotherapy was 60.4, 49.4 and 46.8%, respectively.
Unadjusted median OS from diagnosis in the AOK PLUS and GWQ populations in treated patients was 13.7 (interquartile range [IQR], 6.8–32.9) and 13.8 months (IQR, 7.1–41.7) months, and in untreated patients was 3.0 (IQR, 1.2–10.8) months and 3.6 months (IQR, 1.2–18.8), respectively.
These findings suggest that a substantial proportion of patients with mUC in Germany remained undertreated during the study period.
Urothelial carcinoma (UC) accounts for 90% of bladder cancer cases; it is considered the tenth most commonly diagnosed cancer globally and was responsible for 200,000 deaths worldwide in 2018 [1,2]. The disease is the ninth most common cause of cancer-induced mortality. In the European Union, nearly 204,000 people were diagnosed with bladder cancer in 2020, and 67,000 patients died as a result of the disease [3]. While bladder cancer incidence and mortality rates (MRs) vary across European countries, bladder cancer is one of the most commonly observed cancers in Germany, where 31,040 people were diagnosed with the disease in 2018 (18,270 patients with invasive disease; 12,770 with noninvasive papillary tumors and/or cancer in situ); 26% of those diagnosed were women [4]. The burden is highest among older patients. The median age of disease onset is 73 years, and the risk of developing UC increases with age [5].
In Germany, ≈29% of patients with UC have locally advanced or metastatic disease at diagnosis, according to the 7th edition of the tumor, node, metastasis (TNM) staging system [4]. In addition, 30% of patients diagnosed with muscle-invasive UC develop metastasis, resulting in a poor prognosis at this stage [6]. Previous international studies have reported a median overall survival (OS) of ≈4 months in patients who were not treated with systemic anticancer agents [7,8]. However, in patients receiving platinum-based chemotherapy (PB-CT) and no maintenance treatment, a median OS of up to 16.2 months and median progression-free survival of up to 7.6 months was observed in previous studies [9–12].
Observed advantages in OS associated with the use of systemic treatments led to guideline recommendations that all eligible patients receive cisplatin- or carboplatin-based CT as first-line (1L) treatment [13]. Non–platinum-containing regimens are considered in patients who cannot tolerate any PB-CT because of renal impairment or other comorbidities [1,13]. In recent years, the treatment landscape has transformed as a result of the approval of immunotherapy (IO) agents with checkpoint inhibitors for use as 1L, second-line (2L) for locally advanced or metastatic UC (la/mUC), and adjuvant treatments in patients with muscle-invasive UC (MIUC) [14–18]. Previous studies have indicated that IOs result in improved outcomes compared with standard treatments in the 2L setting [13,19].
Since 2017, four IO agents have been approved to treat UC in Germany including atezolizumab and pembrolizumab, which are used as 1L monotherapies in patients with la/mUC who are PD-L1–positive and not suitable for cisplatin [15,16,20]. Atezolizumab, pembrolizumab, and nivolumab were also approved as 2L treatments for la/mUC in adults after failure of prior PB-CT [17]. In 2021, and thus outside the study period of the current study, avelumab was approved for use as 1L maintenance therapy in patients who are progression-free following 1L PB-CT and is now considered the standard of care, replacing the previous ‘watch-and-wait’ approach [13,21–23]. Enfortumab vedotin, an antibody-drug conjugate, was approved by the European Commission in 2022 as a treatment option for adults after failure of prior platinum-containing treatment and a PD-1 or PD-L1 inhibitor [24].
Regardless of the recent developments in la/mUC treatment, observational evidence shows that many patients with mUC are still undertreated. In Europe, the proportion of patients who are not receiving systemic treatment is thought to range from 40% (Spain) to 70–74% (UK) [25]. So far, no descriptive comparisons between the OS of untreated and treated patients have been conducted in Germany. Additionally, insufficient information is available on the share of untreated patients and on the treatment patterns and OS among patients with mUC who are receiving recently approved IO agents.
Thus, this study sought to explore real-world treatment rates, treatment patterns and outcomes in patients with mUC in Germany using claims data provided by statutory sickness funds.
Materials & methods
Study design & data source
This study was a retrospective noninterventional cohort analysis of patients with UC and incident metastatic disease that used data from 01/01/2013 to 31/12/2020. This study involved two anonymized health insurance claims datasets provided by AOK PLUS and GWQ ServicePlus AG under the formal agreement and legal basis of §75, Tenth Book of the Social Code (SGB X). No informed consent or ethical approval from an institutional review board was required to implement this retrospective study, which only used anonymized, nonidentifiable data. AOK PLUS is the sixth largest German sickness fund and covers ≈3.5 million insured patients in the regions of Saxony and Thuringia in central-eastern Germany, representing 50% of the local population and approximately 4–5% of the overall population of statutory insured patients in Germany. GWQ is an institutional convener of sickness funds that has access to health insurance record data from ≈12 million insured patients from smaller health insurance funds. Patients included in the GWQ dataset reside in various regions throughout Germany. Due to relevant approvals from participating sickness funds, the final GWQ dataset used in this study covered ≈4.5 million publicly insured patients. Together, both datasets represent ≈10% of the total German population.
Patient population
First, a base set of eligibility criteria was applied to identify patients with incident mUC diagnoses (main cohort), as indicated below:
At least one inpatient and/or two outpatient specialist diagnoses of UC (International Statistical Classification of Diseases and Related Health Problems [ICD-10] C65–C68 [neoplasms of the urinary organs; excl. kidney]) between 01/01/2015–31/12/2019.
At least one inpatient or confirmed outpatient diagnosis of metastasis (ICD-10 C77–C79 excluding C79.0 and C79.1) from any specialist within the period lasting from 3 months before the first observed UC code to 6 months after the last observed UC code (first metastasis code served as the index date).
No metastases codes within the 24-month baseline (BL) period (before index metastasis diagnosis).
At least 18 years old at the index date.
Continuously insured for at least 24 months before and 12 months after the index date, with death as the only exception.
No diagnosis of other malignant neoplasms (ICD-10 C34 - lung; C18 - colon; C19 - rectosigmoid junction, C20 - rectum) from 24 months before and 12 months after the index date.
A time limitation for the diagnosis of metastasis, i.e., requiring metastasis codes to be observed in close proximity to the last UC diagnosis, was included to avoid potential misclassification of UC cases with metastasis from other cancers. Other malignant neoplasms were excluded because a similar treatment regimen for these tumors is indicated; this could confound the results observed in our population of patients with mUC.
Following the application of the general eligibility criteria, three additional subcohorts were delineated based on additional requirements:
Untreated cohort: patients did not receive at least one of the systemic treatment options (PB-CT, non–PB-CT, or IO) at any time after their incident mUC diagnosis.
-
Treated cohort: patients were required to receive at least one of the following mUC 1L systemic treatments within 12 months after the index date. The treatment group was determined according to the first treatment or treatment combination received after the mUC index date:
-
○PB-CT (i.e., cisplatin/carboplatin monotherapy or combination treatment or oxaliplatin combination treatment).
-
○IO (i.e., pembrolizumab, atezolizumab, or nivolumab).
-
○Non–PB-CT (i.e., gemcitabine, vinflunine, paclitaxel mono- or combination treatment, or docetaxel monotherapy).
