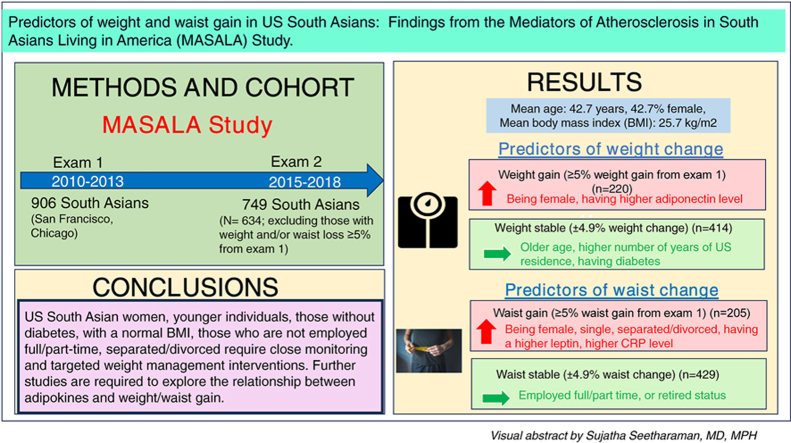
Abstract
Background
Weight and waist gain are significant concerns in adulthood. Both weight and waist gain are particularly important among South Asians, known to have an increased risk of developing chronic cardiometabolic complications at any body mass index compared to other racial and ethnic groups. The aim of this study was to investigate factors predicting weight and waist gain in a longitudinal cohort of South Asians living in the US (United States).
Methods
This was a prospective analysis using data from exam 1 (2010–2013) and exam 2 (2015–2018) of the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study, a prospective cohort study of South Asians (recruited from San Francisco and Chicago), with a mean 4.8 years of follow-up.
Results
Of 634 participants studied (42.7 % women, mean age 55 years, BMI 25.7 kg/m2, weight 70.4 kg at exam 1), 34.7 % had gained ≥5 % weight and 32.3 % gained ≥5 % waist at exam 2. In the adjusted models, older age, higher number of years of US residence, and having diabetes were associated with lower odds of weight gain; being female and having higher adiponectin were associated with higher odds of weight gain. Being female and being employed full/part time or being retired predicted lower likelihood of waist gain. Being single, separated/divorced, having a higher leptin and a higher C-reactive protein level predicted higher likelihood of waist gain.
Conclusions
The current study identified several social, demographic, and clinical factors that can serve as targets for obesity interventions among US South Asians. In addition, this study also raises hypotheses about associations of adipokine levels with weight and waist gain.
Keywords: Adiponectin, Leptin, South asians, Weight gain, Waist gain
Graphical abstract
1. Introduction
Weight gain is a significant health concern during adulthood. Research has shown that 98 % of men and 92 % of women experience an upward trajectory with an estimated weight gain of 0.53 kg (1.17 lbs.) per year [1]. Adults who gain the most weight have the highest risk for cardiovascular and other obesity-related conditions [1]. Not only weight, but the risk for central adiposity as measured by waist circumference (WC), also increases during adulthood, and increases the risk of obesity-related morbidity and mortality [2,3]. Both weight and waist gain are particularly important among South Asians, known to have an increased risk of developing chronic cardiometabolic complications at any BMI compared to other racial and ethnic groups [4,5]. Understanding factors that predict weight and waist gain are important for prevention efforts in this high-risk population.
Past studies have found that determinants of weight and waist change during adulthood are multifactorial and can be influenced by sociodemographic factors [[6], [7], [8]], dietary factors [[9], [10], [11], [12], [13]], behaviors such as alcohol use and smoking [12,13], physical activity levels [7,11], metabolic conditions such as diabetes [6,14], and psychological factors [15]. Similar studies examining weight and waist change over time are limited among South Asian Americans, an underrepresented group in research studies.
Work from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study [16] has demonstrated that South Asians have a high-risk phenotype for developing diabetes and hypertension at a lower BMI [[17], [18], [19], [20]] compared to non-Hispanic White, Black, Hispanic, and Chinese American populations. The body composition in South Asians is sometimes termed the “thin fat” phenotype, describing a lower BMI and low lean body mass, with higher amount of visceral fat and adipose tissue in ectopic sites such as the liver and skeletal muscle [21]. Therefore, measurement of waist circumference more so than measurement of weight is very important in this population. In addition to a less favorable body composition, a cross sectional study using the MASALA and Multi Ethnic Study of Atherosclerosis (MESA) data showed that compared to other racial and ethnic groups, US South Asians had lower adiponectin and higher resistin levels, both of which have been implicated in increasing risk for insulin resistance and obesity [18]. Less is known about whether body composition, adipokine levels, and metabolic factors influence weight and waist gain in US South Asians.
Addressing paucity of literature, we aimed to prospectively investigate factors associated with weight and waist gain change over time, in a community-based cohort of South Asian Americans, to help elucidate potential subgroups at higher risk for targeted prevention strategies.
2. Research design and methods
2.1. Study design
This was a prospective cohort study design using data from exam 1 (2010–2013) and exam 2 (2015–2018) of the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study, a prospective cohort study of South Asians recruited from the San Francisco Bay Area at the University of California, San Francisco (UCSF), and the greater Chicago area at Northwestern University (NU). Details of the MASALA study design, recruitment, and sampling methods have been previously published [16]. A total of 906 subjects were recruited between October 2010 and March 2013 and 749 (83 %) participants returned to complete exam 2 with a mean 4.8 years of follow-up [16].
The institutional review boards at both the University of California, San Francisco and Northwestern University approved the study protocol and informed consent was obtained from all participants at each exam. The current analysis was approved by the Institutional review board at the University of California, San Francisco.
2.2. Study participants
Eligibility criteria included participants self-identifying with a South Asian background (three out of four grandparents born in one of the following countries: India, Pakistan, Bangladesh, Nepal, or Sri Lanka, ages between 40 and 84 years, ability to speak and read English, Hindi, or Urdu, and have no known cardiovascular disease [16]. Exclusion criteria included individuals who reported nitroglycerin medication use; had active cancer; shortened life expectancy< 5 years; impaired cognitive ability; plans to move out of the geographic vicinity of the study site in the next 5 years; living in a nursing home; or weighed >300 lbs. due to computed tomography scanner weight limits [16]. Similar procedures for the physical examination and laboratory measures were conducted at baseline and follow-up [16].
Our analytic sample consisted of participants who had complete data on weight, height, and waist circumferences at both exams.
2.3. Study measurements
2.3.1. Outcome variables
Anthropometry. Participant's weight (kg) was measured using a digital weighing scale, and height (cm) was recorded using a stadiometer. BMI (kg/m2) was calculated based on participants' height and weight [16]. A continuous BMI measure and a categorical BMI measure were created based on Asian obesity categories by the World Health Organization: Overweight (BMI 23 to <27.5 kg/m2) and obesity (BMI 27.5 kg/m2 or more) [22]. Waist circumference (WC, cm) was measured using a flexible measuring tape at the level of the umbilicus level of the umbilicus or at the level between the lower ribs and anterior superior iliac spine for those with larger abdominal girth [16]. A categorical WC measure was created to classify individuals who had abdominal obesity based on Asian category (men ≥90 cm and women ≥80 cm) [23]. Percentage weight and waist circumference change between exams 2 and 1 were calculated. Participants were categorized as having weight or waist gain (≥5 % weight or waist gain from exam 1) or being weight or waist stable (±4.9 % weight or waist change from exam 1). Among 748 participants, we excluded 34 (4.5 %) with weight loss ≥5 % and 51 (6.8 %) with waist loss ≥5 % and 29 (3.8 %) with both weight and waist loss weight loss ≥5 % from exam 1, for this analysis, leaving a final analytic sample of 634 participants.
