Abstract:
Respiratory syncytial virus (RSV) is the major cause of bronchiolitis among children under 5 years of age worldwide, accounting for a prevalence of 25%–88% in Saudi Arabia. Although no effective treatment for the virus exists, passive immunoprophylaxis reduced RSV hospitalizations in high-risk children. With recent advances in immunization, the Saudi Initiative of Bronchiolitis Diagnosis, Management, and Prevention panel screened recent relevant international guidelines, locally published data, and expert consensus to update guidelines for RSV prevention, taking into consideration the resources, timing, varying health profiles, and RSV burden in Saudi Arabia. The panel updated its recommendations to include immunization of infants, mothers, and older adults. Practical guidelines were prepared to facilitate the administration of the short-acting and newly developed long-acting RSV monoclonal antibodies (mAb) during the regular follow-ups of high-risk infants in specialized clinics. In addition, long-acting mAb was highlighted as all-infant protection in the routine immunization calendar.
Keywords: High-risk children, immunization, practice guidelines, respiratory syncytial virus monoclonal antibodies
The Saudi Initiative of Bronchiolitis Diagnosis, Management, and Prevention (SIBRO) was published in July 2018 to provide pediatricians and general practitioners with national guidelines for the latest and best evidence-based practices.[1] The SIBRO panel consisted of a multidisciplinary team of experts led by the Saudi Pediatrics Pulmonology Society, a subsidiary of the Saudi Thoracic Society. The Saudi Societies of Neonatology, Critical Care, and Infectious Diseases aided it. It gives many evidence-based responses to questions regarding the local epidemiology, burden, diagnosis, and management of bronchiolitis.
Only supportive therapy and a few therapeutic interventions are evidence based and have been proven effective. The respiratory syncytial virus (RSV), the major pathogen responsible for bronchiolitis, accounts for approximately 70% of cases worldwide, whereas its prevalence in Saudi Arabia varies widely (25%–88%).[2,3]
RSV prevention with palivizumab in high-risk patients has been effective and well known since 1998.[4] Recently, new updates for prevention that included the development of new modalities of passive and active immunization have emerged.[5] The SIBRO panel screened recent literature and international guidelines and updated its guidelines for RSV prevention concerning local burden, sociodemographic characteristics, and resources. Guidelines on other aspects of bronchiolitis diagnosis and management will be updated when ready.
Virology
RSV was identified in a group of chimpanzees and named the chimpanzee coryza agent in 1956.[6] This pathogen is a highly contagious respiratory virus classified into two subtypes – RSV-A and RSV-B, based on G protein variations.[7] RSV is the main cause of acute respiratory tract infections (such as bronchitis and pneumonia) among children under 5 years of age.[8]
Burden of Respiratory Syncytial Virus
The global burden of RSV is estimated to be 33.1 million episodes of RSV-related acute lower respiratory tract infection (LRTI), resulting in about 3.2 million (2.7–3.8) hospital admissions and 59,600 (48,000–74,500) inhospital deaths in children aged below 5 years. Approximately 45% of hospital admissions and inhospital deaths due to RSV-ALRI occur in infants <6 months.[9] All infants, including healthy term-born infants, are predisposed to severe RSV-related LRTI. Approximately 79% of healthy infants and toddlers under the age of 2 years were hospitalized with RSV.[10]
In Saudi Arabia, many single center cross-sectional and retrospective studies have reported the burden of bronchiolitis and RSV on Saudi Arabia’s health-care system. For example, a 6-year study in Saudi investigated 643 hospitalized cases of ALRI.[11] Among all samples, respiratory viruses were detected in 309 samples. RSV was identified most frequently in 295 of the positive samples, accounting for 95.5% of all viral agents. In 245 cases, the patient was <1 year. The highest rate of RSV infection was identified in infants during the first 6 months of life (P < 0.03).[11]
Furthermore, in another Saudi study, 70 out of 200 (35%) nasopharyngeal aspirates tested positive for RSV infection. Upon typing of the positive samples using duplex real-time polymerase chain reaction, 57.1% of them were found to be type A viruses and 42.9% were found to be type B viruses. These results validate the implication of both virus subtypes in RSV infection of Saudi children during winter, with a slight dominance of type A viruses.[12]
A third Saudi study found that 883 (8.3%) out of 10,617 patient specimens screened for respiratory viruses tested positive. Of these, 733 were positive for RSV, 62 for influenza, 79 for parainfluenza, and 9 for adenovirus.
The age distribution of the patients showed that 92% of infections occurred in infants aged ≤1 year. RSV is an important cause of LRTIs in infants, and it often results in hospitalization. RSV infections occur primarily during annual outbreaks during winter months. In the abovementioned study, RSV infections occurred between November and February, with a peak in January. Those results agree with other studies conducted in Saudi Arabia, in which RSV was identified as the most common cause of bronchiolitis.[13]
In a fourth Saudi study involving 282 specimens, 128 (45.4%) tested positive for RSV. Most of the positive specimens came from patients aged below 1 year (51.3%), and there was a statistically significant association between RSV infection and age <2 years (47.2%, P = 0.019).