The codes used to define these treatments are detailed in Supplementary Table 1.
-
○
Delayed-treated cohort: patients received at least 1 1L systemic treatment for mUC after the first 12 months post index.
In addition, a sensitivity analysis was performed to compare characteristics and outcomes in patients with mUC who had no other primary tumors (ICD-10 C00-C75, without consideration of malignant melanoma of the skin, other malignant neoplasms of the skin, and malignant neoplasm of the prostate) with those in the main cohort. The criteria used to identify patients included in the sensitivity cohort were the same as those for the main cohort, except that all patients with any further primary malignant neoplasms were excluded within the 24 months before and 12 months after the index date.
Data analysis
Patients were observed for at least 12 months after incident mUC diagnosis (index) or until death. Patient characteristics, treatment rates and treatment patterns were described during the 24-month BL and 12-month follow-up periods for all cohorts; in the delayed-treated cohort, outcomes were also observed during the entire follow-up time. In the treated cohort, treatment lines, time to first systemic treatment, treatment discontinuation and treatment switch were assessed during the follow-up period.
Treatment lines were captured using proxies. 1L treatment initiation was defined as the first date on which a patient received an Anatomical Therapeutic Chemical (ATC) code or operations and procedures key (German: ‘Operationen- und Prozedurenschlüssel’, OPS) for any systemic treatment for mUC. Furthermore, combination treatments were assumed if prescriptions for multiple agents were observed within 30 days of each other. An exception to the 30-day rule was made in the non–PB-CT cohort; the time was extended to 90 days to account for potential treatment interruptions due to adverse events.
Treatment discontinuation was assumed in cases where one of the following events was observed: switch to another systemic treatment (defined by no further prescription of the respective agents from the current treatment line, followed by a prescription code for one of the other treatment options), death, or treatment gap (defined as no receipt of any systemic treatment for at least 90 days; a gap of 60 days was considered in a sensitivity analysis). Subsequent treatment lines were indicated either by switching to another systemic treatment or by resuming the same treatment after a treatment gap.
A logistic regression was used to test for indicators of receiving treatment within the main mUC cohort. The approaches were in line with previous studies in relation to included covariates [26,27] and recommendations from clinicians involved in this study. During follow-up, a Kaplan–Meier (KM) analysis was used to calculate OS from the mUC index date. The unadjusted time to death from the date of initial mUC diagnosis was reported and compared in treated and untreated cohorts. Based on the outcomes in treated patients, time to death from 1L initiation was calculated and compared among the treatment subcohorts.
Key outcomes were compared between the treated and untreated mUC cohorts and between the three treatment groups using statistical tests, including a t-test, chi-squared test, Fisher's exact test, or McNemar test. All reported p-values were based on two-sided tests, and variables with a corresponding p-value < 0.05 were considered significant.
Data analyses were performed separately for each claims database. In the second step, outcomes were combined using a meta-analysis approach [28,29]. All relevant data queries, descriptive or comparative analyses, and meta-analyses were performed using Microsoft SQL Server 2019 (AOK PLUS) / 2016 (GWQ), Microsoft Excel 2021 (AOK PLUS) / 2019 (GWQ), Stata version 17.0 (AOK PLUS) / 15.1 (GWQ) software, and R version 4.2.0.
Results
Study sample
In total, 3226 patients (sensitivity, 2350) were included in the main mUC cohort, of which 1892 (58.6%) remained untreated; 1286 patients (39.9%) received systemic treatment in the first 12 months. Moreover, 48 patients (1.5%) who received mUC treatments after 1 year following the incident mUC diagnosis were identified. Although these patients were included in the main mUC cohort, they were omitted from treatment-related outcomes analyses presented in the current study (Figure 1). Among treated patients, 825 (64.2%), 139 (10.8%) and 322 (25.0%) were allocated to the PB-CT, IO and non–PB-CT cohorts, respectively (Figure 1 & Table 1). The mean (standard deviation [SD]) age of patients in the main mUC cohort was 73.8 (10.8) years, and most patients were male (70.8%). The mean (SD) Charlson Comorbidity Index (CCI) score was 6.3 (3.8) in the main cohort; the mean (SD) Elixhauser Comorbidity Index score was 17.6 (11.4; Table 2). The most frequently observed surgical interventions related to UC during the BL period were transurethral bladder resection (51.6%) followed by cystectomy (13.3%; Table 2). In the untreated cohort, generally, higher average age and fewer male patients were observed compared with the treated cohort (77.3 vs 68.8 years; 68.7 vs 74.0% male patients). Among the treated subcohorts, patients receiving IO had the highest average mean age (IO, 72.7 years; non–PB-CT, 72.0 years; PB-CT, 66.9 years) and were more often female compared with patients receiving non–PB-CT and PB-CT (28.1 vs 24.2% and 26.3%).
Figure 1.

Patient selection and cohort delineation.
1L: First-line; CT: Chemotherapy; mUC: Metastatic urothelial carcinoma.
Table 1.
1L treatment regimens (mutually exclusive).
| Treatment regimens | Patients treated with 1L treatment, n (%) (n = 1286) |
|---|---|
| All patients receiving PB-CT | 825 (64.2) |
| Cisplatin or carboplatin + gemcitabine | 304 (23.6) |
| Paclitaxel + carboplatin | 14 (1.1) |
| Paclitaxel + carboplatin + gemcitabine | 2 (0.2) |
| Oxaliplatin + gemcitabine | 2 (0.2) |
| Cisplatin or carboplatin (mono) | 12 (0.9) |
| Moderately complex and intensive block CT (OPS 8-543; MVAC or gemcitabine + cisplatin) | 491 (38.2) |
| All patients receiving IO† | 139 (10.8) |
| Atezolizumab | 21 (1.6) |
| Pembrolizumab | 71 (5.5) |
| Nivolumab‡ | 47 (3.7) |
| All patients receiving other non–PB-CT | 322 (25.0) |
| Gemcitabine | 79 (6.1) |
| Paclitaxel + gemcitabine | 10 (0.8) |
| Paclitaxel | 8 (0.6) |
| Docetaxel | 23 (1.8) |
| Vinflunine | 7 (0.5) |
| Vinflunine + gemcitabine | 6 (0.5) |
| Noncomplex CT (OPS 8-542; gemcitabine or paclitaxel + gemcitabine or paclitaxel) | 189 (14.7) |
IOs for use in the 1L and 2L settings were first approved in the European Union beginning in late 2017.
During the patient inclusion period (2015–2019), nivolumab was not approved as a 1L IO and was only approved for use in the 2L setting in the European Union.
1L: First-line; 2L: Second-line; IO: Immunotherapy; mono: Monotherapy; MVAC: Methotrexate + vinblastine + doxorubicin + cisplatin; OPS: Operations and procedures key; PB-CT: Platinum-based chemotherapy.
Table 2.