2.3.2. Predictor variables
We included self-reported social-demographic information, behavioral and psychological characteristics, and clinical measures that were collected from the participants during their baseline exam by bilingual trained staff [16].
Social-demographic variables. Participant's age in years, sex (male or female), place of birth (US or outside of US), number of years of US residence for non-US born participants, marital status (single, married, separated or divorced, or widowed), occupational status (not working, working part or full-time, unemployed, or retired), education level (less than, equal to, or greater than a Bachelor's degree), and health insurance status (yes or no).
Behavioral variables. Tobacco use was assessed by asking participants their smoking status (never, former, or current smoker). Alcohol use was assessed by asking about alcohol drink consumption per week. Sedentary behavior was assessed by asking participants the number of minutes per week they watch television. Physical activity was assessed by asking participants the number of minutes of moderate exercise they did during a week using the Typical Weeks Activity Survey [24]. Dietary intake over the previous year was assessed using the Study of Health Assessment and Risk in Ethnic Groups (SHARE) food frequency questionnaire, which has been validated among South Asians in Canada [25]. Fasting behaviors were assessed by asking the participants if they fasted once or more per week, once or more per month or once a year or never. Frequency of eating out was assessed by asking if they ate out 2–3 times per week or once or less than once per week.
Psychological factors. Several psychological factors were analyzed that were assessed using Spielberger trait anxiety scale, the Center for Epidemiologic Studies Depression Scale (CES-D), Spielberger anger scale, and chronic psychological burden [[26], [27], [28]].
Body Composition. Abdominal computed tomography (CT) scans (Philips Medical Systems, Andover, MA; Toshiba Medical Systems, Tustin, CA; Siemens Medical Solution, Malvern, PA) were used to determine abdominal visceral fat area and abdominal intermuscular fat area [16]. Visceral and subcutaneous abdominal fat were measured at the L4–L5 level using the Medical Image Processing, Analysis, and Visualization (MIPAV) software at the University of California, San Diego body composition reading center [29]. Visceral fat was defined as those pixels within the appropriate Hounsfield Unit (HU) range and within the contour of the visceral cavity. The four abdominal/back muscle groups from which abdominal intermuscular fat was measured included the psoas, paraspinous, oblique, and rectus muscles. These muscles were highlighted by the readers and then deleted from the calculation of the subcutaneous fat area.
Fatty Liver disease. CT images for liver fat attenuation (higher attenuation implying lower fat in the liver) were interrogated using the MIPAV software at the vertebral level of T12–L1. Fatty liver was defined at hepatic attenuation ≤40 HUs.
Adipokines/inflammatory markers. A panel of blood tests were undertaken to assess the adipokine and inflammatory marker profile of participants that included measurement of leptin, adiponectin, resistin, and high sensitivity C-reactive protein (CRP) levels after a 12-h fast. Adiponectin and resistin levels were measured using the Millipore Luminex adipokine panel A (EMD Millipore, Billerica, MA). The intraassay coefficient of variations (CV) was 2.34 %–4.12 % for adiponectin and 3.25 %–5.03 % for resistin. Serum leptin levels were measured in duplicate (RIA for total Leptin and ELISA for high molecular weight Leptin; Linco, St Charles, MO, USA) and the intra-assay coefficient of variation was 6.0 %.
2.3.3. Metabolic profile
Glucose metabolism. A series of tests were conducted to measure dysglycemia in participants including a HbA1c. Among those who were not on any diabetes medicines, an oral glucose tolerance test was conducted to measure glucose and insulin levels. This included a fasting glucose test (measured using the hexokinase method) to determine glucose levels at baseline, followed by a glucose test at 2 h after ingestion of a 75-g glucose challenge. Participant's glycemic status was defined according to American Diabetes Association criteria. Normal glycemia was defined as having a FPG <100 mg/dL and 2-h glucose <140 mg/dL. Prediabetes was defined as having a fasting plasma glucose between 100 and 125 mg/dL or 2-h glucose of 140–199 mg/dL. Type 2 diabetes was defined as use of diabetes medications, or fasting plasma glucose ≥126 mg/dL, and/or a 2-h post challenge glucose ≥200 mg/dL.
Fasting serum samples were batched for insulin, measured by the sandwich immunoassay method (Roche Elecsys 2010; Roche Diagnostics, Indianapolis, IN). The homeostasis model assessment (HOMA)-IR was used to measure IR and calculated as [Insulin0 (mIU/mL) x Glucose0 (mmol/L)/22.5]. The homeostasis model assessment of Beta cell function (HOMA-b) which was used calculated as [20 x Insulin0 (mIU/mL)/Glucose0 (mmol/L)-3.5].
Lipid panel. A fasting lipid profile was obtained that included total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol was calculated.” Blood pressure. Seated resting blood pressure was measured three times using an automated blood pressure monitor (V100 Vital Signs Monitor; GE Healthcare, Fairfield, CT) and the average of the last two readings were used for analysis [16]. Hypertension was defined as a systolic blood pressure of 140 mm Hg or greater and/or diastolic blood pressure of 90 mm Hg or greater or use of antihypertensive medication.
3. Statistical analysis
We used data from exam 1 (2010–2013) and exam 2 (2015–2018) of the MASALA study. Dr. Alka Kanaya, MD coordinated data management and data quality assurance, Dr. Sujatha Seetharaman performed the statistical analysis and Dr. Isabel Elaine Allen, PhD, supervised the statistical analysis. Initially, we generated univariate summaries with frequencies and percentages for categorical variables and means, median, standard deviations, minimum, and maximum for continuous variables. Bivariate relationships were examined between all potential exposure variables with participants' weight and waist circumference change. Chi-squared statistics were calculated between categorical variables, Analysis of Variance and Student's t-tests were used to compare continuous variables, and correlations were calculated between continuous variables. Stepwise multivariable logistic regression methods were used to examine the association of weight gain using metabolic, body composition, psychological factors, behaviors, and sociodemographic factors from exam 1, after adjusting for age, sex, baseline BMI, and years lived in the US. Similarly, we performed stepwise linear regression methods using to examine the prospective association of waist circumference change in exam 2 using exposures from exam 1, after adjusting for age, sex, marital and occupational status. We also explored pertinent interaction effects in the adjusted models for whether sex, baseline BMI status, and glucose tolerance status modified the effect of any of the significant risk factors for weight and waist gain. Analysis was performed using Stata software, version 17 (Stata Corp. 2021, College Station, TX).