The clinical observations from 128 toddlers who tested positive for RSV showed that RSV infection was significantly associated with bronchopneumonia (56.7%, P = 0.001) and bronchiolitis (55.4%, P = 0.002). In addition, 47% and 36.7% of the infected toddlers were hospitalized for 1–4 and 5–8 days, respectively. The clinical manifestations, among which a cough and tachypnea were the most frequent, occurred in 100% and 98% of the toddlers, respectively. They also presented with fever (81%), wheezing, crepitation, and retraction, representing 66% of patients. Three deaths were reported.[14]
Furthermore, in a fifth study (116), 19.3% of the nasopharyngeal aspirate samples for 4575 inpatients and outpatients with acute respiratory symptoms tested positive for RSV. Approximately 55% of the patients were male and 45% were female. The majority (58.9%) of the patients were aged 0–6 months, followed by the >6–12-month age group (19.8%). Seasonal variation showed that most RSV cases occurred during winter and early spring.[15]
Risk factors for RSV infection in Saudi children admitted to the pediatric intensive care unit (PICU) of two tertiary hospitals were investigated,[16,17] and prematurity was associated with increased RSV infection severity. Approximately 37% of infants admitted to the PICU were premature at birth. Moreover, children with pulmonary pathologies and cardiovascular abnormalities were also more prone to RSV infection. Fortunately, the mortality rate was <2%, and mortality was associated with severe comorbidities.[17,18]
One study conducted in the Eastern Province of Saudi Arabia between January 2015 and February 2022 showed that the overall percentage of RSV detection was 26.3% (336/1279) among the tested individuals. RSV infection was more common among children below 5 years and elders aged above 60 years. Two-thirds of the cases required hospitalization. The average duration of hospitalization due to RSV infection was 6.5 days.[19]
Another retrospective cohort study aimed to compare the demographic and clinical characteristics of children with bronchiolitis admitted to the general ward and PICU between May 2016 and May 2021 revealed that the most common causative virus was RSV (54.9%), and approximately 75% of them were healthy term infants.[17]
Prevention
Nonpharmacological
Since there is still no effective antiviral treatment for RSV, RSV prevention remains critical. Comprehensive hygiene etiquette is efficacious and cost-effective in preventing RSV spread and should always be advocated as a prophylactic measure.[20]
In addition, second-hand smoking heightens the risk of severe RSV infection requiring hospitalization, especially in late preterm infants.[21] Accordingly, measures to reduce and prevent second-hand smoking are another cornerstone in RSV prevention. On a different note, breastfeeding (even in association with formula milk) reduces the risk of hospitalization for bronchiolitis during the 1st year of life.[22] Thus, breastfeeding should be encouraged. Adherence to infection control practices is the basis for reducing the incidence of health-care-associated RSV disease.
Pharmacological
Effective therapeutics for RSV infection remain elusive, and while vaccines to prevent RSV infection in high-risk adults have recently become available, safe and effective vaccines for children are still in early-phase clinical trials. Therefore, prophylactic interventions remain the best strategy to avoid the acute and chronic complications of the disease, particularly in infants and young children.[23] The World Health Organization recommends the evaluation of the new anti-RSV monoclonal antibodies (mAb) as a prevention strategy and their insertion into routine immunization calendars.[24] Palivizumab, nirsevimab, and other investigational mAb candidates like clesrovimab and most RSV vaccine candidates share the same mechanism of targeting the RSV fusion (F) protein.[25]
Monoclonal antibodies
These recommendations will be updated as new evidence becomes available.
Timing
The recommendations in this section apply to both short-acting and long-acting mAbs.
Variations in the onset and offset of the RSV season in different regions of Saudi Arabia may affect the timing of (mAb) administration. Season onset can be determined by active surveillance identifying the first of 2 consecutive weeks that the RSV molecular assay test positivity rate is ≥3% or the antigen detection positivity rate is ≥10%.[26] Based on prepandemic COVID-19 patterns, mAbs could be administered in most regions of Saudi Arabia from October through the end of March. However, providers can adjust administration schedules based on local epidemiology[27]
High-risk infants hospitalized or still in the NICU during the season should get the recommended schedule of mAbs[28]
Furthermore, infants who are eligible at the beginning of the season and ready for discharge from the NICU should receive the first dose up to 72 h before discharge[28]
For eligible infants based on age and risk, it should be administered shortly (3 weeks) before the start of their first RSV season[28]
To realize the full benefits of mAbs before each season, it is recommended that age-eligible infants be recalled at the start of the RSV season before they become ineligible based on age if nirsevimab is available[5]
Administering mAbs through the end of the season is important because the risk of severe disease is highest during the first few months of life[5]
Since natural immunity to RSV is incomplete and reinfection occurs throughout life, high-risk infants recovered from RSV-related upper respiratory tract infection or LRTI should continue receiving mAbs for the season[29]
The panel recommends that immunoprophylaxis can be considered if there is an interseasonal (i.e., outside the season) RSV outbreak, as per the season onset definition[5]
mAbs do not interfere with the immune response to live or inactivated vaccines. The childhood immunization schedule should be followed for all children, regardless of mAb use[30]
The SIBRO panel recommends RSV mAb as a prevention strategy that should be inserted into routine immunization calendars.[31,32]
High-risk population
The SIBRO defined certain infants and young children at increased risk of hospitalization for RSV infection to guide palivizumab administration. The SIBRO screened the international recommendations and considered local sociodemographic, geographical distribution, and local published literature data to update high-risk infants based on the best available evidence. Such infants are considered eligible for palivizumab administration. In addition, it is used for long-acting mAbs for infants aged >12 months [Table 1]. Moreover, health-care professionals in remote regions with no pediatric intensive care beds or in a community with known high rates of severe RSV among older infants and toddlers should use their clinical judgment to include these infants in the immunoprophylaxis program.
Table 1.