Patient characteristics and comorbidities.
| Patient characteristics | Main cohort |
Sensitivity cohort |
Untreated cohort |
Treated cohort |
PB-CT cohort |
IO cohort |
Non–PB-CT cohort |
|
|---|---|---|---|---|---|---|---|---|
| N = 3226 | n = 2350 | n = 1892 | n = 1286 | n = 825 | n = 139 | n = 322 | ||
| Age (at index date) | Mean (SD) | 73.8 (10.8) | 73.8 (10.8) | 77.3 (9.8) | 68.8 (10.4) | 66.9 (10.5) | 72.7 (10.0) | 72.0 (8.8) |
| Sex | M % / F % | 70.8 / 29.2 | 74.0 / 26.0 | 68.7 / 31.3 | 74.0 / 26.0 | 73.7 / 26.3 | 71.9 / 28.1 | 75.8 / 24.2 |
| Index year (year of first mUC diagnosis) | ||||||||
| 2015 | n (%) | 600 (18.6) | 438 (18.6) | 380 (20.1) | 212 (16.5) | 148 (17.9) | 0 (0) | 64 (19.9) |
| 2016 | n (%) | 649 (20.1) | 461 (19.6) | 383 (20.2) | 260 (20.2) | 186 (22.5) | 3 (2.2) | 71 (22.1) |
| 2017 | n (%) | 695 (21.5) | 513 (21.8) | 406 (21.5) | 272 (21.2) | 173 (21.0) | 33 (23.7) | 66 (20.5) |
| 2018 | n (%) | 595 (18.4) | 427 (18.2) | 352 (18.6) | 230 (17.9) | 129 (15.6) | 45 (32.4) | 56 (17.4) |
| 2019 | n (%) | 687 (21.3) | 511 (21.7) | 371 (19.6) | 312 (24.3) | 189 (22.9) | 58 (41.7) | 65 (20.2) |
| Follow-up period (in days) | Mean (SD) | 420.2 (490.2) | 413.6 (491.3) | 322.7 (485.1) | 544.7 (465.7) | 597.4 (363.9) | 389.3 (302.2) | 476.6 (444.8) |
| Censorship reason | ||||||||
| Death | n (%) | 2533 (78.5) | 1825 (77.7) | 1576 (83.3) | 926 (72.0) | 573 (69.5) | 105 (75.5) | 248 (77.0) |
| End of insurance | n (%) | 33 (1.0) | 15 (0.6) | 19 (1.0) | 14 (1.1) | 11 (1.3) | 0 (0) | 3 (0.9) |
| End of follow-up | n (%) | 660 (20.5) | 510 (21.7) | 297 (15.7) | 346 (26.9) | 241 (29.2) | 34 (24.5) | 71 (22.1) |
| Charlson Comorbidity Index (24-month BL) | Mean (SD) | 6.3 (3.8) | 6.1 (3.7) | 6.8 (3.9) | 5.5 (3.5) | 5.1 (3.4) | 6.5 (3.7) | 6.2 (3.7) |
| Median (min–max) | 6 (0–19) | 5 (0–18) | 6 (0–19) | 5 (0–18) | 4 (0–18) | 6 (0–16) | 5 (0–18) | |
| Elixhauser Comorbidity Index (24-month BL) | Mean (SD) | 17.6 (11.4) | 17.2 (11.3) | 19.3 (11.6) | 15.2 (10.7) | 13.8 (10.3) | 19.0 (11.8) | 17.3 (10.7) |
| Median (min–max) | 16 (-7–66) | 16 (-7–66) | 18 (-7–66) | 14 (-3–53) | 11 (-3–48) | 17 (-3–53) | 16 (0–52) | |
| UC-related interventions (24-month BL) | ||||||||
| Transurethral resection of the bladder | n (%) | 1666 (51.6) | 1371 (58.3) | 923 (48.8) | 719 (55.9) | 488 (59.2) | 65 (46.8) | 166 (51.6) |
| Cystectomy | n (%) | 430 (13.3) | 356 (15.2) | 214 (11.3) | 211 (16.4) | 129 (15.6) | 29 (20.9) | 53 (16.5) |
| Lymph node dissection | n (%) | 163 (5.1) | 115 (4.9) | 79 (4.2) | 84 (6.5) | 56 (6.8) | 10 (7.2) | 18 (5.6) |
| Radiotherapy | n (%) | 111 (3.4) | 70 (3.0) | 76 (4.0) | 36 (2.8) | 23 (2.8) | 8 (5.8) | 12 (3.7) |
| Bladder replacement | n (%) | 89 (2.8) | 77 (3.3) | 22 (1.2) | 65 (5.1) | 52 (6.3) | 4 (2.9) | 9 (2.8) |
| Ureterocutaneostomy | n (%) | 73 (2.3) | 57 (2.4) | 51 (2.7) | 22 (1.7) | 10 (1.2) | 4 (2.9) | 8 (2.5) |
| Partial bladder resection | n (%) | 62 (1.9) | 35 (1.5) | 26 (1.4) | 35 (2.7) | 15 (1.8) | 8 (5.8) | 5 (1.6) |
BL: Baseline; F: Female; IO: Immunotherapy; M: Male; max: Maximum; min: Minimum; mUC: Metastatic urothelial carcinoma; PB-CT: Platinum-based chemotherapy.
Treatment rates & patterns
The overall treatment rate observed in this study was 39.9%. The proportion of treated patients slowly increased over time, from 35.3% in 2015 to 45.4% in 2019; this is partly explained by an increase in observed IO use related to market launches (Figure 2). During the 12-month follow-up period, the most commonly received systemic mUC treatments among treated patients across all treatment lines were moderately complex and intensive block CT (50.7%), noncomplex CT (41.8%) and gemcitabine (22.4%). In contrast to 1L treatments, a patient could have received more than 1 of the treatments during follow-up. In general, the mean (SD) time to 1L treatment initiation was 1.9 (2.2) months in the treated cohort, with an average (SD) treatment duration of 3.1 (3.3) months until discontinuation. The average (SD) time to treatment was the longest in the IO cohort compared with the PB-CT and non–PB-CT cohorts (IO, 3.4 [3.3]; PB-CT, 1.7 [1.9]; non–PB-CT, 1.7 [2.0] months), as was the mean (SD) 1L treatment duration (5.4 [7.3], 3.0 [2.1], and 2.6 [2.5] months). In addition, less than half (36.8%) of treated patients started a 2L treatment during follow-up; patients with IO as their 1L treatment had the fewest 2L treatments (15.8%), mainly caused by treatment discontinuation due to death or a 90-day treatment gap. Most patients who received PB-CT as 1L treatment and then started a 2L treatment during the follow-up period switched to IO (26.4%; Supplementary Figure 1). Moreover, the mean (SD) time to 2L treatment from the last outpatient prescription or hospital discharge date of 1L treatment was 6.1 (7.1) months in the treated cohort. Time to 2L treatment initiation was shortest in the IO cohort, with 2.1 (1.8) months, compared with 6.4 (7.4) months in the PB-CT cohort and 5.8 (6.3) months in the non–PB-CT cohort. Only a small proportion of all treated patients started a third-line (3L) treatment during the follow-up period (9.3%), and 11.8% of patients with PB-CT received 3L treatment, compared with 5.9% of patients with non–PB-CT and 2.9% of IO-treated patients. The overall low numbers are possibly due to the high MR recorded in patients with mUC (Figure 3).
Figure 2.

Proportion of total patients receiving systemic treatment by index year.
IO: Immunotherapy; PB-CT: Platinum-based chemotherapy.
Figure 3.