4. Results
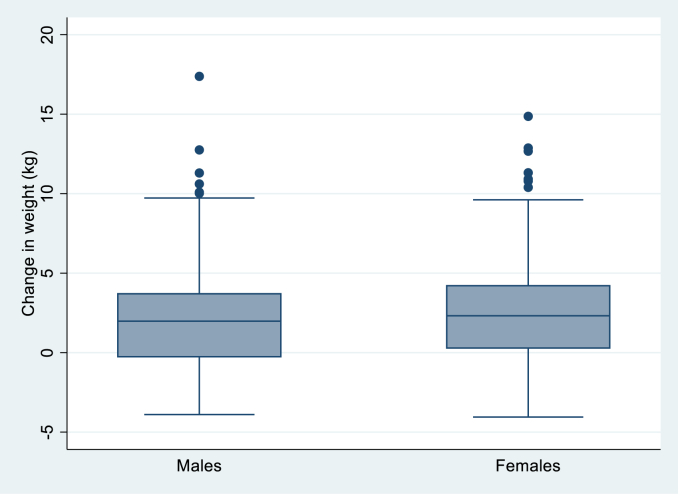
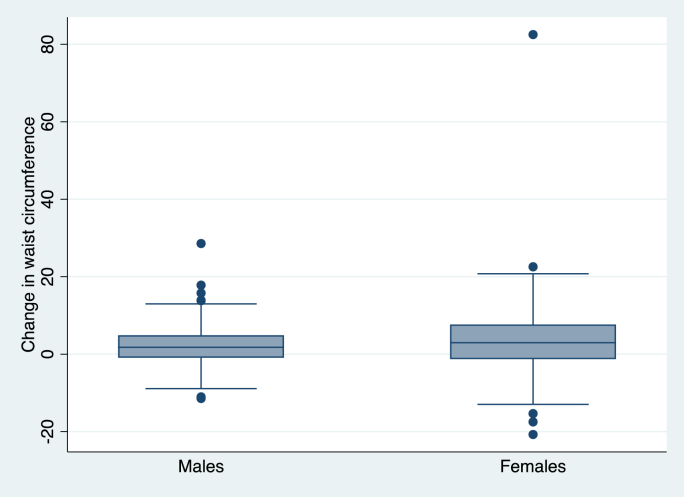
Among the 634 participants studied (42.7 % women, mean age 55 years, BMI 25.7 kg/m2, weight 70.4 kg at exam 1), 414 (65.3 %) were weight stable (±4.9 % weight change from exam 1) and 220 (34.7 %) had weight gain (≥5 % weight gain from exam 1); 429 (67.7 %) participants were waist stable (±4.9 % waist change from exam 1) and 205 (32.3 %) participants had waist gain (≥5 % waist gain from exam 1) (Table 1). Females had slightly higher weight and wasit gain in exam 2 compared to males (Fig. 1, Fig. 2)
Table 1.
MASALA participant characteristics by weight and waist change groups, 2010–2013 (N = 634).
| Anthropometry | Weight Stable ±4.9 % weight change∗ n = 414 (65.3 %) | Weight gain ≥5 % weight gain∗ n = 220 (34.7 %) | Waist Stable ±4.9 % waist change∗ n = 429 (67.7 %) | Waist gain ≥5 % waist gain∗ n = 205 (32.3 %) |
|---|---|---|---|---|
| BMI (by Asian Category) at exam 1 | ||||
| Normal BMI (BMI< 23) | 84 (20.3 %) | 72 (32.7 %) | 103 (24.0 %) | 53 (24.6 %) |
| Overweight (BMI 23-<27.5) | 202 (48.8 %) | 100 (45.5 %) | 210 (48.9 %) | 92 (44.9 %) |
| With obesity (BMI 27.5 or more) | 128 (30.9 %) | 48 (21.8 %) | 116 (27.0 %) | 60 (29.3 %) |
| Weight at exam 2 (kg) | 71.9 (64.2–79.2) | 72.3 (63.3–88.8) | ||
| Waist circumferance (cm) at exam 2 | 95 (89-0.5- 101.9) | 94.3 (88.4–103.4) | ||
| BMI (kg/m2) at exam 2 | 25.9 (23.8–28.6) | 26.5 (24.2–29.6) | ||
| Absolute Weight change (kg) | 0.7 (1.9) | 5.6 (2.3) | 1.7 (2.7) | 3.9 (3.5) |
| Absolute Waist circumferance change (cm) | 2.2 (6.0) | 5.9 (5.2) | 0.6 (2.6) | 9.6 (6.5) |
| Absolute BMI change (kg/m2) | 0.4 (1.1) | 2.2 (1.1) | 0.7 (1.2) | 1.6 (1.6) |
| Predictor variables | ||||
|
Sociodemographic characteristics Age at exam 2 (years) |
60.4 (9.3) | 57.5 (9.2) | 59.2 (9.3) | 59.7 (9.5) |
| Female | 150 (36.2 %) | 121 (55 %) | 157 (36.6 %) | 114 (55.6 %) |
| Place of birth | ||||
| India | 340 (82.1 %) | 191 (86.8 %) | 358 (83.5 %) | 173 (84.4 %) |
| Other South Asian country | 64 (15.5 %) | 22 (10.0 %) | 58 (13.5 %) | 28 (13.7 %) |
| Other diaspora country | 10 (2.4 %) | 7 (3.2 %) | 13 (3.9 %) | 4 (1.9 %) |
| Years lived in the US | 28.3 (11.0) | 25.1 (10.8) | 27.4 (10.9) | 26.8 (11.3) |
| Education | ||||
| ≤ High school | 25 (6.0 %) | 11 (4.8 %) | 24 (5.6 %) | 11 (5.4 %) |
| < bachelor's degree | 20 (4.8 %) | 8 (3.5 %) | 19 (4.4 %) | 8 (3.9 %) |
| ≥bachelor's degree | 369 (89.1 %) | 406 (91.7 %) | 386 (89.9 %) | 186 (90.7 %) |
| Marital status | ||||
| Married/living as married/with partner | 381 (92.0 %) | 20 (92.3 %) | 400 (93.2 %) | 184 (89.8 %) |
| Separated/divorced | 9 (2.2 %) | 8 (3.7 %) | 8 (1.9 %) | 9 (4.4 %) |
| Widowed | 17 (4.1 %) | 8 (3.7 %) | 15 (3.5 %) | 10 (4.9 %) |
| Single | 7 (1.7 %) | 1 (0.5 %) | 6 (1.4 %) | 2 (0.9 %) |
|
Occupation Not working/unemployed |
56 (13.5 %) | 39 (17.7 %) | 48 (11.2 %) | 47 (22.9 %) |
| Employed full-time/part-time | 290 (70.0 %) | 161 (73.2 %) | 320 (74.6 %) | 131 (63.9 %) |
| Retired | 68 (16.4 %) | 20 (9.1 %) | 61 (14.2 %) | 27 (13.2 %) |
| Health Insurance | 384 (92.8 %) | 209 (95.4 %) | 401 (93.5 %) | 192 (94.1 %) |
| Behavioral characteristics Cigarette smoking status | ||||
| Never/Former smoker | 399 (96.4 %) | 214 (97.3 %) | 412 (96.0 %) | 201 (98 %) |
| Current smoker | 15 (3.6 %) | 6 (2.7 %) | 17 (3.7 %) | 4 (1.9 %) |
| 1+ alcoholic drinks/week | 164 (39.6 %) | 60 (27.3 %) | 163 (38.0 %) | 61 (29.8 %) |
| TV watching, minutes/week | 420 (210–840) | 420 (210–840) | 420 (210–840) | 420 (210–840) |
| Moderate physical activity (MET-min/week) | 2906.3 (1635–4920) | 3097.5 (1518.8–5302.5) | 2992.5 (1620–5100) | 2940 (1530–4702.5) |
| Vegetarian diet | 142 (34.8 %) | 90 (41.2 %) | 152 (36.0 %) | 80 (39.4 %) |
| Fasting | ||||
| About once or more/week | 36 (8.7 %) | 31 (14.1 %) | 45 (10.5 %) | 22 (10.7 %) |
| About 1–2 times/month | 69 (16.7 %) | 27 (12.3 %) | 66 (15.4 %) | 30 9 (14.6 %) |
| Once a year/or almost never | 309 (74.6 %) | 162 (73.6 %) | 318 (74.1 %) | 153 (74.6 %) |
| Eating out 2 or 3 times a week | 44 (10.6 %) | 33 (15 %) | 50 (11.7 %) | 27 (13.2 %) |
| Once or < once a week | 370 (89.4 %) | 187 (85.0 %) | 379 (88.3 %) | 178 (86.8 %) |
| Psychological characteristics | ||||
| Spielberger anger score | 15.8 (3.7) | 16.1 (3.7) | 15.8 (3.8) | 16.2 (3.6) |
| CES-Depression score | 7.1 (6.4) | 7.8 (7.5) | 7.0 (6.6) | 8.0 (7.1) |
| Social support score | 25.1 (4.8) | 24.7 (4.9) | 25.1 (4.7) | 24.5 (5.1) |