High-risk patients
| Patient segment | Recommendations | Level of evidence |
|---|---|---|
| Early preterm (≤28 weeks, 6 days GA) | ≤12 months of age | 1B |
| Mid preterm (29 weeks GA, 0 days to 32 weeks, 6 days GA) | ≤6 months of age | 1B |
| Late preterm (33 weeks, 0 days weeks GA to 35 weeks, 0 days GA) | ≤6 months of age at the start of the RSV season OR born during RSV season with at least one of the following risk factors attendance at childcare | 1B |
| Children <5 years of age who live permanently in the same household (including siblings) | ||
| Exposure to environmental air pollutants | ||
| Infants and children with CLD | <12 months for all; <24 months if still receiving medications for CLD within 6 months from the beginning of the epidemic season | 1B |
| Infants and children with hemodynamically significant CHD | <12 months for all; <24 months if still receiving medications for the cardiac condition<6 months from the beginning of the epidemic season Postoperative dose after cardio bypass | 1B |
| Children with anatomic pulmonary abnormalities or neuromuscular disorder | <24 months may be considered for infants with impaired ability to handle respiratory secretions | 3B |
| Immunocompromised children | <24 months may be considered for children who are profoundly immunocompromised during the RSV season | 2B |
| Children with Down syndrome | Recommended in children with accompanying qualifying heart disease, CLD, airway clearance issues, or prematurity (<35 weeks, 0 days GA) | 2B |
| Children with cystic fibrosis | <12 months with clinical evidence of CLD and/or nutritional compromise <24 months with manifestations of severe lung disease OR weight for length <10th percentile | 2A |
| Special situations: If an infant receiving prophylaxis experiences a breakthrough of RSV | If an infant who is receiving prophylaxis experiences a breakthrough of RSV, the monthly prophylaxis should continue as planned until a maximum of 5 doses have been administered | 3B |
RSV=Respiratory syncytial virus, OR=Odds ratio, CLD=Chronic lung disease, GA=Gestational age, CHD=Congenital heart disease
Modality
Monoclonal antibodies
Short-acting: Palivizumab
Palivizumab is a humanized mAb produced by recombinant DNA technology. It is a composite of human (95%) and murine (5%) antibody sequences.[2] It binds to the F protein of RSV, which plays a role in viral attachment and mediates fusion, effectively neutralizing the virus and preventing its entry into the cell. Palivizumab was licensed in June 1998 by the US Food and Drug Administration for the reduction of severe LRTIs caused by RSV in certain risk groups
The efficacy and safety of palivizumab have been demonstrated in many prospective, retrospective, and registry studies and confirmed by systematic reviews and meta-analyses[1,3,33]
Among all the clinical studies, the most reliable evidence comes from three Phase III RCTs: the IMpact-RSV trial (1998),[34] the study by Feltes et al. on critical congenital heart disease,[35] and the study by Blanken et al.[36] These studies highlighted the fact that palivizumab is an effective form of prophylaxis that significantly reduces RSV-related hospitalization rates by 38%–80%, positively affecting several outcomes such as hospitalization durations, progression to ICU admission, duration of oxygen support, and mortality in a high-risk population. Moreover, the rates of occurrence of wheezing episodes during the 1st year of life and asthma later on and mortality in high-risk populations were reduced.[3,37,38] The rate of hospitalization due to respiratory illness was also reduced, especially in low- and middle-income countries (LMICs). The AAP has updated the guidelines several times for better usage of this medication[4,39,40,41]
Palivizumab is administered intramuscularly at a dosage of 15 mg/kg. It is packaged in 100 mg vials, and when a given vial is opened, it should be used within 6 h. It should be administered every month during the season up to five doses.[42] The new liquid form allows less time for preparation and less waiting time for administration. Palivizumab solution for injections does not contain preservatives, is for single use, and should be administered immediately after the dose is drawn into the syringe.[43] In circumstances where the season extends beyond usual, up to seven doses of palivizumab have been reported to be safe and effective[40]
In general, there were few differences in the incidence of adverse events (AEs) among patients who had received palivizumab and those who had received a placebo. The most common adverse effects were erythema at the injection site, fever, or diarrhea. The discontinuation of palivizumab due to drug-related AEs is rare[44]
-
Effect of palivizumab restriction in the latest AAP guidelines
Several reports showed the resurgence of admission of premature infants born at 29–35 gestational weeks since the AAP-recommended restriction in the US and other countries and centers that implemented such restrictions.[45] Moreover, in Saudi Arabia, a study reported an increase of almost three times in the risk of admission to the PICU.[17] Many national guidelines did not follow and put their own recommendations. Finally, per new local data, more groups of high-risk infants that are moderately preterm (29–33-week gestation) and have Down’s syndrome should be re-enforced for palivizumab administration as per Table 1 and documentation as per Table 2.
Table 2.
Recommendations for nirsevimab immunoprophylaxis
| *The recommended interval between the last dose of palivizumab and a dose of nirsevimab (in high risk infants) is 1 month (similar to the interval if the infant were to receive another dose of palivizumab)[5] | |||
| &The recommendations for nirsevimab for high-risk children apply to infants and children recommended to receive palivizumab[1] | |||
| To realize the full benefits of mAbs before each season, it is recommended that age-eligible infants be recalled at the start of the RSV season before they become ineligible based on age if nirsevimab is available | |||
| Administering nirsevimab through the end of the season is important because the risk of severe disease is highest during the first few months of life[31] | |||
| #Maternal vaccination efficacy is not established in | |||
| Women with high-risk pregnancies such as multiple pregnancy, pregnancy-induced or chronic diseases, evidence of placental insufficiency, or fetus/newborn with major congenital anomaly | |||
| Infants who have undergone cardiopulmonary bypass or extracorporeal membrane oxygenation, leading to loss of maternal antibodies | |||
| Infants with a substantially increased risk for severe RSV disease (e.g., hemodynamically significant congenital heart disease, intensive care admission, and requiring oxygen at discharge) | |||
| Infant born within 14 days of administering the maternal RSV vaccine[46] | |||
| High-risk infants hospitalized or still in NICU during the season should get the recommended schedule. Also, eligible infants at the beginning of the season and ready for discharge from NICU should receive the first dose up to 72 hours before discharge[28] | |||
| The mAb included palivizumab and nirsevimab does not interfere with the immune response to live or inactivated vaccines. The childhood immunization schedule should be followed for all children, regardless of mAb use[30] | |||
|
| |||
| Age | Needle length | Injection site | |
|
| |||