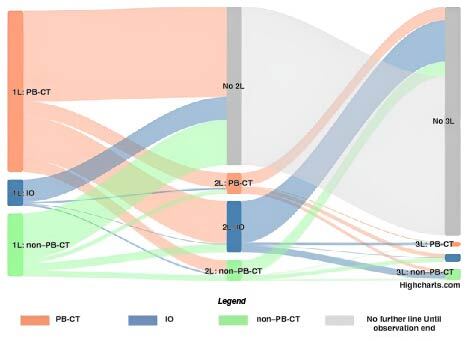
Sankey diagram–treatment sequences by treatment subcohorts.
More detailed figures can be found in Supplementary Figure 1. Four patients from the total treated cohort (n = 1286) were excluded from this observation because their treatment lines could not clearly be identified; all treatment line observations shown are for a gap definition of 90 days.
1L: First-line; 2L: Second-line; 3L: Third-line; IO: Immunotherapy; PB-CT: Platinum-based chemotherapy.
Treatment discontinuation
Patients were considered to have discontinued 1L treatment if they experienced a measured treatment gap, death, or a documented treatment switch. Most treated patients discontinued 1L treatment in the first year of observation after mUC diagnosis (97.0%) when assuming a treatment gap of 90 days (60-day sensitivity, 97.4%). Furthermore, a lower proportion of patients who discontinued 1L treatment was observed in the IO cohort (87.1%; 60-day sensitivity, 87.8%) as compared with the PB-CT (98.1%; 60-day sensitivity, 98.6%) and non–PB-CT (98.5%; 60-day sensitivity, 98.8%) cohorts.
Treatment discontinuation within the first year was most commonly indicated by a treatment gap and was observed in 47.0% (60-day sensitivity, 52.6%) of treated patients. The proportion of patients with treatment gaps was highest in the PB-CT cohort (50.7%; 60-day sensitivity, 55.5%), followed by the non–PB-CT (48.1%; 60-day sensitivity, 55.3%) and IO (22.3%; 60-day sensitivity, 29.5%) cohorts. Most patients receiving IO discontinued due to death (49.6%; 60-day sensitivity, 43.9%); a lower rate of discontinuation due to death was observed in the PB-CT (18.6%; 60-day sensitivity, 14.2%) and non–PB-CT (33.9%; 60-day sensitivity, 27.3%) cohorts.
Indicators of receiving systemic treatment
A multivariable logistic regression was used to assess predictors of treatment in the main mUC cohort, in which the outcome variable was defined as having received treatment within the first 12 months (Table 3). Within our model, younger age, lower CCI score, index diagnosis in 2019 compared with 2015, receipt of UC-related interventions during BL (e.g., cystectomy, transurethral resection of the bladder, or radiotherapy), inpatient mUC diagnosis, fewer hospitalizations during BL, and male sex were found to be significant factors that were positively associated with a patient's likelihood of receiving systemic treatments in the first 12 months after diagnosis. In addition, care level was analyzed only for the AOK PLUS database (due to data availability). The probability of being treated increased with decreasing care levels.
Table 3.
Indicators for receiving treatment based on a multivariate logistic regression (treated vs untreated cohorts).
| Predictors of treatment | Odds ratio | p-value | Standard error | 95% CI |
|---|---|---|---|---|
| Care level (24-months BL; ordinal [no care level; low/medium level of care; high level of care]) – AOK PLUS only | 0.36 | <0.001 | 0.05 | 1.92–3.38 |
| Age at index (continuous) | 0.93 | <0.001 | 0.01 | 0.92–0.94 |
| Previous UC-related treatments, surgeries, and interventions (24-month BL; binary) | 1.65 | <0.001 | 0.12 | 1.37–2.00 |
| Charlson Comorbidity Index (24-month BL; continuous) | 0.97 | 0.011 | 0.01 | 0.93–1.00 |
| Outpatient diagnostic setting (binary) | 1.28 | 0.013 | 0.11 | 1.05–1.54 |
| Number of hospitalizations (24-month BL; continuous) | 0.97 | 0.027 | 0.02 | 0.94–1.00 |
| Female sex (binary) | 0.83 | 0.032 | 0.16 | 0.69–0.98 |
| Index year (reference year: 2015; categorical) | ||||
| 2016 | 1.26 | 0.117 | 0.17 | 0.97–1.62 |
| 2017 | 1.16 | 0.288 | 0.15 | 0.90–1.49 |
| 2018 | 1.24 | 0.148 | 0.17 | 0.96–1.61 |
| 2019 | 1.66 | 0.002 | 0.21 | 1.29–2.13 |
| Previous primary malignant carcinomas (24-month BL; binary) | 1.10 | 0.317 | 0.12 | 0.92–1.31 |
| Number of outpatient visits (24-month BL; continuous) | 1.01 | 0.162 | 0.01 | 1.00–1.01 |
p-values <0.05 are shown in bold font.
BL: Baseline; UC: Urothelial carcinoma.
Overall survival & mortality
The proportion of patients still alive was assessed in treated and untreated patients at 3, 6, 9 and 12 months after index mUC diagnosis. At 3 months, survival was nearly twice as high in the treated cohort compared with the untreated cohort (91.9 vs 51.6%), and the magnitude of the difference increased at the 12-month mark (56.1 vs 26.1%). In the treated cohort, patients receiving PB-CT had the highest proportion of surviving patients in all predefined measurement windows, with 95.6% alive at 3 months and 60.4% at 12 months. This was followed by the non–PB-CT cohort (3 months, 85.4%; 12 months, 49.4%) and IO cohort (3 months, 84.9%; 12 months, 46.8%). Finally, the MR per patient-year was higher in untreated patients compared with treated patients (0.95 vs 0.48), and patients treated with PB-CT had the lowest MR (0.42), followed by the non–PB-CT (0.60) and IO (0.72) cohorts (Table 4). Given that reporting of median OS figures was not possible using meta-analysis approaches outlined in the methodology, median OS numbers are reported separately for both databases (Table 4). Within the context of KM analyses, the median (interquartile range [IQR]) OS during follow-up in the main mUC cohort was 5.9 (1.8–19.1) months for AOK PLUS and 9.1 (2.5–31.2) months for GWQ from index mUC diagnosis. Furthermore, the unadjusted median (IQR) OS in untreated patients from index mUC diagnosis was 3.0 (1.2–10.8) months for AOK PLUS and 3.6 (1.2–18.8) months for GWQ. In the treated cohort, median (IQR) OS was notably longer and amounted to 13.7 (6.8–32.9) months for AOK PLUS and 13.8 (7.1–41.7) months for GWQ (Figure 4). Treated patients had a median (IQR) OS from initiation of 1L treatment of 11.6 (4.9–31.5) months for AOK PLUS and 11.7 (5.1–37.6) months for GWQ. Differences were found in OS from 1L initiation between the three treatment subcohorts, and patients receiving PB-CT recorded the best survival outcomes, with a median (IQR) OS of 12.9 (6.2–33.1) months for AOK PLUS and 13.8 (7.4–49.4) months for GWQ. Furthermore, the median (IQR) OS was 4.1 (1.8–14.1) months for AOK PLUS and 8.2 (2.8–not estimable) for GWQ in the IO cohort, and 11.2 (4.4–36.0) months for AOK PLUS and 6.5 (2.6–15.5) months for GWQ in the non–PB-CT cohort.
Table 4.