| Emotional burden score | 0.8 (1.0) | 0.9 (1.1) | 0.8 (1.0) | 0.9 (1.1) |
| Spielberger anxiety score | 15.8 (4.3) | 15.9 (4.5) | 15.8 (4.4) | 16.0 (4.2) |
| Body composition measures | ||||
| Abdominal subcutaneous fat area (cm2) | 235.9 (95.4) | 224.7 (89.4) | 229.8 (90.0) | 236.7 (100.4) |
| Abdominal visceral fat area (cm2) | 141.7 (50.9) | 120.7 (57.2) | 134.8 (52.4) | 133.6 (57.4) |
| Liver attenuation (HU) | 54.1 (10.4) | 57.6 (10.2) | 54.8 (9.7) | 56.3 (11.8) |
| Intermuscular fat area (cm2) | 21.8 (8.9) | 19.8 (7.9) | 20.8 (8.7) | 21.9 (8.2) |
| Abdominal lean muscle mass area (cm2) | 97.0 (24.5) | 90.9 (23.8) | 97.0 (24.5) | 89.1 (23.7) |
| Adipokines/inflammatory markers | ||||
| Leptin (pg/ml) | 15,668 (13,117) | 15,056 (11,755) | 14,511 (11,374) | 17,463 (14,851) |
| Adiponectin level (ng/dL) | 10,848 (6429.4) | 13,276 (6774.1) | 11,170 (6774.0) | 12,798 (6242.2) |
| Leptin (pg/ml) | 15,668 (13,117) | 15,056 (11,755) | 14,511 (11,374) | 17,463 (14,851) |
| Resistin level (pg/dL) | 21,563 (10025.9) | 21,213 (8056.2) | 21,513 (9827.6) | 21,289 (8384.9) |
| CRP level (ug/mL) | 2.3 (4.1) | 2.4 (3.8) | 2.3 (3.9) | 2.6 (4.1) |
| Metabolic characteristics | ||||
| Prediabetes | 224 (54.1 %) | 131 (59.6 %) | 238 (55.5 %) | 117 (57.1 %) |
| Diabetes | 122 (29.5 %) | 25 (11.3 %) | 111 (25.9 %) | 36 (17.6 %) |
| Hypertension | 182 (43.9 %) | 60 (27.1 %) | 167 (39.9 %) | 73 (35.6 %) |
| Fatty Liver | 44 (10.7 %) | 12 (5.5 %) | 39 (9.2 %) | 17 (8.3 %) |
| Total cholesterol (mg/dL) | 186 (162–208.5) | 182.5 (163–209.5) | 187 (162–209) | 183 (162–209) |
| LDL (mg/dL) | 110 (89–133) | 109 (90–134) | 111 (90–135) | 107 (88–129) |
| HDL (mg/dL) | 47 (39–55) | 50.5 (41–59) | 47 (40–55) | 50 (40–59) |
| Triglycerides (mg/dL) | 125 (93–160) | 104.5 (78.5–144) | 120 (88–158) | 112 (88–150) |
| Insulin (pmol/L) | 61 (45–92.9) | 49.9 (34.5–75) | 59 (41.8–87.9) | 55.6 (85.8) |
| Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (pmol/L∗ mg/dL) | 2.6 (1.8–4.0) | 1.9 (1.3–3.1) | 2.4 (1.7–3.7) | 2.3 (1.5–3.7) |
| Homeostasis model assessment of Beta-cell function (HOMA-B) ([pmol/L]/[mg/dL]) | 108.4% (69.6–158.9) | 99.8 % (74.6–140.4) | 102.9 (70.2–148.1) | 107.4 (71.6–153.8) |
All values represent mean ± SD or n (%), or median (IQR) as appropriate.
∗Weight and waist change from exam 2 to exam 1.
Fig. 1.
Graph showing change in weight by sex between exam 1 and exam 2.
Fig. 2.
Graph showing change in waist by sex between exam 1 and exam 2.
Bivariate analysis associated with weight increase included having a higher adiponectin per SD (β 0.31; 95 % 0.06–0.56; p = 0.016). Factors associated with weight decrease included retired work status (β 1.22; 95 % CI -1.94 to −0.49; p = 0.001), having one alcoholic drink per week (β 0.52; 95 % CI -1.04 to −0.01; p = 0.046), having prediabetes (β 0.42; 95 % CI -1.03–0.20; p = 0.183), having diabetes (β 2.09; 95 % CI -2.81 to −1.37; p < 0.001), or having hypertension (β 0.83; 95 % CI -1.33–0.33; p = 0.001).
Bivariate analyses associated with waist gain included being separated or divorced (β 2.95; 95 % CI 0.10–5.8; p = 0.043) or being widowed (β 7.60; 95 % CI 3.47–11.73; p < 0.001); being unemployed (β 3.03; 95 % CI 1.53–4.53; p < 0.001), having a higher emotional burden (β 0.45; 95 % CI 0.004–0.91; p = 0.048) or having a higher CRP level (β 0.30; 95 % CI 0.18–0.41; p < 0.001). Factors associated with waist decrease included being a former smoker (β 1.72; 95 % CI -3.06 to −0.38; p = 0.012) or having one alcoholic drink per week (β 1.48; 96 % CI -2.45 to −0.51; p = 0.003).
Supplementary Description.
Supplementary Table 1 shows adipokine and CRP levels by sex, glycemic status, and Asian BMI category. We found that adiponectin, leptin and CRP levels were higher in females compared to males. However, we found that while adiponectin levels were higher among those with a normal BMI
and without diabetes, leptin and CRP levels were higher in individuals with overweight, obesity, with pre-diabetes and diabetes (Supplemental Table 1).
4.1. Predictors of weight gain
In the adjusted logistic regression models, higher number of years of US residence (OR 0.98; 95 % CI 0.96–0.99, p = 0.041) and having diabetes (OR 0.38; 95 % CI 0.21–0.70; p = 0.002) at exam 1 were associated with lower odds of weight gain in exam 2. Female sex (OR:1.51; 95 % CI 1.02–2.24; p = 0.040) and having a higher adiponectin level (OR 1.39 per SD; 95 % CI 1.13–1.71; p = 0.002) were associated with higher odds of weight gain (Table 2).
Table 2.