| IM injection, use a 22–25-gauge needle[5] | Newborns (1st 28 days) | ⅝” | Anterolateral thigh muscle |
| Infants (1–12 months) | 1” | Anterolateral thigh muscle | |
| Toddlers (1–2 years) | 1–1¼” | Anterolateral thigh muscle | |
| ⅝–1” | Deltoid muscle of arm | ||
RSV=Respiratory syncytial virus, CLD=Chronic lung disease, IM=Intramuscular, mAbs=Monoclonal antibodies, NICU=Neonatal intensive care unit
Long-acting monoclonal antibodies
-
Nirsevimab
- Nirsevimab is given as a single injection lasting at least 5 months, compared with monthly injections with the older RSV monoclonal product, palivizumab. Children who have received nirsevimab should not receive palivizumab in the same RSV season[5]
-
Eligibility considerations regarding nirsevimab were as follows:
- ALL infants aged 12 months born during or going into their first RSV season are recommended to receive one dose of nirsevimab (50 mg for infants <5 kg and 100 mg for infants ≥5 kg)
- Clinicians should target the administration of nirsevimab in the 1st week of life for infants born shortly before and during the RSV season
- Nirsevimab is administered as an intramuscular injection using a single dose, prefilled syringe
- It is dosed by weight and age (50 mg if <5 kg; 100 mg if ≥5 kg; 200 mg [2 mg × 100 mg]) for high-risk children entering their second RSV season
- It is stored in a refrigerator at 2°C–8°C and may be stored at room temperature (20°C–25°C) for up to 8 h. Illnesses or febrile diseases are not contraindications to nirsevimab
- If an eligible infant received palivizumab and the health-care facility decided to start nirsevimab, with the recommended interval between the last dose of palivizumab and a dose of nirsevimab being 1 month
- Nirsevimab recommendations are the same, regardless of prior RSV infection or RSV-associated hospitalization. In the case of acute/recent RSV infection (with or without fever), patients with documented current RSV infection should defer nirsevimab until recovery from the acute illness.[5]
-
Nirsevimab efficacy and safety
- The safety and efficacy of nirsevimab were supported by three clinical trials.[47,48,49] In a randomized, double-blinded, placebo-controlled trial that included 1453 preterm infants (born at 29–35 weeks of gestation) who were born during or going into their first RSV season. Of the 1453 preterm infants, 969 received a single dose of nirsevimab and 484 received a placebo. Among infants who received nirsevimab, 25 (2.6%) experienced MA RSV LRTI compared with 46 (9.5%) infants who received a placebo with a reduction of MA RSV LRTI by approximately 70% relative to placebo. Subgroup analysis among infants weighing <5 kg revealed an efficacy of ~86%. These results informed future trials to use weight-banded dosing of 50 mg for those who weigh <5 kg and 100 mg for those weighing ≥5 kg. In another RCT, the primary analysis group within the trial included 1490 term and late preterm infants (born at a gestational age of at least 35 weeks), 994 of whom received a single dose of nirsevimab and 496 of whom received a placebo. Weight-banded dosing was used as described. Among infants who were treated with nirsevimab, 12 (1.2%) experienced MA RSV LRTI compared with 25 (5.0%) infants who received a placebo. Nirsevimab reduced the risk of MA RSV LRTI by approximately 75% relative to placebo.[49] Furthermore, in a randomized, double-blind, active (palivizumab)-controlled, multicenter trial, in toddlers up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. The trial enrolled 925 preterm infants and infants with chronic lung disease of prematurity or congenital heart disease. The efficacy (as defined by immune bridging) and safety profile of nirsevimab were similar to that of palivizumab.[47] Finally, in a pragmatic, real-world effectiveness trial, the 8058 infants aged ≤12 months were born at a gestational age of 29 weeks and were entering their first RSV season in France, Germany, or the United Kingdom receive either a single intramuscular injection of nirsevimab or standard care before or during the RSV season shows LRTI, which corresponded to a nirsevimab efficacy of 83.2% (95% confidence interval [CI]: 67.8–92.0; P < 0.001). Very severe RSV-associated LRTI occurred in five infants (0.1%) in the nirsevimab group and 19 (0.5%) of them in the standard care group, which represented a nirsevimab efficacy of 75.7% (95% CI: 32.8–92.9; P = 0.004)[48]
- The safety profile of nirsevimab was favorable across efficacy studies, with no imbalance in AEs observed between the nirsevimab and palivizumab groups. In the FDA review, it was noted that there were numerically more deaths in the nirsevimab arm than in the control arm but deemed that these deaths were completely unrelated to nirsevimab (e.g., cardiac disease, gastroenteritis, and trauma). Possible side effects of nirsevimab include rash and injection-site reactions. Nirsevimab should not be given to infants and children with a history of serious hypersensitivity reactions to nirsevimab active ingredients or any of its excipients[47,49]
- Technical considerations: Technical considerations for mAbs and nirsevimab administration are shown in Table 3
- Cost-effectiveness: An all-infant immunization strategy with nirsevimab could substantially reduce the health and economic burden for US infants during their first RSV season. Under the current standard of care, RSV caused 529 915 RSV-MALRTIs and 47 281 hospitalizations annually, representing $1.2 billion (2021 US dollars [USDs]) in costs. Universal immunization of all infants with nirsevimab is expected to reduce 290 174 RSV-MALRTI, 24 986 hospitalizations, and expenditures worth $612 million 2021 USD[50]
- In Saudi Arabia, the decision-analytic model to estimate nirsevimab impact on RSV-related health events and costs compared with the standard of practice (SoP) for infants in Saudi Arabia. The model stratified infants by their month of birth and estimated health and cost outcomes for the first RSV season. To create a model and assess the effectiveness of preventative measures, the authors used various sources of data, such as published literature, publicly available information, and expert opinions. The data considered included demographics, seasonality, epidemiology, health event risk, prevention effectiveness, and coverage rates. The model estimated that under the current SoP, RSV results in 17,179–19,607 hospitalizations (including 2,932–3,625 PICU and 172–525 MV admissions), 57,654–191,115 ER visits, 219,053–219,970 PC visits, 14 deaths and 12,884–14,705 cases of recurrent wheezing, and a total cost of SAR 480–619 million. Universal nirsevimab immunoprophylaxis was estimated to avert 57.9% of hospitalizations (57.9% of PICU admissions and 57.9% MV episodes), 53.3% of ER visits, 53.3% of PC visits, 57.9% of episodes of recurrent wheezing, four deaths, and result in savings of SAR 274–343 million in total health-care cost. Compared with current SoP, the nirsevimab immunoprophylaxis strategy in the KSA for all infants during their first RSV season was estimated to significantly decrease health-care resource use and the economic burden associated with RSV[51]
-
Clesrovimab
- Long-acting mAbs: MK-1654 (clesrovimab) (Merck & Co. Inc.) is also an extended half-life mAb currently undergoing Phase-IIb and III clinical trials. It targets antigenic site-IV in both pre-F and post-F forms,[51] in contrast to nirsevimab, which binds to the antigenic site 0 present only in the prefusion conformation of the F protein.
