Abstract
Introduction:
Prolonged cardiac monitoring after cryptogenic stroke or embolic stroke of undetermined source (ESUS) is necessary to identify atrial fibrillation (AF) that requires anticoagulation. Wearable devices may improve AF detection compared to conventional management. We aimed to review the evidence for the use of wearable devices in post-cryptogenic stroke and post-ESUS monitoring.
Methods:
We performed a systematic search of PubMed, EMBASE, Scopus and clinicaltrials.gov on 21 July 2022, identifying all studies that investigated the use of wearable devices in patients with cryptogenic stroke or ESUS. The outcomes of AF detection were analysed. Literature reports on electrocardiogram (ECG)-based (external wearable, handheld, patch, mobile cardiac telemetry [MCT], smartwatch) and photoplethysmography (PPG)-based (smartwatch, smartphone) devices were summarised.
Results:
A total of 27 relevant studies were included (two randomised controlled trials, seven prospective trials, 10 cohort studies, six case series and two case reports). Only four studies compared wearable technology to Holter monitoring or implantable loop recorder, and these studies showed no significant differences on meta-analysis (odds ratio 2.35, 95% confidence interval [CI] 0.74–7.48, I2 = 70%). External wearable devices detected AF in 20.7% (95% CI 14.9–27.2, I2 = 76%) of patients and MCT detected new AF in 9.6% (95% CI 7.4%–11.9%, I2 = 56%) of patients. Other devices investigated included patch sensors, handheld ECG recorders and PPG-based smartphone apps, which demonstrated feasibility in the post-cryptogenic stroke and post-ESUS setting.
Conclusion:
Wearable devices that are ECG or PPG based are effective for paroxysmal AF detection after cryptogenic stroke and ESUS, but further studies are needed to establish how they compare with Holter monitors and implantable loop recorder.
Keywords: Cardiac monitoring, cryptogenic stroke, embolic stroke of undetermined source, wearable devices
INTRODUCTION
Cryptogenic stroke is a subtype of ischaemic stroke with no determined cause or more than one competing cause, accounting for 30%–40% of ischaemic stroke.[1] Embolic stroke of undetermined source (ESUS) is a subgroup of non-lacunar cryptogenic stroke with no identified cause of stroke despite extensive investigation, including ≥24 h of cardiac monitoring, no occlusive large vessel atherosclerosis and high-risk cardioembolic sources.[2] One of the potential causes of ESUS or cryptogenic stroke is paroxysmal atrial fibrillation (AF), which requires management with anticoagulation; therefore, adequate cardiac monitoring is necessary. This is confirmed by large randomised controlled trials (RCT) such as the Cryptogenic Stroke and Underlying Atrial Fibrillation (CRYSTAL-AF) study, where 6 months of monitoring with implantable loop recorders (ILR) increased AF detection (hazard ratio 6.4) as compared to conventional follow-up.[3] The EMBRACE trial found that non-invasive electrocardiogram (ECG) monitoring with a wearable belt for 30 days increased AF detection with an absolute difference of 12.9% compared to 24-h Holter monitor,[4] suggesting that wearable devices are effective for cardiac monitoring after cryptogenic stroke.
Recent developments in cardiac monitoring on wearable and mobile devices have allowed prolonged ECG monitoring that is less invasive and more convenient.[5] Real-time transmission of data and automatic analysis using machine learning-based algorithms have further increased the timeliness of AF diagnosis.[6] Mobile health devices were associated with significant increases in AF detection compared to control groups in four RCTs on unselected patients with indications for cardiac monitoring.[7] With the US Food and Drug Administration (FDA) approval of wearable devices, including smartwatches, wearable belts, vests and patches for the detection of rhythm abnormalities, they may play an increasing role in cardiac monitoring after-cryptogenic stroke or ESUS. Therefore, we aimed to review the current literature on evidence for the use of wearable devices in post-cryptogenic stroke and post-ESUS monitoring.