Multivariable Stepwise Binary Logistic and Linear Model for predictors of Weight Change Among South Asian Americans in the MASALA Study.
| Variable | Logistic regression for Weight Change ≥5 % |
Linear regression for Weight Increase (per kg) |
||
|---|---|---|---|---|
| aOR (95 % CI) | p-value | Beta coefficient (95 % CI) | p-value | |
| Age | 0.98 (0.96–1.01) | 0.100 | 0.05 (-0.08–-0.02) | 0.004 |
| Female Sex | 1.51 (1.02–2.24) | 0.040 | 0.13 (−0.42–0.67) | 0.650 |
| BMI at exam 1 | 0.96 (0.91–1.01) | 0.157 | 0.02 (−0.04–0.09) | 0.489 |
| Years lived in the US | 0.98 (0.96–0.99) | 0.041 | −0.01 (−0.04–0.01) | 0.381 |
| Glucose tolerance status: normal | Reference | Reference | ||
| Prediabetes | 0.85 (0.54–1.33) | 0.475 | −0.23 (−0.87–0.41) | 0.483 |
| Diabetes | 0.38 (0.21–0.70) | 0.002 | −1.60 (-2.39–-0.82) | < 0.001 |
| Hypertension | 0.70 (0.45–1.06) | 0.087 | −0.19 (−0.75–0.37) | 0.513 |
| Adiponectin (per SD) | 1.39 (1.13–1.71) | 0.002 | 0.34 (0.05–0.62) | 0.019 |
| Interaction testing | ||||
|
BMI∗∗ category x sex Overweight males |
0.42 (0.22–0.82) | 0.011 | −0.73 (−1.61–0.15) | 0.104 |
| Overweight females | 0.51 (0.24–1.07) | 0.076 | −0.60 (−1.64–0.45) | 0.262 |
| Males with obesity | 0.19 (0.06–0.57) | 0.003 | −1.87 (-3.26–-0.47) | 0.009 |
| Females with obesity | 0.38 (0.13–1.16) | 0.089 | −1.08 (−2.58–0.43) | 0.161 |
| Normal BMI | Reference | Reference | ||
|
BMI ∗∗x glucose tolerance Overweight, no diabetes |
0.56 (0.22–1.41) | 0.216 | 0.07 (−1.22–1.37) | 0.911 |
| Overweight, prediabetes | 0.36 (0.18–0.70) | 0.003 | −1.04 (-1.95–-0.14) | 0.024 |
| Overweight, diabetes | 0.66 (0.21–2.05) | 0.475 | −0.54 (−1.92–0.84) | 0.443 |
| Obesity, no diabetes | 0.17 (0.04–0.65) | 0.010 | −2.39 (-4.18–-0.59) | 0.009 |
| Obesity, prediabetes | 0.35 (0.12–1.01) | 0.052 | −1.05 (−2.44–0.35) | 0.141 |
| Obesity, diabetes | 0.10 (0.02–0.54) | 0.007 | −2.18 (-3.99–-0.38) | 0.018 |
| Normal BMI and no diabetes | Reference | Reference | ||
|
BMI∗∗x adiponectin (per SD) Overweight |
0.74 (0.58–0.93) | 0.010 | −0.21 (−0.53–0.10) | 0.176 |
| Obesity | 0.65 (0.43–0.97) | 0.034 | −0.35 (−0.89–0.18) | 0.199 |
| Normal BMI | Reference | Reference | Reference | Reference |
| Adiponectin x sex | ||||
| Females | 0.80 (0.54–1.18) | 0.262 | 0.07 (−0.46–0.60) | 0.794 |
| Males | Reference | Reference | ||
| BMI ∗∗x waist change groups (stable versus gain) | ||||
| Normal BMI and waist gain | 2.99 (1.41–6.39) | 0.004 | 1.31 (0.35–2.27) | 0.007 |
| Overweight and waist stable | 0.44 (0.23–0.84) | 0.013 | −0.91 (-1.71–-0.11) | 0.025 |
| Overweight and waist gain | 1.57 (0.76–3.25) | 0.224 | 4.59 (0.48–2.33) | 0.003 |
| Obesity and waist stable | 0.20 (0.07–0.61) | 0.005 | −1.06 (-3.10–-0.54) | 0.005 |
| Obesity and waist gain | 1.06 (0.34–3.27) | 0.918 | 4.94 (−0.89–1.92) | 0.469 |
| Normal BMI and waist stable | Reference | Reference | ||
aOR: Adjusted odds ratios from multivariable logistic regression models adjusting for age, sex, BMI, number of years lived in the US at exam 1.
BMI∗∗: by Asian BMI category.
Linear regression analysis also showed that having diabetes was associated with clinically meaningful weight loss in exam 2 (β −1.60; 95 % CI -2.39 to −0.82; p < 0.001) (Table 2). Older age (β 0.05; 95% CI -0.08 to −0.02; p = 0.004) and higher adiponectin level (β 0.34 per SD; 95 % CI 0.05–0.62; p = 0.019) were associated with weight gain in exam 2 (Table 2).
We examined associations between being on diabetes medications (metformin, insulin) in exam 1 and weight change in exam 2. We found that among the 624 individuals, 77 were taking metformin and 10 were taking insulin for diabetes. Among those on metformin, 65 individuals (84.42 %) were in the weight stable group in exam 2. Only 2 (20 %) individuals on insulin were in the weight gain group in exam 2.
4.2. Predictors of waist gain
Logistic regression models showed that being employed full/part time (OR 0.57; 95 % CI 0.35–0.94; p = 0.027) or being retired (OR 0.51; 95 % CI 0.26–1.02; p = 0.056) were associated with a lower odds of waist increase (Table 3), and only female sex (OR1.82; 95 % CI 1.16–2.87; p = 0.009) was associated with greater odds of waist increase (Table 3).
Table 3.
Multivariable Stepwise Linear and Binary Logistic Model for predictors of Waist Change Among South Asian Americans in the MASALA Study.