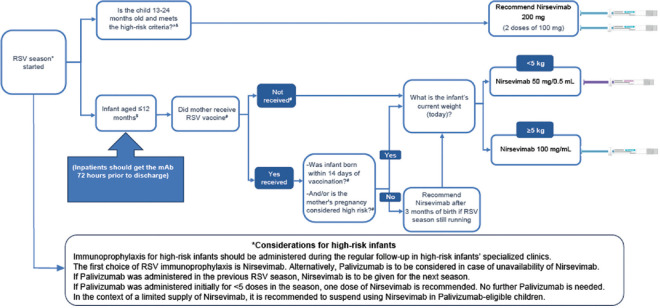
Figure 1.
Nirsevimab administration guide
Table 3.
Immunoprophylaxis technical considerations[5]
| Type of mAbs | Action | Recommendation | Comments |
|---|---|---|---|
| All short and long-acting | Immunoprophylaxis to be included in the National program immunization enrolment | For all infants except high-risk infants | Immunoprophylaxis for high-risk infants should be administered in specialized clinics |
| mAbs be co-administered with other routine vaccines | Yes. Simultaneous administration of mAb with age-appropriate vaccine is recommended | mAbs are not expected to interfere with the immune response, safety, and reactogenicity of other vaccines | |
| If an infant is diagnosed with an acute RSV illness, give a dose of mAbs to help reduce the severity of the illness | mAbs have not been studied as a treatment in infants with RSV and are not licensed for the treatment of RSV disease | mAbs should be given to recovering infants after 4 weeks from the onset of acute RSV illness | |
| High-risk infants who are hospitalized or still in NICU during the season | Should get the recommended schedule | ||
| Eligible infants at the beginning of the season and ready for discharge from NICU | Should receive the first dose up to 72 h before discharge | ||
| Nirsevimab | The institution decided to start nirsevimab, and a high-risk infant already received palivizumab <5 doses | Should receive nirsevimab. No further Palivizumab is needed | The recommended interval between the last dose of palivizumab and a dose of nirsevimab (in high-risk infants) is no later than 1 month |
| Splitting a 100 mg manufacturer-filled syringe into two 50 mg doses | No, nirsevimab 100 mg doses are approved for single use, it is a serious administration error | Manufacturer-filled syringes are Prepared with a single dose and sealed under sterile conditions Do not contain a preservative to help prevent the growth of microorganisms Intended for ONE patient for ONE injection Never administer medications from the same syringe to more than one patient, even if the needle is changed |
|
| If a high-risk child mistakenly received a 100 mg dose of nirsevimab instead of the 200 mg dose | Another half-dose should be administered as soon as possible but no later than the end of the season. This counts as a 200 mg dose |
mAbs=Monoclonal antibodies, RSV=Respiratory syncytial virus, NICU=Neonatal intensive care unit
Monoclonal antibody recommendations
The SIBRO panel recommends the following:
All infants should receive the long-acting mAbs for RSV immunoprophylaxis as per the recommendations [Figure 1]
-
High-risk infants, as per SIBRO guidelines in Table 1
- To be followed regularly in specialized clinics
- The first choice of the immunoprophylaxis modality is long-acting mAbs. Alternatively, short-acting mAbs (monthly doses during RSV season) should be considered in the case of long-acting mAb unavailability
- If a short-acting mAb was administered in the previous RSV season, the panel recommends the administration of long-acting mAbs for the next season
- However, if no long-acting mAb is available, short-acting ones should be administered as previously recommended
- If an eligible infant received short-acting mAbs and the health facility decided to start long-acting mAbs, the recommended interval between the last dose of short-acting mAbs and a dose of long-acting mAbs is no later than 1 month
- If the short-acting mAb was administered initially for the season and < 5 doses were administered, the infant should receive one dose of the long-acting mAb. No further short-acting ones should be administered.
Vaccination
During the last two decades, trials with various phases were conducted to test the efficacy and safety of anti-RSV vaccination. Most severe RSV infections occur in infancy and do not produce lifelong immunity; hence, re-infections are common.[1] Moreover, RSV infection in older adults is underestimated. The development of RSV vaccination, therefore, targeted the life span as early as during pregnancy, infancy, and older adults. Moreover, various routes (intramuscular, intradermal, and nasal) were examined with the aim of reducing the cost and ensuring its more widespread use, especially in LMICs. Although various mechanisms were studied, only a few of them succeeded to get FDA approval after phase II/III trials. These include maternal and older adults’ vaccines.[52,53,54,55,56]
Maternal vaccination
The US Food and Drug Administration approved the Maternal RSV Vaccine, which aims to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by RSV. It is administered as a single dose at 32 through 36 weeks of gestation. In a previous RCT, the efficacy rate against MA RSV LRTI in infants was 51.3% (29.4%–66.8%) that against RSV-associated hospitalization was 56.8% (10.1%–80.7%). Maternal vaccination includes a warning about the imbalance in preterm births in maternal vaccine recipients (5.7%) compared with those who received a placebo (4.7%).[5] Currently, available data are insufficient to establish or exclude a causal relationship between preterm birth and maternal vaccination.[57] However, another Phase 3 trial (RSVPreF3 Mat) was halted due to an imbalance of preterm births, and the sponsoring company has abandoned its assessment in pregnancy.[5] Moreover, maternal vaccines tend to protect only infants born just before and during the RSV epidemic season.[58] Moreover, the trials conducted to study the efficacy of the maternal vaccine were conducted in healthy women with singleton pregnancies; hence, vaccination with a high risk of impaired placental transfer should be studied, and infants born to such mothers should be considered unprotected.[46] Moreover, the protection of maternal vaccination may only last for 3–4 months; hence, infants beyond this age and still in season should receive mAbs.[58] Finally, considerable real-world data is indicating a suboptimal coverage for maternal vaccines, such as pertussis and influenza.[59]
The panel recommends a postmarketing survey with real-world effectiveness data on the maternal vaccine, with special considerations for safety during pregnancy. The maternal vaccine could also be considered for areas where nirsevimab is in short supply or otherwise unavailable.