METHODS
We performed a comprehensive literature search on PubMed, EMBASE, Scopus and clinicaltrials.gov from inception until 21 July 2022. The keywords and Medical Subject Headings searched were (wear* OR portable OR mobile OR smart*) AND (heart OR cardio* OR cardiac OR coron* OR arrhyth* OR atrial AND fibrillation) AND stroke [see Table S1, Supplemental Digital Appendix (614.5KB, tif) ]. Additional articles were identified on screening the references of included articles. The inclusion criteria were primary studies on adult patients with (a) cryptogenic stroke or ESUS and (b) wearable technology used to monitor the heart. The exclusion criteria were studies on unspecified ischaemic stroke and non-human studies. Invasive cardiac monitoring devices such as ILR were not considered wearable devices in this study, but studies using ILR in the control group were included (i.e., comparing wearables to ILR). Studies on routine Holter monitoring were excluded, but those on mobile cardiac telemetry (MCT) using mobile or smartphone technology, such as for real-time data transmission, were included. Reviews, letters and correspondences were also excluded. Titles and abstracts were screened by two researchers, and discrepancies were resolved by discussion. Full texts were screened, and data were extracted on a predesigned form. Data extracted included study type, demographics, type of wearable device, control population, duration of monitoring, AF detection and anticoagulation use. Diagnosis of AF was defined as continuous AF for >30 s on cardiac monitoring or >10 s on 12-lead ECG.[8]
Table S1.
Search strategy
| Database | Search term | Results |
| Pubmed | (wear* OR portable OR mobile OR smart*) AND (heart OR cardio* OR cardiac OR coron* OR arrhyth* OR atrial AND fibrillation) AND stroke | 413 |
| Scopus | TITLE-ABS-KEY (wear* OR portable OR mobile OR smart*) AND (heart OR cardio* OR cardiac OR coron* OR arrhyth* OR atrial AND fibrillation) AND stroke | 514 |
| Embase | (wear* OR portable OR mobile OR smart*) AND (heart OR cardio* OR cardiac OR coron* OR arrhyth* OR atrial AND fibrillation) AND stroke | 754 |
Risk of bias was assessed using the Joanna Briggs Institute appraisal check list for case reports and case series,[9] Newcastle–Ottawa Scale for cohort studies,[10] Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool for non-randomised trials[11] and Cochrane Risk of Bias 2 (RoB 2) tool for randomised controlled studies[12] [see Tables S2-S6, Supplemental Digital Appendix (614.5KB, tif) ].
Table S2.
Risk of bias assessment of randomized controlled trials using the Risk of Bias 2 tool (ROB 2)
| First author | Year | Risk of bias arising from the randomization process | Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | Risk of bias due to deviations from the intended interventions (effect of adhering to intervention) | Missing outcome data | Risk of bias in measurement of the outcome | Risk of bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Gladstone | 2014 | Low | Low | Moderate | Low | Low | Low | Low |
| Wouters | 2022 | Unclear | Moderate | Moderate | Moderate | Low | Low | Moderate |
Table S6.
Risk of bias assessment of case reports using the Joanna Briggs Institute (JBI) critical appraisal check list
| First author | Year | Were patient’s demographic characteristics clearly described? | Was the patient’s history clearly described and presented as a timeline? | Was the current clinical condition of the patient on presentation clearly described? | Were diagnostic tests or assessment methods and the results clearly described? | Was the intervention(s) or treatment procedure(s) clearly described? | Was the post- intervention clinical condition clearly described? | Were adverse events (harms) or unanticipated events identified and described? | Does the case report provide takeaway lessons? |
|---|---|---|---|---|---|---|---|---|---|
| Patel | 2021 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Wouters | 2022 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Table S3.
Risk of bias assessment of non-randomized trials using the ROBINS-I tool
| First author | Year | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|---|---|---|
| Lang | 2022 | Critical | Serious | Low | Low | Low | Serious | Moderate | Critical |
| Lumikari | 2020 | Moderate | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Magnusson | 2020 | Moderate | Moderate | Low | Moderate | Moderate | Moderate | Low | Moderate |
| Pagola | 2017 | Moderate | Moderate | Low | Moderate | Moderate | Moderate | Low | Moderate |
| Pagola | 2016 | Serious | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Sampaio | 2018 | Low | Moderate | Low | Low | Low | Moderate | Low | Moderate |
Table S4.