| Logistic regression for Waist gain ≥5 % |
Linear regression for increase in waist (cm) |
|||
|---|---|---|---|---|
| Variable | aOR (95 % CI) | p-value | Beta Coefficient (95 % CI) | p-value |
| Age | 1.02 (0.99–1.04) | 0.171 | 0.002 (−0.06–0.06) | 0.932 |
| Female sex | 1.82 (1.16–2.87) | 0.009 | 1.07 (−0.14–2.27) | 0.083 |
| BMI | 1.00 (0.94–1.06) | 0.966 | −0.04 (−0.19–−0.12) | 0.632 |
| Marital status | ||||
| Separated/divorced | 2.26 (0.84–6.07) | 0.106 | 2.69 (-0.08–5.47) | 0.057 |
| Widowed | 0.95 (0.38–2.35) | 0.911 | 0.40 (−2.10–2.90) | 0.753 |
| Single | 0.52 (0.09–2.80) | 0.449 | 4.90 (0.79–9.01) | 0.019 |
| Occupational status | ||||
| Employed full-time/part-time | 0.57 (0.35–0.94) | 0.027 | −1.32 (-2.70–0.05) | 0.059 |
| Retired | 0.51 (0.26–1.02) | 0.056 | −1.07 (−2.91–0.75) | 0.255 |
| Leptin (per SD) | 1.06 (0.83–1.34) | 0.664 | 0.72 (0.06–1.38) | 0.032 |
| CRP (per SD) | 1.01 (0.85–1.20) | 0.929 | 0.82 (0.34–1.29) | 0.001 |
| Interaction testing BMI∗∗x sex | ||||
| Overweight males | 0.87 (0.44–1.71) | 0.680 | −1.55 (-3.22–0.13) | 0.070 |
| Overweight females | 0.77 (0.38–1.56) | 0.461 | −2.14 (-4.11–-0.18) | 0.032 |
| Males with obesity | 1.27 (0.46–3.52) | 0.652 | −3.35 (-5.98–-0.73) | 0.012 |
| Females with obesity | 0.64 (0.23–1.84) | 0.411 | −3.45 (-6.28–-0.62) | 0.017 |
| BMI ∗∗x leptin (per SD) | ||||
| Overweight individuals | 0.77 (0.45–1.31) | 0.337 | −0.95 (−2.39–0.50) | 0.198 |
| Individuals with obesity | 0.78 (0.42–1.44) | 0.430 | −0.56 (−2.23–1.11) | 0.508 |
| Leptin x sex | ||||
| Females | 1.10 (0.66–1.82) | 0.719 | 1.62 (0.36–2.89) | 0.012 |
| Males | Reference | Reference | ||
| BMI∗∗x CRP | ||||
| Overweight individuals | 1.59 (0.68–3.71) | 0.287 | 0.62 (−0.46–1.70) | 0.262 |
| Individuals with obesity | 1.51 (0.61–3.74) | 0.377 | 1.65 (0.55–2.76) | 0.003 |
| CRP x sex | ||||
| Females | 0.94 (0.67–1.33) | 0.740 | 1.27 (0.34–2.19) | 0.007 |
| Males | Reference | Reference | ||
| BMI ∗∗x weight change (gain vs. stable) | ||||
| Normal BMI and weight gain | 3.29 (1.60–6.78) | 0.001 | 3.45 (1.70–5.19) | < 0.001 |
| Overweight and weight stable | 0.96 (0.46–1.99) | 0.907 | −1.30 (−2.93–0.33) | 0.118 |
| Overweight and weight gain | 3.49 (1.63–7.48) | 0.001 | 2.34 (0.52–4.16) | 0.012 |
| Obesity and weight stable | 1.06 (0.37–3.07) | 0.911 | −2.62 (-5.08–-0.15) | 0.037 |
| Obesity and weight gain | 6.23 (1.90–20.41) | 0.003 | 1.31 (−1.55–4.16) | 0.369 |
| Normal BMI and weight stable | Reference | Reference | ||
aOR: Adjusted odds ratios from multivariable logistic regression models adjusting for age, sex, BMI, marital and occupational status in exam 1.
BMI∗∗: by Asian BMI category.
In the adjusted linear regression models, being employed full/part time (β −1.32; 95 % CI -2.70–0.05; 0.059) was associated with a decrease in waist (Table 3). Being separated/divorced (β 2.69 95 % CI -0.08–5.47; p = 0.057), being single (β 4.90; 95 % CI 0.79–9.01; p = 0.019), having a higher leptin level (β 0.72; 95 % CI 0.06–1.38; p = 0.032), and a higher CRP level (β 0.82; 95 % CI 0.34–1.29; p = 0.001) were associated with waist increase (Table 3).
4.3. Interaction testing
We found important evidence for interactions for weight gain. Men with obesity at baseline had a lower likelihood of weight gain compared to women with normal BMI. Regardless of glucose tolerance status, individuals with overweight and obesity had a lower likelihood of weight gain compared to those with a normal BMI (Table 2). Individuals with overweight and obesity, who were in the waist stable group had lower weight gain compared to normal weight individuals in the waist gain group (Table 2).
For waist gain interactions, we found that regardless of sex, individuals with overweight and obesity had a lower waist gain compared to those with a normal BMI (Table 3). Females with a higher CRP level and higher leptin level had a greater waist increase in exam 2. Regardless of baseline BMI, all individuals with weight gain also had waist gain.
5. Discussion
This is the first study to prospectively examine factors influencing weight and waist change in a long-term cohort of US South Asians, known to have an increased risk of developing chronic cardiometabolic complications even at a lower BMI. After adjusting for demographic characteristics, being female and having higher adiponectin levels were associated with a greater odds of weight gain, while older age, longer US residency, and having diabetes were associated with lower odds of weight gain. Conversely, higher leptin and CRP levels, as well as being separated/divorced or single, were linked to greater waist circumference changes. Employment (full or part-time) was associated with lower odds of waist gain. Individuals who gained weight also experienced waist gain, regardless of their baseline BMI. Conversely, those with an overweight or obesity baseline BMI who maintained stable waist measurements showed a decrease in weight during exam 2.
We found a significant effect of adipokines and CRP levels on weight change. Adiponectin, a “beneficial and anti-inflammatory adipokine”, is thought to be protective against insulin resistance and type 2 diabetes [30]. In the current study, higher baseline adiponectin levels were surprisingly associated with weight gain. Further subgroup analysis (not shown) demonstrated that in both the weight gain and weight stable groups, higher adiponectin levels were correlated with lower mean BMI and a longer residence in the US. However, higher adiponectin was correlated to higher prediabetes and hypertension but lower diabetes prevalence in the weight stable group. In the weight gain group, higher adiponectin levels correlated to lower prediabetes and diabetes and a slightly higher hypertension prevalence. Although we did not have early adulthood data in MASALA, our study findings indicate that individuals with higher adiponectin levels at baseline have lived in the US for longer, and therefore may have had much of their lifetime weight gained beforehand, as evidenced by the higher metabolic complications of prediabetes and hypertension. Previous studies exploring the relationship between adiponectin levels and subsequent weight change have generally not found significant associations, except for the Nurses' Health Study [34], which showed a positive association between higher adiponectin levels and weight gain in relatively “healthy” women who did not develop diabetes over a long follow-up period. Other studies that did not observe this association were conducted in specific populations, such as Pima Indians [31], Afro Jamaican adults [32], and elderly White populations [33]. Additionally, the Nurses' Health Study had limitations, including reliance on BMI calculated from self-reported weight and height and predominantly included “healthy” women without a prior diagnosis of chronic diseases like diabetes [34]. Consistent with the Nurses' Health Study, we observed that relatively “healthy” females without diabetes and normal BMI had the highest baseline adiponectin levels and were most likely to gain weight. These findings suggest a potential complex interaction between adiponectin and weight gain, warranting further research to determine if adiponectin can serve as a biomarker for weight change in various populations. Moreover, previous studies have identified associations between higher leptin and CRP levels and waist gain across multiple racial and ethnic groups, including South Asians, which aligns with our findings [[35], [36], [37], [38]]. Therefore, higher leptin and CRP levels could be potential biomarkers for waist gain, indicating future cardiovascular and metabolic risk.
In the current study, findings varied across different weight groups and by sex. We found that women were more at risk for both weight and waist gain compared to men. Specifically, men with overweight and obesity had a decreased likelihood of weight gain compared to women in any BMI category, placing women at a higher risk for developing obesity-related comorbidities such as type 2 diabetes. These results contrast with a prior study by Jackson et al. which used data from the US Health and Retirement Study and the UK English Longitudinal Study of Ageing, and found that women were more likely to experience weight loss compared to men [39]. Our findings suggest that weight and waist change trajectories may differ between middle- and older-aged South Asian men and women. Further studies are needed to explore these differences. These findings may have important implications for sex-specific weight management strategies, particularly for middle-aged and older South Asian women.