Survival and mortality outcomes in patients with mUC.
| Main mUC cohort |
Sensitivity cohort |
Untreated |
Treated |
PB-CT |
IO |
Non–PB-CT |
||
|---|---|---|---|---|---|---|---|---|
| N = 3226 | n = 2350 | n = 1892 | n = 1286 | n = 825 | n = 139 | n = 322 | ||
| Survival (measured at different time periods after index mUC diagnosis) | ||||||||
| 3 months | n (%) | 2207 (68.4) | 1569 (66.8) | 977 (51.6) | 1182 (91.9) | 789 (95.6) | 118 (84.9) | 275 (85.4) |
| 6 months | n (%) | 1752 (54.3) | 1236 (52.6) | 694 (36.7) | 1010 (78.5) | 694 (84.1) | 92 (66.2) | 224 (69.6) |
| 9 months | n (%) | 1473 (45.7) | 1039 (44.2) | 566 (29.9) | 859 (66.8) | 594 (72.0) | 79 (56.8) | 186 (57.8) |
| 12 months | n (%) | 1264 (39.2) | 904 (38.5) | 494 (26.1) | 722 (56.1) | 498 (60.4) | 65 (46.8) | 159 (49.4) |
| Mortality rate (follow-up period) | ppy | 0.67 | 0.69 | 0.95 | 0.48 | 0.42 | 0.72 | 0.60 |
| OS based on Kaplan–Meier estimation (in months; follow-up period) | ||||||||
| Time to death from mUC index date | AOK PLUS (mdn [IQR]) | 5.9 (1.8–19.1) | – | 3.0 (1.2–10.8) | 13.7 (6.8–32.9) | – | – | – |
| GWQ (mdn [IQR]) | 9.1 (2.5–31.2) | 3.6 (1.2–18.8) | 13.8 (7.1–41.7) | |||||
| Time to death from treatment initiation | AOK PLUS (mdn [IQR]) | – | – | – | 11.6 (4.9–31.5) | 12.9 (6.2–33.1) | 4.1 (1.8–14.1) | 11.2 (4.4–36.0) |
| GWQ (mdn [IQR]) | 11.7 (5.1–37.6) | 13.8 (7.4–49.4) | 8.2 (2.8-NE) | 6.5 (2.6–15.5) | ||||
IO: Immunotherapy; IQR: Interquartile range; mdn: Median; mUC: Metastatic urothelial carcinoma; NE: Not estimable; OS: Overall survival; PB-CT: Platinum-based chemotherapy; ppy: Per patient-year.
Figure 4.

Kaplan–Meier Curves–time to death from mUC index date for the treated and untreated cohorts.
(A) AOK PLUS and (B) GWQ.
mUC: Metastatic urothelial carcinoma.
Discussion
This study is the first of its kind and seeks to address existing research gaps by providing an overview of the real-world treatment of newly diagnosed patients with mUC in Germany. Through an investigation of real-world treatment rates, patterns and outcomes during the period in which new IOs became available for use, this research builds on the findings achieved in previous studies, which were often limited to patients receiving PB-CT and non–PB-CT only. Data from ≈8.5 million insured patients (10–11% of the German population) were used; therefore, a high degree of population coverage and representativeness is provided. While AOK PLUS covers eastern Germany with Saxony and Thuringia, the highest percentage of GWQ-insured patients are in southern and western Germany, especially in Bavaria, Baden-Württemberg, Hesse and Rhineland-Palatinate. The study shows similarities with a previous retrospective observational cohort study that focused on the 1L and 2L treatment patterns in German adults who were receiving PB-CT and non–PB-CT and were diagnosed with advanced or metastatic UC [10]. However, as the study period lasted from 2009–2016, the treatment landscape for patients with mUC has developed drastically, and since then, 4 IO agents have been approved in Germany [13,19]. The current study included three of the newly approved treatments and descriptively compared patient characteristics, clinical outcomes, systemic treatment rates, treatment patterns and indicators of treatment in patients receiving IO to other treatments.
A high proportion of undertreated patients was observed in the current study; more than half of the main mUC cohort had not received any mUC systemic anticancer treatment. The number of patients receiving mUC systemic treatment increased by 10% between 2015 and 2019. It was shown that among other factors, younger age, lower CCI score, a later index year, receipt of UC-related interventions during BL, inpatient mUC diagnosis, fewer hospitalizations during BL and male sex were associated with a higher likelihood of receiving systemic treatment.
Similar results of low treatment rates and observed factors related to the likelihood of receiving treatment, including patient characteristics such as age and sex, have been found in previous studies [25–27,30,31]. In addition to the drivers explored in this study, research indicates that other factors (which cannot be assessed using claims data), such as clinician choice, patient or caregiver preference, and impaired renal function, may play a role in high levels of observed undertreatment among patients with mUC [7]. In a bivariate analysis conducted by M.A. Dinan et al., the demographic characteristics of USA patients with la/mUC who received 1L CT were compared with untreated patients [26]. Their findings suggest that being married, having fewer than two comorbidities, being Black and being treated in an academic center or teaching hospital were positively associated with a patient's likelihood of receiving treatment.
Another, possibly less common reason in the context of mUC, why treatment is foregone in the untreated patient cohort could be that clinicians decide not to treat a patient because they may feel that the therapeutic benefit is disproportionate to the adverse events the patient may experience, as many patients with mUC are older and already have a high comorbidity index [32]. This may be particularly relevant when considering treatment with single-dose cisplatin [33].
Future studies should investigate why certain patients with mUC in Germany go undertreated to understand the best method for addressing treatment inequality.
Most observed treated patients received PB-CT, followed by non–PB-CT and IO, due to existing guidelines suggesting PB-CT as the 1L standard of care in mUC treatment [1,13,19,20]. On average, patients receiving IO continued to receive 1L treatment for longer than patients who received CT. However, in our study, only 15.8% of patients receiving 1L IO received 2L treatment, and 2.9% received 3L treatment; almost twice as many patients in the non–PB-CT cohort received 2L (28.0%) and 3L (5.9%) treatment. In addition, the PB-CT cohort had the highest proportion of patients receiving 2L (43.8%) and 3L (11.8%) treatment. These findings were in line with a previous German study, which focused on the 1L and 2L treatment patterns in patients receiving PB-CT or non–PB-CT [10].
The unadjusted survival estimations in this study showed that patients with PB-CT had longer OS than patients receiving non–PB-CT or IO. However, on average, patients receiving PB-CT were younger and had fewer comorbidities; more tolerable agents, such as IO, were prescribed to older and frailer patients. In addition, we observed the longest time to treatment initiation in the IO cohort, which may be related to the delayed initiation of treatment due to the determination of the PD-L1 status, which is required prior to IO initiation. The increasing number of patients treated may suggest that patients assessed as unsuitable for CT may have received one of the newly approved IOs during the course of their disease. Thus, the reported OS rates should not be compared between treatments. Such a comparison would require a multivariable approach that considers all major confounders, such as age, sex and comorbidities, but also clinical parameters that are not available in the claims datasets used in this study.
Our reported OS rates for the PB-CT and non–PB-CT subcohorts align with those of previous studies [9,10]. Additionally, even if no study reporting OS in patients receiving IO has been published in Germany, our IO-related OS findings are well in line with recent international studies reporting OS in platinum-ineligible patients and patients treated with IO [34,35]. Additionally, former research confirmed that treated patients had improved survival rates from mUC diagnosis when compared with untreated patients [8,9,25]. As some new IO agents, such as avelumab 1L maintenance, were approved outside this study period (2021), future studies should investigate how the use of newly introduced treatments may impact observed OS figures [22].