Older adults’ vaccination
The US FDA licenses two RSV vaccines for use in adults aged ≥60 years in the United States RSVPreF3 (Arexvy, GSK) and RSVpreF (Abrysvo, Pfizer). The Saudi FDA also approved the latter. Vaccination should be administered as a single dose before the onset of the RSV season based on shared clinical decision-making.[60] Pending regulatory approvals, an mRNA-based RSV vaccine, mRNA-1345, encoding the stabilized RSV prefusion F glycoprotein, was investigated in phase 2-3 with 83.7% (66-92) against RSV-associated lower respiratory tract disease.[61]
Recommendations about prevention during the post-COVID era
Neonates are particularly susceptible to COVID-19, as 10% of coronavirus patients are pediatric patients, with 40% of them being <2 years old. As such, experts predict challenging times, with the potential emergence of another COVID-19 wave, in addition to delays in the release of the seasonal flu vaccine. Conversely, the SIBRO’s initiative to release clear RSV diagnosis and management guidelines has significantly reduced the number of pediatric cases of severe bronchiolitis as per anecdotal accounts from experts. These guidelines will help address potential issues from another COVID-19 wave.
The COVID-19 pandemic has highlighted the importance of epidemiological studies in the KSA. The KSA spared no expense to contain the pandemic and invested heavily in spreading awareness and enforcing precautionary protocols. This proactive approach can be applied to extremely low-birth-weight infants, where investment from the state can be a cost-effective measure to limit RSV spread. This investment can take the form of free RSV prophylaxis, which would help relieve the financial burden on the infant’s family. RSV mortality, which can be exacerbated by the pandemic and its ensuing health-care institution overload, is an issue in developing countries. The fear of going to healthcare facilities during the pandemic and misinterpreting the curfew rules made it challenging for vulnerable patients to access essential preventive healthcare services. Some of the recommendations to manage these challenges include increasing the number of RSV immunoprophylaxis program clinics, drive-thru visits, and home vaccinations and encouraging swift referrals to specialists in the RSV immunoprophylaxis program. These solutions are not without faults, as additional training would be required for health-care personnel to administer home vaccinations, and adding an RSV immunoprophylaxis program to the regular immunization schedule would add to the burden on the health-care system. The new liquid form used in the RSV immunoprophylaxis, and the mandated insurance coverage of high-risk infants hoped to improve compliance and reduce disparity, respectively. The inclusion of the RSV immunoprophylaxis doses into the regular immunization schedule can help mitigate RSV-related mortality; however, further examination of the benefits and feasibility of this recommendation is required. The short-term success of RSV immunoprophylaxis is threatened by the emergence of another COVID-19 wave, in addition to delays in the release of the seasonal flu vaccine. This presents an immense challenge to health-care experts. State investments in RSV epidemiological studies and free vaccinations can help alleviate the brunt of the pandemic.[27]
Conclusion
RSV is one of the most common causes of LRTI-related hospitalization, especially in healthy term infants. As there is still no effective antiviral treatment for RSV, prevention is the most effective strategy against RSV infections. An immunoprophylaxis strategy in the KSA for all infants during their first RSV season is strongly recommended to dramatically reduce the RSV burden and use of health-care resources, as well as the RSV-associated economic burden.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Joseph Domachowske, Professor of Pediatrics, SUNY Upstate Medical University, for his valuable suggestion and guide. Furthermore, we thank Enago for the editing service.
References
- 1.Alharbi AS, Alqwaiee M, Al Hindi MY, Mosalli R, Al Shamrani A, Alharbi S, et al. Bronchiolitis in children: The Saudi initiative of bronchiolitis diagnosis, management, and prevention (SIBRO) Ann Thorac Med. 2018;13:127–43. doi: 10.4103/atm.ATM_60_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group I-RS. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7. [PubMed] [Google Scholar]
- 3.Garegnani L, Styrmisdóttir L, Roson Rodriguez P, Escobar Liquitay CM, Esteban I, Franco JV. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11:CD013757. doi: 10.1002/14651858.CD013757.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1442–6. [PubMed] [Google Scholar]
- 5.Jones JM, Fleming Dutra KE, Prill MM, Roper LE, Brooks O, Sánchez PJ, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: Recommendations of the advisory committee on immunization practices – United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:920–5. doi: 10.15585/mmwr.mm7234a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domachowske JB, Bonville CA, Rosenberg HF. Animal models for studying respiratory syncytial virus infection and its long term effects on lung function. Pediatr Infect Dis J. 2004;23:S228–34. doi: 10.1097/01.inf.0000144672.81955.a4. [DOI] [PubMed] [Google Scholar]
- 7.Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One. 2017;12:e0175792. doi: 10.1371/journal.pone.0175792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umoren R, Odey F, Meremikwu MM. Steam inhalation or humidified oxygen for acute bronchiolitis in children up to three years of age. Cochrane Database Syst Rev. 2011;(Issue 1) doi: 10.1002/14651858.CD006435.pub2. Art. No.: CD006435. [DOI] [PubMed] [Google Scholar]
- 10.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–8. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 11.Bukhari EE, Elhazmi MM. Viral agents causing acute lower respiratory tract infections in hospitalized children at a tertiary care center in Saudi Arabia. Saudi Med J. 2013;34:1151–5. [PubMed] [Google Scholar]