Our study findings on weight decrease among individuals with diabetes align with previous research showing that a diagnosis of T2D in adults is associated with progressive weight loss following the diagnosis [40,41]. The use of diabetes medications, such as metformin—which can cause modest weight loss in some individuals—could explain the improved weight trajectories in some participants, as most individuals on metformin were in the weight-stable group. This study found no significant effect of insulin use on weight change. Additionally, we observed that, independent of glucose tolerance status, individuals with obesity were less likely to gain weight, and overweight individuals with prediabetes also exhibited this trend. Jackson et al. [39] reported that individuals with obesity had a higher likelihood of clinically meaningful weight loss in the future. It is possible that these individuals with obesity/overweight and with diabetes had more frequent health checks, were more mindful of their weight status, were adopting healthy lifestyle behaviors and/or seeking pharmacological treatments to manage their weight and were less likely to experience further weight gain compared to those who had not yet developed the complications of overweight or obesity.
These findings have significant implications for obesity management interventions targeting South Asians, a group at high risk for developing obesity-related complications. The observed effects of social capital, such as marital and occupational status, on changes in waist circumference are noteworthy, as these social factors can either facilitate or hinder the adoption of healthy lifestyle behaviors. These insights are crucial for health promotion efforts, suggesting that targeted interventions for these higher-risk individuals may help reduce the development of central adiposity and, consequently, mitigate the occurrence of obesity and its associated health consequences.
5.1. Limitations
Study findings should be considered in light of several limitations. Firstly, the MASALA study is representative of primarily middle-aged to older Asian Indian immigrants in the US, and thus is not generalizable to other South Asian subgroups in the US. Secondly, the MASALA study excluded individuals with a body weight greater than 300 lbs. due to CT scanner capacity and thus limiting our ability to assess weight and waist gain patterns in these individuals with the highest levels of obesity. Thirdly, the population in this study was homogenous with high socioeconomic attainment, limiting generalizability to individuals with lower educational or income. The duration of being overweight or having obesity and data regarding intentional versus unintentional weight loss or gain and participation in a weight control program were not collected in the study.
5.1.1. Strengths
Offsetting these limitations were several strengths. The MASALA study is the first and the largest study in South Asians in the US to prospectively evaluate weight and waist gain in South Asians. The study utilized systematic and standardized data collection including use of measured weight and height to calculate accurate BMI. In addition to clinical, behavioral, and psychological measures, the MASALA study included other important variables such as several radiographic measures of body composition, adipokine levels, and metabolic conditions such as diabetes, fatty liver disease, and hypertension.
Overall, our study findings suggest that the greatest potential for weight or waist gain was observed in individuals with lower BMI or waist circumference at baseline, less time in the US, and lack of adipose tissue dysfunction at baseline, as indicated by higher levels of adiponectin levels. Despite the higher adiponectin levels, these individuals had a BMI consistent with being overweight and had already developed obesity complications (e.g., prediabetes, hypertension) and thus had more potential for weight or waist gain compared to participants who already had more advanced overweight/obesity.
6. Conclusions
In a prospective cohort of US South Asians, the current study identified several social, demographic, and clinical factors that can serve as targets for obesity interventions in this high-risk population. Additionally, the study also raises hypotheses about the associations of adipokine levels with weight and waist gain.
Key takeaway points
-
•
Among a prospective cohort of US South Asians, older age, longer US residency, and having diabetes were associated with a lower odds of weight gain. Conversely, being female and having higher adiponectin levels were associated with a higher odds of weight gain.
-
•
Employment (full or part-time) or being retired reduced the likelihood of waist gain. In contrast, being female, single, separated/divorced, and having higher levels of leptin and CRP were linked to a higher likelihood of waist gain.
-
•
US South Asian women, younger individuals, those without diabetes, those with a normal BMI, and those not employed full-time or who are single/separated/divorced should be closely monitored and provided with weight management interventions. Further studies are needed to explore the relationship between adipokines and future weight and waist gain, as current findings are preliminary and hypothesis-generating.
Author contribution
Dr. Alka Kanaya, MD is the principal investigator of the MASALA study and she conceptualized and designed the study; Dr. Sujatha Seetharaman, MD, MPH conducted the statistical analysis, drafted the initial manuscript, and critically revised it; Dr. Elaine Isabel Allen supervised the statistical analysis; Dr. Meghana Gadgil, MD, Dr. Shylaja Srinivasan, MD, MAS, and Lisa Swartz Topor, MD, MMSc, critically reviewed, revised, and approved the manuscript.
Sources of funding
The MASALA study was supported by Grants R01HL093009 and 1K24HL112827 from the National Heart, Lung, And Blood Institute and the UCSF-CTSI grants UL1RR024131 and UL1TR001872 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. The authors thank the other investigators, the staff, and the participants of the MASALA study for their valuable contributions.
Ethical adherence and ethical review
The current study was a post hoc analysis of data from the MASALA study, a prospective clinical trial that involved human test subjects or volunteers. The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, received approval from the institutional review boards at both the University of California, San Francisco, and Northwestern University. Informed consent was obtained from all participants at each exam. The current analysis was approved by the Institutional review board at the University of California, San Francisco.
Declaration of artificial intelligence (AI) and AI-assisted technologies
During the preparation of this work the author(s) used AI in preparation of the submission to improve language and readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study for their valuable contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2024.100118.
Contributor Information
Sujatha Seetharaman, Email: sujathasri.seetharaman@ucsf.edu.
Isabel Elaine Allen, Email: Isabel.allen@ucsf.edu.
Meghana Gadgil, Email: meghana.gadgil@ucsf.edu.
Shylaja Srinivasan, Email: shylaja.srinivasan@ucsf.edu.
Lisa Swartz Topor, Email: lisa_swartz_topor@brown.edu.
Alka M. Kanaya, Email: alka.kanaya@ucsf.edu.