In our study, about one-quarter of treated patients received non–PB-CT. More than half of the patients assigned to the non–PB-CT cohort received noncomplex CT, and about another quarter of patients was assigned due to the receipt of gemcitabine monotherapy. Widespread use of non–PB-CT was not supported by European Union and German guidelines published at the time of patient inclusion (2015–2019), which recommended PB-CT as a 1L standard of care for all fit patients [36,37]. Somewhat lower numbers were observed in the non–PB-CT cohort in previous studies [10,38]; however, fewer agents were included in the non–PB-CT cohort, and no generic OPS codes were observed in these studies. In addition, the proportion of patients receiving non–PB-CT decreased only slightly, even after the first IO agents were approved in 2017 (2015: 10.7%; 2019: 9.5%). However, it should be noted that IO agents were not included in guidelines in the European Union and Germany for use in platinum-ineligible patients until 2020 [13,19,20].
This study observed an increasing use of IO agents beginning in 2016, with a peak of 8.4% in 2019 after the approval of atezolizumab and pembrolizumab for 1L treatment in 2017. The approval of new and well-tolerated treatments for older patients and those with multiple morbidities may be related to the decrease in the number of untreated patients over time.
The literature suggests that IO may be increasingly used in older patients with concomitant diseases due to its good tolerability and related approval for patients ineligible for 1L PB-CT; therefore, the approval of new IOs may provide further options for patients who would otherwise go untreated. Further research on the drivers of undertreatment should explore factors contributing to treatment decision-making in mUC, given that these agents may be used to address unmet treatment needs in key mUC population subdemographics [39,40].
However, this study's observation period ended before avelumab 1L maintenance was available, and the treatment landscape is rapidly evolving, as evidenced by the recent approvals of enfortumab vedotin as monotherapy for patients with la/mUC who have previously received PB-CT and a PD-1 or PD-L1 inhibitor, and nivolumab as adjuvant monotherapy in patients with MIUC with tumor cell PD-L1 expression ≥1%, who are at high risk of recurrence after undergoing radical resection of MIUC [18,24]. Therefore, further research is warranted to examine the ongoing evolution of treatment and should consider the use of the most effective sequencing on survival outcomes in Germany.
Strengths & limitations
The main strength of this claims data study is the analysis of an extensive dataset provided by AOK PLUS and GWQ databases covering all relevant inpatient and outpatient care. Due to the nature of our study, our results were unlikely to be affected by any selection bias. Moreover, our study design is less susceptible to missing data since German claims databases typically include information on all filled prescriptions and refills at the patient level, irrespective of the prescribing clinician. Nevertheless, we acknowledge some limitations of our analysis.
As no data on the classification of malignant tumors are provided in German claims data, a proxy provided by clinicians treating patients with mUC in Germany was used for the identification of patients with mUC. Because this study is the first of its kind, we acknowledge that this algorithm has not been formally validated and that no previous studies using German claims data were identified. Moreover, the specific codes used to identify patients with mUC (i.e., OPS codes) may reflect German-specific clinical practices only and may limit the findings' generalizability to other countries.
A gap definition of 90 days was used to identify the discontinuation of observed agents and treatment lines. The results were similar to those observed in the sensitivity analysis, using a 60-day gap definition. However, as no information on the reason for the treatment gap was available, we acknowledge that the observed gap could be due to a planned end of treatment rather than discontinuation. In this context, we would also like to point out that the reason why a patient continues with the same treatment after 1L could be due to the gap definition in this study and does not necessarily represent an actual change of treatment line. This is particularly relevant for patients with PB-CT. However, the treatment gap definition developed together with clinicians can be considered a robust approach.
The current study compared unadjusted OS figures descriptively between the treated and untreated cohorts. This comparison may be potentially biased due to the time that treated patients had to have survived to receive treatment. However, it was found that the best way to compare OS between cohorts was to start from the same BL. In addition, the average time to treatment initiation was less than 2 months in treated patients, indicating only a slight potential bias. It was not possible to assess the change in OS over time with the availability of IO treatments (e.g., analysis of OS by year of diagnosis) because of inherent bias caused by shorter follow-up in patients who were treated more recently, which results in a lower probability of death. Detailed analyses of patients who received IO treatment was not possible because of the small size of this cohort.
For uniform OPS codes for different CT, a treatment assignment decision had to be made based on the description of their use in the OPS catalog and the experience of the clinicians involved in this study. Thus, there is some uncertainty regarding the group classification, as it was not possible to observe which treatments were administered when generic OPS codes were coded. In addition, as German claims databases contain data from routine practice, they may have missing data and coding errors in relation to outpatient diagnoses. Nevertheless, the coding of the databases is generally considered to be of high quality [41,42].
Since AOK PLUS and GWQ databases could not be linked within the context of this study, they were analyzed individually, and results were presented aggregately using a meta-analysis approach. However, we acknowledge that reporting some aggregated figures was not feasible for all outcomes (e.g., median OS).
Conclusion
Our findings suggest that a substantial proportion of patients with mUC in Germany, a high-income country with a high clinician density, remained undertreated, even despite this fairly recent analysis. Generally, low median OS was observed, even in patients who received 1L PB-CT, which was associated with the best survival outcomes in the era before avelumab 1L maintenance; this is generally consistent with the high MR observed in this population globally. However, it appears that patients who initiate systemic treatment have numerically higher OS, and a positive trend in treatment rates was observed over time. This improvement may be related to the introduction of IO agents for the treatment of patients previously considered ineligible for PB-CT, as well as greater awareness of other effective treatments, and will hopefully lead to more eligible patients being included in the treatment pool and a broader implementation of the current treatment guidelines in patients with mUC.
Supplementary Material
Acknowledgments
We would like to acknowledge that some of the results presented in this manuscript have been previously presented in poster format at the ASCO-GU congress on February 16–18, 2023 [43,44]. We would like to thank Prof. Günter Niegisch for presenting this work and for the valuable feedback received from attendees.
Funding Statement
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945) and was previously conducted under an alliance between Merck and Pfizer. G Niegisch has conducted symposia for Roche, MEDAC, Pfizer, Bristol Myers Squibb, and AstraZeneca; was part of advisory boards for the following companies: Roche, Sanofi, Bristol Myers Squibb, Merck, Pfizer, MEDAC, and Janssen; and received reimbursement for travel costs and congress registrations from Roche, Pfizer, Merck, and Bristol Myers Squibb.
Author contributions
G Niegisch and M-O Grimm: methodology; validation; writing – review & editing; F Hardtstock: conceptualization; methodology; investigation; formal analysis; writing – review & editing; J Krieger: conceptualization; data curation; investigation; writing – original draft; writing – review & editing; resources; A Starry: data curation; investigation; writing – review & editing; S Guenther, U Osowski, and T Wilke: conceptualization; writing – review & editing; B Deiters and U Maywald: data curation; writing – review & editing; M Kearney: conceptualization; writing – review & editing.