- 12.Al Majhdi FN, Al Jaralla A, Elaeed M, Latif A, Gissmann L, Amer M. Prevalence of respiratory syncytial virus infection in Riyadh during the winter season 2007-2008 and different risk factors impact. Int J Virol. 2009;5:154–63. [Google Scholar]
- 13.Akhter JA, Al Johani S, Dugaishm F, Al Hefdi R, Al Hassan I. Etiology of respiratory viral infections using rapid virus isolation methods at a tertiary care center in Riyadh, Saudi Arabia. Saudi Pharm J. 2009;17:177–81. [Google Scholar]
- 14.Meqdam MM, Subaih SH. Rapid detection and clinical features of infants and young children with acute lower respiratory tract infection due to respiratory syncytial virus. FEMS Immunol Med Microbiol. 2006;47:129–33. doi: 10.1111/j.1574-695X.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- 15.Shier M. Respiratory syncytial virus infection in patients visiting King Khalid University Hospital. Menoufia Med J. 2005;18:11–6. [Google Scholar]
- 16.Al Muhsen SZ. Clinical profile of respiratory syncytial virus (RSV) bronchiolitis in the intensive care unit at a tertiary care hospital. Curr Pediatr Res. 2010;14:75–80. [Google Scholar]
- 17.Osman S, Alaa Adeen A, Hetta O, Alsiraihi A, Bader M, Aloufi A, et al. Epidemiology and risk factor analysis of children with bronchiolitis admitted to the intensive care unit at a tertiary care center in Saudi Arabia. Children (Basel) 2023;10:646. doi: 10.3390/children10040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockman LJ, Curns AT, Anderson LJ, Fischer Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 19.Alkharsah KR. The scope of respiratory syncytial virus infection in a tertiary hospital in the Eastern province of Saudi Arabia and the change in seasonal pattern during and after the COVID-19 pandemic. Medicina (Kaunas) 2022;58:1623. doi: 10.3390/medicina58111623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamp IA. Cincinnati children's hospital medical center. IRB. 2005;2013:5717. [Google Scholar]
- 21.Nicolai A, Ferrara M, Schiavariello C, Gentile F, Grande ME, Alessandroni C, et al. Viral bronchiolitis in children: A common condition with few therapeutic options. Early Hum Dev. 2013;89(Suppl 3):S7–11. doi: 10.1016/j.earlhumdev.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandini S, Corvaglia L, Alessandroni R, Aquilano G, Marsico C, Spinelli M, et al. Respiratory syncytial virus infection in infants and correlation with meteorological factors and air pollutants. Ital J Pediatr. 2013;39:1. doi: 10.1186/1824-7288-39-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegzyn C, Toh LK, Notario G, Biguenet S, Unnebrink K, Park C, et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: A systematic review. Infect Dis Ther. 2014;3:133–58. doi: 10.1007/s40121-014-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow E, Adetifa I, Chaiyakunapruk N, Cherian T, Fell DB, Graham BS, et al. WHO preferred product characteristics for monoclonal antibodies for passive immunization against respiratory syncytial virus (RSV) disease in infants –Key considerations for global use. Vaccine. 2022;40:3506–10. doi: 10.1016/j.vaccine.2022.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M, Zheng ZZ, Chen M, Modjarrad K, Zhang W, Zhan LT, et al. Discovery of a Prefusion Respiratory Syncytial Virus F-Specific Monoclonal Antibody That Provides Greater In Vivo Protection than the Murine Precursor of Palivizumab. J Virol. 2017;91:e00176–17. doi: 10.1128/JVI.00176-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Midgley CM, Haynes AK, Baumgardner JL, Chommanard C, Demas SW, Prill MM, et al. Determining the seasonality of respiratory syncytial virus in the United States: The impact of increased molecular testing. J Infect Dis. 2017;216:345–55. doi: 10.1093/infdis/jix275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alharbi AS, Alzahrani M, Alodayani AN, Alhindi MY, Alharbi S, Alnemri A. Saudi experts'recommendation for RSV prophylaxis in the era of COVID-19: Consensus from the Saudi Pediatric Pulmonology Association. Saudi Med J. 2021;42:355–62. doi: 10.15537/smj.2021.42.4.20200769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Harbi AS. War against respiratory syncytial virus. An 8-year experience at a tertiary hospital. Saudi Med J. 2018;39:1200–6. doi: 10.15537/smj.2018.12.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KM, Bloom HH, Mufson MA, Chanock RM. Natural reinfection of adults by respiratory syncytial virus. Possible relation to mild upper respiratory disease. N Engl J Med. 1962;267:68–72. doi: 10.1056/NEJM196207122670204. [DOI] [PubMed] [Google Scholar]
- 30.Barnett ED, Lynfield R, Sawyer MH American Academy of Pediatrics, American Academy of Pediatrics, Committee on Infectious Diseases. Red Book: 2021-2024 Report of the Committee on Infectious Diseases. 32nd ed. Washington DC: American Academy of Pediatrics; 2021. [Google Scholar]
- 31.Harris E. FDA approves RSV monoclonal antibody for infants and young children. JAMA. 2023;330:586. doi: 10.1001/jama.2023.13137. [DOI] [PubMed] [Google Scholar]
- 32.Triomphe T, Reic I, Mader S. European Commission Grants First Approval Worldwide of Beyfortus®(nirsevimab) for Prevention of RSV Disease in Infants. Paris: Sanofi; 2022. [Google Scholar]
- 33.Castelnuovo G, Pietrabissa G, Manzoni GM, Cattivelli R, Rossi A, Novelli M, et al. Cognitive behavioral therapy to aid weight loss in obese patients: Current perspectives. Psychol Res Behav Manag. 2017;10:165–73. doi: 10.2147/PRBM.S113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langley JM, LeBlanc JC, Wang EE, Law BJ, MacDonald NE, Mitchell I, et al. Nosocomial respiratory syncytial virus infection in Canadian pediatric hospitals: A pediatric investigators collaborative network on infections in Canada study. Pediatrics. 1997;100:943–6. doi: 10.1542/peds.100.6.943. [DOI] [PubMed] [Google Scholar]