Abbreviations
- WC
Waist circumference
- BMI
Body mass index
- MASALA
Mediators of Atherosclerosis in South Asians Living in America
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Malhotra R., Ostbye T., Riley C.M., Finkelstein E.A. Young adult weight trajectories through midlife by body mass category. Obesity. 2013;21(9):1923–1934. doi: 10.1002/oby.20318. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I., Katzmarzyk P.T., Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 3.Shelgikar K.M., Hockaday T.D., Yajnik C.S. Central rather than generalized obesity is related to hyperglycaemia in Asian Indian subjects. Diabet Med. 1991;8(8):712–717. doi: 10.1111/j.1464-5491.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 4.Pandit K., Goswami S., Ghosh S., Mukhopadhyay P., Chowdhury S. Metabolic syndrome in South Asians. Indian J Endocrinol Metab. 2012;16(1):44–55. doi: 10.4103/2230-8210.91187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah A.D., Vittinghoff E., Kandula N.R., Srivastava S., Kanaya A.M. Correlates of prediabetes and type II diabetes in US South Asians: findings from the Mediators of Atherosclerosis in South Asians living in America (MASALA) study. Ann Epidemiol. 2015;25(2):77–83. doi: 10.1016/j.annepidem.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogre V., Abedandi R., Salifu Z.S. Correlates and predictors of increasing waist circumference in patients with type 2 diabetes mellitus: a cross-sectional study. Int Sch Res Notices. 2014;2014 doi: 10.1155/2014/318569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D., Hou W., Wang F., Arcan C. Factors affecting obesity and waist circumference among US adults. Prev Chronic Dis. 2019;16 doi: 10.5888/pcd16.180220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva M., Weiderpass E., Licaj I., Rylander C. Factors associated with high weight gain and obesity duration: the Norwegian women and cancer (NOWAC) study. Obes Facts. 2018;11(5):381–392. doi: 10.1159/000492002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Wang H., Zhang B., Popkin B.M., Du S. Elevated fat intake increases body weight and the risk of overweight and obesity among Chinese adults: 1991-2015 trends. Nutrients. 2020;12(11) doi: 10.3390/nu12113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira M.A., Kartashov A.I., Ebbeling C.B., Van Horn L., Slattery M.L., Jacobs D.R., Jr., et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365(9453):36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 11.Viner R.M., Cole T.J. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI between 16 year and 30 years in the 1970 British Birth Cohort. Int J Obes. 2006;30(9):1368–1374. doi: 10.1038/sj.ijo.0803183. [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D., Hao T., Rimm E.B., Willett W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacInnis R.J., Hodge A.M., Dixon H.G., Peeters A., Johnson L.E., English D.R., et al. Predictors of increased body weight and waist circumference for middle-aged adults. Publ Health Nutr. 2014;17(5):1087–1097. doi: 10.1017/S1368980013001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh Y., Kim S.H., Nam G.E., Park H.S. Weight gain, comorbidities, and its associated factors among Korean adults. J Kor Med Sci. 2023;38(12) doi: 10.3346/jkms.2023.38.e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball K., Crawford D. An investigation of psychological, social and environmental correlates of obesity and weight gain in young women. Int J Obes. 2006;30(8):1240–1249. doi: 10.1038/sj.ijo.0803267. [DOI] [PubMed] [Google Scholar]
- 16.Kanaya A.M., Kandula N., Herrington D., Budoff M.J., Hulley S., Vittinghoff E., et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol. 2013;36(12):713–720. doi: 10.1002/clc.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gujral U.P., Vittinghoff E., Mongraw-Chaffin M., Vaidya D., Kandula N.R., Allison M., et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann Intern Med. 2017;166(9):628–636. doi: 10.7326/M16-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah A.D., Kandula N.R., Lin F., Allison M.A., Carr J., Herrington D., et al. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes. 2016;40(4):639–645. doi: 10.1038/ijo.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Up Gujral, Narayan K.M.V., Kandula N.R., Liu K., Kanaya A.M. Incidence of diabetes and prediabetes and predictors of glycemic change among South Asians in the USA: the MASALA study. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2019-001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaya A.M., Herrington D., Vittinghoff E., Ewing S.K., Liu K., Blaha M.J., et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–1628. doi: 10.2337/dc13-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leary S., Fall C., Osmond C., Lovel H., Campbell D., Eriksson J., et al. Geographical variation in neonatal phenotype. Acta Obstet Gynecol Scand. 2006;85(9):1080–1089. doi: 10.1080/00016340600697447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whoe Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Darsini D., Hamidah H., Notobroto H.B., Cahyono E.A. Health risks associated with high waist circumference: a systematic review. J Public Health Res. 2020;9(2):1811. doi: 10.4081/jphr.2020.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ainsworth B.E., Irwin M.L., Addy C.L., Whitt M.C., Stolarczyk L.M. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 25.Kelemen L.E., Anand S.S., Vuksan V., Yi Q., Teo K.K., Devanesen S., et al. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J Am Diet Assoc. 2003;103(9):1178–1184. doi: 10.1016/s0002-8223(03)00985-4. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C.D. Preliminary manual for the state-trait anger scale (STAS) Palo Alto CCPP. 1980 [Google Scholar]
- 27.1:386-401 RLTC-Dsas-rdsfrtgpAPM.
- 28.Bromberger J.T., Matthews K.A. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychol Aging. 1996;11(2):207–213. doi: 10.1037//0882-7974.11.2.207. [DOI] [PubMed] [Google Scholar]
- 29.McAuliffe M. National Institutes of Health; Bethesda, MD: 2009. 4.2.0. Medical image processing a, and visualization (MIPAV) [Google Scholar]
- 30.Wang Z.V., Scherer P.E. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vozarova B., Stefan N., Lindsay R.S., Krakoff J., Knowler W.C., Funahashi T., et al. Low plasma adiponectin concentrations do not predict weight gain in humans. Diabetes. 2002;51(10):2964–2967. doi: 10.2337/diabetes.51.10.2964. [DOI] [PubMed] [Google Scholar]
- 32.Bennett N.R., Boyne M.S., Cooper R.S., Royal-Thomas T.Y., Bennett F.I., Luke A., et al. Impact of adiponectin and ghrelin on incident glucose intolerance and on weight change. Clin Endocrinol. 2009;70(3):408–414. doi: 10.1111/j.1365-2265.2008.03344.x. [DOI] [PubMed] [Google Scholar]
- 33.Langenberg C., Bergstrom J., Laughlin G.A., Barrett-Connor E. Ghrelin, adiponectin, and leptin do not predict long-term changes in weight and body mass index in older adults: longitudinal analysis of the Rancho Bernardo cohort. Am J Epidemiol. 2005;162(12):1189–1197. doi: 10.1093/aje/kwi338. [DOI] [PubMed] [Google Scholar]
- 34.Hivert M.F., Sun Q., Shrader P., Mantzoros C.S., Meigs J.B., Hu F.B. Higher adiponectin levels predict greater weight gain in healthy women in the Nurses' Health Study. Obesity. 2011;19(2):409–415. doi: 10.1038/oby.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monti V., Carlson J.J., Hunt S.C., Adams T.D. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J Am Diet Assoc. 2006;106(6):822–828. doi: 10.1016/j.jada.2006.03.015. quiz 9-30. [DOI] [PubMed] [Google Scholar]
- 36.Marques-Vidal P., Bochud M., Bastardot F., Luscher T., Ferrero F., Gaspoz J.M., et al. Association between inflammatory and obesity markers in a Swiss population-based sample (CoLaus Study) Obes Facts. 2012;5(5):734–744. doi: 10.1159/000345045. [DOI] [PubMed] [Google Scholar]
- 37.Faam B., Zarkesh M., Daneshpour M.S., Azizi F., Hedayati M. The association between inflammatory markers and obesity-related factors in Tehranian adults: tehran lipid and glucose study. Iran J Basic Med Sci. 2014;17(8):577–582. [PMC free article] [PubMed] [Google Scholar]
- 38.Forouhi N.G., Sattar N., McKeigue P.M. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord. 2001;25(9):1327–1331. doi: 10.1038/sj.ijo.0801723. [DOI] [PubMed] [Google Scholar]
- 39.Jackson S.E., Beeken R.J., Wardle J. Predictors of weight loss in obese older adults: findings from the USA and the UK. Obes Facts. 2014;7(2):102–110. doi: 10.1159/000362196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Fine Olivarius N., Siersma V.D., Koster-Rasmussen R., Heitmann B.L., Waldorff F.B. Weight changes following the diagnosis of type 2 diabetes: the impact of recent and past weight history before diagnosis. results from the Danish Diabetes Care in General Practice (DCGP) study. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looker H.C., Knowler W.C., Hanson R.L. Changes in BMI and weight before and after the development of type 2 diabetes. Diabetes Care. 2001;24(11):1917–1922. doi: 10.2337/diacare.24.11.1917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.