Financial & competing interests disclosure
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945) and was previously conducted under an alliance between Merck and Pfizer. G Niegisch has conducted symposia for Roche, MEDAC, Pfizer, Bristol Myers Squibb, and AstraZeneca; was part of advisory boards for the following companies: Roche, Sanofi, Bristol Myers Squibb, Merck, Pfizer, MEDAC, and Janssen; and received reimbursement for travel costs and congress registrations from Roche, Pfizer, Merck, and Bristol Myers Squibb. M-O Grimm reports providing a consulting or advisory role for AstraZeneca, Bristol Myers Squibb, Ipsen, MSD, ONO, Pfizer, Astellas, and EUSA; has received reimbursement for travel and accommodations expenses from Bristol Myers Squibb and Merck; has received honoraria from Astellas, AstraZeneca, Bristol Myers Squibb, MEDAC, MSD, ONO, Novartis, Pfizer, Ipsen, Merck, and EUSA; and has received research funding from Bristol Myers Squibb (Inst) and Intuitive Surgical (Inst). F Hardtstock, J Krieger, and A Starry participated in this study as staff members of Cytel; the work of Cytel in this study was financed by Merck. U Osowski is employed by Merck Healthcare Germany GmbH, Weiterstadt, Germany, an affiliate of Merck KGaA. M Kearney is employed by Merck Healthcare KGaA, Darmstadt, Germany, and holds stocks/shares in Merck, Novartis, and UCB Biopharma SPRL. S Guenther was employed by Merck Healthcare KGaA, Darmstadt, Germany, at time of study. B Deiters has nothing to declare. U Maywald has nothing to declare. T Wilke is the managing director of IPAM e.V. and has received honoraria from various companies, including Novo Nordisk, Janssen Pharmaceuticals, Boehringer Ingelheim Pharma, Bayer Health Care, Novartis, Sanofi, Pfizer, GSK, and Cytel. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Editing support was provided by Sophie Saunders from Nucleus Global and was funded by Merck (CrossRef Funder ID: 10.13039/100009945).
Ethical conduct of research
This study was non-interventional, had a retrospective design, and used only anonymized, non-identifiable data from two health insurance claims datasets (provided by AOK PLUS and GWQ ServicePlus under formal agreement). In accordance with German law (§75, Tenth Book of the Social Code [SGB X]) and policies of the institutions conducting the analysis, no informed consent from patients or ethical approval from an institutional review board was required.
Because of the non-interventional, retrospective nature of this study and because our analysis involved an anonymized dataset, no ethical review was required. This complies with national guidelines and recommendations [43,44]. Because of the anonymized and retrospective nature of the data, informed consent was not obtained from the patients.
Data sharing statement
Data supporting this study's findings are not publicly available due to privacy and ethical restrictions related to the use of patient-level data. Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck's (CrossRef Funder ID: 10.13039/100009945) Data Sharing Policy. All requests should be submitted in writing to Merck's data-sharing portal (www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Witjes JA, Bruins HM, Carrión Aet al. . EAU guidelines on muscle-invasive and metastatic bladder cancer. 2022. Available from: https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer ; •• A comprehensive grasp of the evidence-based management and clinical recommendations for muscle-invasive and metastatic bladder cancer hinges on a thorough examination of the guidelines outlined by the European Association of Urology (EAU).
- 2.Wild CP, Weiderpass E, Stewart BWet al. . World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France: International Agency for Research on Cancer; 2020. Available from: www.iarc.who.int/cards_page/world-cancer-report/ [Google Scholar]
- 3.World Health Organization . Bladder fact sheet. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf
- 4.Robert Koch-Institut . Cancer in Germany 2019/2020. Berlin, Germany; 2021. Available from: www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_inhalt.html [Google Scholar]
- 5.National Cancer Institute . Cancer Stat Facts: Bladder Cancer. Bethesda, MD; 2022. Available from: https://seer.cancer.gov/statfacts/html/urinb.html [Google Scholar]
- 6.de Wit M, Bauernhofer T, Bokemeyer Cet al. . Leitlinie Blasenkarzinom (Urothelkarzinom). Berlin: DGHO Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e.V.; 2017. Available from: https://repository.publisso.de/resource/frl:6419224/data [Google Scholar]
- 7.Fisher MD, Shenolikar R, Miller PJet al. . Treatment patterns and outcomes in stage IV bladder cancer in a community oncology setting: 2008–2015. Clin. Genitourin. Cancer 2018;16(6):e1171–e1179. [DOI] [PubMed] [Google Scholar]
- 8.Aly A, Johnson C, Yang Set al. . Overall survival, costs, and healthcare resource use by line of therapy in Medicare patients with newly diagnosed metastatic urothelial carcinoma. J. Med. Econ. 2019;22(7):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheeseman S, Thompson M, Sopwith Wet al. . Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front. Oncol. 2020;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niegisch G, Gerullis H, Lin SWet al. . A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J. Cancer 2018;9(8):1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Niegisch et al.‘s study was among the pioneering research efforts in Germany to investigate treatment patterns, including first-line and second-line treatments, as well as overall survival among patients with locally advanced or metastatic urothelial carcinoma (la/mUC). The study relied on electronic medical record data to assess treatment approaches involving platinum and non–platinum-based chemotherapy and offers valuable insights for tracking the evolution of la/mUC treatment strategies and their associated outcomes over time.
- 11.ClinicalTrials.gov . Study of pembrolizumab with or without platinum-based combination chemotherapy versus chemotherapy alone in urothelial carcinoma (MK-3475-361/KEYNOTE-361). Bethesda, MD: National Library of Medicine, US; 2021. [updated 2022 Oct 4]. Available from: https://clinicaltrials.gov/ct2/show/NCT02853305 [Google Scholar]
- 12.ClinicalTrials.gov . Study of atezolizumab as monotherapy and in combination with platinum-based chemotherapy in participants with untreated locally advanced or metastatic urothelial carcinoma (IMvigor130). Bethesda, MD: National Library of Medicine, US; 2016. [updated 2022 Oct 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT02807636 [Google Scholar]
- 13.Powles T, Bellmunt J, Comperat Eet al. . Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021;33(3):244–258. [DOI] [PubMed] [Google Scholar]; •• ESMO Clinical Practice Guidelines are a valuable resource offering crucial recommendations for the diagnosis, staging and management of bladder cancer. These guidelines serve as an essential reference, providing evidence-based insights into the current standards of care and contributing to the comprehensive understanding of mUC management.
- 14.Institute for Quality and Efficiency in Health Care (IQWiG) . Atezolizumab (Urothelkarzinom nach Chemotherapie) - Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2017. Available from: www.iqwig.de/download/a17-52_atezolizumab_nutzenbewertung-35a-sgb-v_v1-0.pdf [Google Scholar]
- 15.Institute for Quality and Efficiency in Health Care (IQWiG) . Pembrolizumab (Urothelkarzinom Erstlinientherapie) - Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2017. Available from: www.iqwig.de/download/a21-34_pembrolizumab_nutzenbewertung-35a-sgb-v_v1-0.pdf [Google Scholar]
- 16.Institute for Quality and Efficiency in Health Care (IQWiG) . Atezolizumab (Urothelkarzinom Erstlinientherapie) – Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2017. Available from: www.iqwig.de/download/a17-51_atezolizumab_nutzenbewertung-35a-sgb-v_v1-0.pdf [Google Scholar]
- 17.Institute for Quality and Efficiency in Health Care (IQWiG) . Nivolumab (Urothelkarzinom) – Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2017. Available from: www.iqwig.de/download/a17-29_nivolumab_nutzenbewertung-35a-sgb-v_v1-0.pdf?rev=117386 [Google Scholar]
- 18.Institute for Quality and Efficiency in Health Care (IQWiG) . Nivolumab (Urothelkarzinom, adjuvant) – Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2022. Available from: www.iqwig.de/download/a22-53_nivolumab_nutzenbewertung-35a-sgb-v_v1-0.pdf [Google Scholar]
- 19.Cathomas R, Lorch A, Bruins HMet al. . The 2021 Updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur. Urol. 2021;81(1):95–103. [DOI] [PubMed] [Google Scholar]; •• This overview presents the latest updates to date from the EAU guidelines for mUC and provides a comprehensive summary of recently approved treatment options. This resource is a vital reference for clinicians, researchers and healthcare professionals seeking to stay current with the evolving landscape of care for patients with mUC.