- 35.Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, Jr., et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–40. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 36.Blanken MO, Rovers MM, Molenaar JM, Winkler Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–9. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 37.Alansari K, Sakran M, Davidson BL, Ibrahim K, Alrefai M, Zakaria I. Oral dexamethasone for bronchiolitis: A randomized trial. Pediatrics. 2013;132:e810–6. doi: 10.1542/peds.2012-3746. [DOI] [PubMed] [Google Scholar]
- 38.Project of the ABIM Foundation, the ACP-ASIM Foundation, the European Federation of Internal Medicine. Medical professionalism in the new millennium: A physicians'charter. Acta Clin Belg. 2002;57:169–71. [Google Scholar]
- 39.Romero JR. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: Results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22:S46–54. doi: 10.1097/01.inf.0000053885.34703.84. [DOI] [PubMed] [Google Scholar]
- 40.Al Alaiyan S, Pollack P, Notario GF. Safety and pharmacokinetics of extended use of palivizumab in Saudi Arabian infants and children. Drugs Context. 2015;4:212270. doi: 10.7573/dic.212270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Florin TA, Byczkowski T, Ruddy RM, Zorc JJ, Test M, Shah SS. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 American Academy of Pediatrics bronchiolitis guidelines. J Pediatr. 2014;165:786–92.e1. doi: 10.1016/j.jpeds.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacaze Masmonteil T, Rozé JC, Fauroux B French Pediatricians'Group of Sunagis Patients'Name-Based Programs. Incidence of respiratory syncytial virus-related hospitalizations in high-risk children: Follow-up of a national cohort of infants treated with palivizumab as RSV prophylaxis. Pediatr Pulmonol. 2002;34:181–8. doi: 10.1002/ppul.10175. [DOI] [PubMed] [Google Scholar]
- 43.Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC, et al. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) MMWR Recomm Rep. 2002;51:1–35. [PubMed] [Google Scholar]
- 44.Checchia PA, Nalysnyk L, Fernandes AW, Mahadevia PJ, Xu Y, Fahrbach K, et al. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: A systematic literature review and meta-analysis. Pediatr Crit Care Med. 2011;12:580–8. doi: 10.1097/PCC.0b013e3182070990. [DOI] [PubMed] [Google Scholar]
- 45.Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: A narrative review. Ann Intern Med. 2020;172:726–34. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kampmann B, Madhi SA, Munjal I, Simões EA, Pahud BA, Llapur C, et al. Bivalent Prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451–64. doi: 10.1056/NEJMoa2216480. [DOI] [PubMed] [Google Scholar]
- 47.Simões EA, Madhi SA, Muller WJ, Atanasova V, Bosheva M, Cabañas F, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: A pooled analysis of randomised controlled trials. Lancet Child Adolesc Health. 2023;7:180–9. doi: 10.1016/S2352-4642(22)00321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med. 2023;389:2425–35. doi: 10.1056/NEJMoa2309189. [DOI] [PubMed] [Google Scholar]
- 49.Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–46. doi: 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- 50.Kieffer A, Beuvelet M, Sardesai A, Musci R, Milev S, Roiz J, et al. Expected impact of universal immunization with nirsevimab against RSV-related outcomes and costs among all US infants in their first RSV season: A static model. J Infect Dis. 2022;226:S282–92. doi: 10.1093/infdis/jiac216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alharbi A, Yousef A, Zubani A, Alzahrani M, Al Hindi M, Alharbi S, et al. Respiratory syncytial virus (RSV) burden in infants in the Kingdom of Saudi Arabia and the impact of all-infant RSV protection: A modeling study. Adv Ther. 2024;41:1419–35. doi: 10.1007/s12325-024-02798-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruckwardt TJ. The road to approved vaccines for respiratory syncytial virus. NPJ Vaccines. 2023;8:138. doi: 10.1038/s41541-023-00734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mejias A, Rodríguez Fernández R, Oliva S, Peeples ME, Ramilo O. The journey to a respiratory syncytial virus vaccine. Ann Allergy Asthma Immunol. 2020;125:36–46. doi: 10.1016/j.anai.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCall MN, Chu CY, Wang L, Benoodt L, Thakar J, Corbett A, et al. Asystems genomics approach uncovers molecular associates of RSV severity. PLoS Comput Biol. 2021;17:e1009617. doi: 10.1371/journal.pcbi.1009617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tischer C, Kostenzer J, Mader S, Zimmermann LJI. Respiratory syncytial virus: Burden, risks, and the way forward-calling for a collaborative approach at World prematurity day 2022. Am J Physiol Lung Cell Mol Physiol. 2022;323:L619–22. doi: 10.1152/ajplung.00302.2022. [DOI] [PubMed] [Google Scholar]
- 56.Mazur NI, Terstappen J, Baral R, Bardají A, Beutels P, Buchholz UJ, et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:e2–21. doi: 10.1016/S1473-3099(22)00291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venkatesan P. First RSV vaccine approvals. Lancet Microbe. 2023;4:e577. doi: 10.1016/S2666-5247(23)00195-7. [DOI] [PubMed] [Google Scholar]
- 58.Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M, et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47:198. doi: 10.1186/s13052-021-01148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pointon L, Howe AS, Hobbs M, Paynter J, Gauld N, Turner N, et al. Evidence of suboptimal maternal vaccination coverage in pregnant New Zealand women and increasing inequity over time: A nationwide retrospective cohort study. Vaccine. 2022;40:2150–60. doi: 10.1016/j.vaccine.2022.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melgar M, Britton A, Roper LE, Talbot HK, Long SS, Kotton CN, et al. Use of respiratory syncytial virus vaccines in older adults: Recommendations of the advisory committee on immunization practices –United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:793–801. doi: 10.15585/mmwr.mm7229a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, et al. Efficacy and safety of a mRNA-based RSV pref vaccine in older adults. New England Journal of Medicine. 2023;389:2233–44. doi: 10.1056/NEJMoa2307079. [DOI] [PubMed] [Google Scholar]