- 20.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) . S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms, Langversion 2.0. 2020. Available from: www.leitlinienprogramm-onkologie.de/leitlinien/harnblasenkarzinom/
- 21.Institute for Quality and Efficiency in Health Care (IQWiG) . Avelumab (Urothelkarzinom) – Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2021. Available from: www.iqwig.de/download/a21-23_avelumab_nutzenbewertung-35a-sgb-v_v1-0.pdf [Google Scholar]
- 22.Powles T, Park SH, Voog Eet al. . Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020;383(13):1218–1230. [DOI] [PubMed] [Google Scholar]
- 23.Bavencio (avelumab) . Summary of product characteristics. Merck Europe B.V., Amsterdam, Netherlands, an affiliate of Merck KGaA; 2023. [Google Scholar]
- 24.Institute for Quality and Efficiency in Health Care (IQWiG) . Enfortumab Vedotin (Urothelkarzinom) – Nutzenbewertung gemäß § 35a SGB V. Cologne, Germany; 2022. Available from: www.iqwig.de/download/a22-61_enfortumab-vedotin_nutzenbewertung-35a-sgb-v_v1-0.pdf [Google Scholar]
- 25.Kearney M, Zhang L, Hubscher Eet al. . Undertreatment in patients with advanced urothelial cancer: systematic literature review and meta-analysis. Future Oncol. 2023:Epub Aug 1. [DOI] [PubMed] [Google Scholar]; •• This Cochrane systematic review is based on clinical guidelines and focuses on real-world evidence in patients with la/mUC who have not received treatment. It offers valuable up-to-date insights and serves as a robust foundation for comparing the previously observed rates of undertreatment in this specific patient population.
- 26.Dinan MA, Georgieva MV, Li Yet al. . Real-world systemic therapy utilization in Medicare patients with locally advanced or metastatic urothelial carcinoma diagnosed between 2008 and 2012. J. Geriatr. Oncol. 2021;12(2):298–304. [DOI] [PubMed] [Google Scholar]
- 27.Ward MM, Ulrich F, Matthews Ket al. . Who does not receive treatment for cancer? J. Oncol. Pract. 2013;9(1):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Castilla B, Aloe AM, Declercq Let al. . Concealed correlations meta-analysis: a new method for synthesizing standardized regression coefficients. Behav. Res. Methods 2019;51(1):316–331. [DOI] [PubMed] [Google Scholar]
- 29.Harbord RM, Higgins JPT. Meta-regression in stata. Stata J. 2008;8(4):493–519. [Google Scholar]
- 30.Davies FJ, Knott C, Kerr Cet al. . Utilising Public Health England datasets to establish a standing cohort of patients with metastatic bladder cancer: initial results and algorithm defining disease progression. Value Health 2020;23:S480–S481. [Google Scholar]
- 31.Knott C, Kearney M, Mahmoudpour Het al. . Factors associated with the receipt of systemic treatment (tx) for metastatic urothelial carcinoma (mUC) in England. Ann. Oncol. 2022;33:S1338. [DOI] [PubMed] [Google Scholar]; • This study provides a significant contribution to the field of mUC using the National Cancer Registration Dataset in the UK. Its data offers a unique perspective on patient undertreatment rates within a European setting and plays a crucial role in pinpointing the underlying reasons for undertreatment in this context.
- 32.Tariman JD, Doorenbos A, Schepp KGet al. . Patient, physician and contextual factors are influential in the treatment decision making of older adults newly diagnosed with symptomatic myeloma. Cancer Treat. Commun. 2014;2(2–3):34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon WA, Seo HK. Optimizing frontline therapy in advanced urothelial cancer. Transl. Androl. Urol. 2020;9(3):983–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geynisman DM, Broughton E, Hao Yet al. . Real-world treatment patterns and clinical outcomes among patients with advanced urothelial carcinoma in the United States. Urol. Oncol. 2022;40(5):195.e1–195.e11. [DOI] [PubMed] [Google Scholar]
- 35.Maraz AC, Nagy B, Macher Tet al. . Nationwide study of real-world treatment patterns and clinical outcomes in patients with metastatic urothelial carcinoma in Hungary. Adv Ther. 2023;40:(12) 5475–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) . S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms, Langversion 1.1. Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF; 2016. Available from: http://leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Blasenkarzinom/LL_Harnblasenkarzinom_Langversion_1.1.pdf [Google Scholar]
- 37.Milowsky MI, Rumble RB, Booth CMet al. . Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016;34(16):1945–1952. [DOI] [PubMed] [Google Scholar]
- 38.Jensen JB, Hauberg D, Hjortsø MDet al. . Treatment pattern and overall survival among patients with locally advanced or metastatic urothelial carcinoma – Results from a complete nationwide unselected real-world registry study in Denmark from 2010 to 2017. Ann. Oncol. 2021;32:S716. [Google Scholar]
- 39.Jodon G, Fischer SM, Kessler ER. Treatment of urothelial cancer in elderly patients: focus on immune checkpoint inhibitors. Drugs Aging 2018;35(5):409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiyama N, Kobayashi T, Narita Set al. . Efficacy and safety of pembrolizumab for older patients with chemoresistant urothelial carcinoma assessed using propensity score matching. J. Geriatr. Oncol. 2022;13(1):88–93. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann J, Weidmann C, Biehle R. [Validation of SHI claims data exemplified by gender-specific diagnoses]. Gesundheitswesen. 2016;78(10):e53–e58. [DOI] [PubMed] [Google Scholar]
- 42.Langner I, Ohlmeier C, Zeeb Het al. . Individual mortality information in the German Pharmacoepidemiological Research Database (GePaRD): a validation study using a record linkage with a large cancer registry. BMJ Open 2019;9(7):e028223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niegisch G, Grimm M-O, Hardtstock Fet al. . Treatment patterns, indicators of receiving systemic treatment, and clinical outcomes in metastatic urothelial carcinoma: a retrospective analysis of real-world data in Germany. J. Clin. Oncol. 2023;41(Suppl. 6):Abstract 464. [Google Scholar]
- 44.Niegisch G, Kearney M, Krieger Jet al. . Factors associated with not receiving systemic treatment (tx) in patients (pts) with metastatic urothelial carcinoma (mUC): results of a retrospective observational study in Germany. Ann. Oncol. 2023;34(Suppl. 2):S1215 (Abstract 2386P). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