Risk of bias assessment of cohort studies using the Newcastle-Ottawa Scale
| First author | Year | Representativeness of the exposed cohort | Selection of the non- exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow- up long enough for outcomes to occur | Adequacy of follow up of cohorts |
|---|---|---|---|---|---|---|---|---|---|
| Khan | 2020 | * | * | * | * | * | * | * | |
| Martinez Tomas | 2021 | * | * | * | * | * | * | ||
| Motolese | 2021 | * | * | * | * | * | * | ||
| Pagola | 2021 | * | * | * | * | ** | * | * | * |
| Flint | 2012 | * | * | * | * | ** | * | * | * |
| Kalani | 2015 | * | * | * | * | ** | * | * | * |
| Kass-Hout | 2018 | * | * | * | * | ** | * | * | |
| Miller | 2013 | * | * | * | * | * | * | * | |
| Krathen | 2021 | * | * | * | * | * | * | * | |
| Matsumura | 2020 | * | * | * | * | * | * | * | * |
Table S5.
Risk of bias assessment of case series using the Joanna Briggs Institute (JBI) critical appraisal check list
| First author | Year | Were there clear criteria for inclusion in the case series? | Was the condition measured in a standard, reliable way for all participants included in the case series? | Were valid methods used for identification of the condition for all participants included in the case series? | Did the case series have consecutive inclusion of participants? | Did the case series have complete inclusion of participants? | Was there clear reporting of the demographics of the participants in the study? | Was there clear reporting of clinical information of the participants? | Were the outcomes or follow up results of cases clearly reported? | Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Was statistical analysis appropriate? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulkova | 2019 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Castrejon | 2022 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Finocchi | 2019 | Yes | Yes | Unclear | Yes | Yes | No | No | Yes | No | Yes |
| Kulach | 2019 | Yes | Unclear | Unclear | No | No | No | No | Yes | No | Yes |
| Lumikari | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pico | 2014 | Yes | Yes | Unclear | Yes | Yes | No | Yes | Yes | No | Yes |
| Tayal | 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Meta-analysis of AF detection using wearable technology was performed on R (version 4.0.0; R Core Team, Vienna, Austria) using DerSimonian and Laird random-effects method. Heterogeneity was assessed using the I2 statistic, and I2%–<25%, 25%–50% and >50% was considered low, moderate and high heterogeneity, respectively. Subgroup analysis based on the type of wearable technology used was performed.
RESULTS
A total of 27 studies on 6489 patients with cryptogenic stroke or ESUS were included. This included two RCTs,[4,13] six prospective trials,[6,14,15,16,17,18] ten cohort studies (six prospective,[19,20,21,22,23,24] four retrospective[25,26,27,28]), seven case series[29,30,31,32,33,34,35] and two case reports[36,37] [see Figure S1 (747.6KB, tif) and Graphical Abstract, Supplemental Digital Appendix (614.5KB, tif) ] Reference graphical abstract. The characteristics of these studies are presented in Table 1. Devices were grouped broadly into ECG-based devices that monitor and transmit ECG trace, such as wearable vests, belts, patches, handheld recorders, smartwatches and MCT, and photoplethysmography (PPG)-based devices such as smartphone apps and smartwatches [Table 2]. The duration of monitoring ranged from 7 to 180 days.
Table 1.
Characteristics of the included studies.
| Author, year, country | Study type | n | Age, years | Male | CHADsVasc | Population | Type of wearable | Comparison | Outcome | Duration of monitoring |
|---|---|---|---|---|---|---|---|---|---|---|
| Bulkova, 2019 | Case series | 3120 | 65±17 | 54% | NR | ESUS within 3 weeks | Continuous ECG recorder (Vitaphone, Faros, ECGPocket) for 3 weeks | None | AF, AVB II–II, NSVT, sinus pauses, SVT | 21.6±5.3 days |
|
| ||||||||||
| Castrejon, 2022 | Case series | 130 | 73±12 | 47% | 3.1±1.7 | Cryptogenic stroke | Nuubo external wearable two-lead ECG monitoring system for 30 days | None | AF, stroke recurrences, SVT, AVB II, VE, NSVT | 28±3 days |
|
| ||||||||||
| Finocchi, 2019 | Case series | 15 | NR | NR | 3±1 | ESUS | Nuubo 30-day ECG monitoring | None | AF | 30 days |
|
| ||||||||||
| Flint, 2012, USA | Prospective cohort | 239 | 64.6±13.8 | 61% | NR | Cryptogenic ischaemic stroke | 30-day CardioPAL SAVI | None | PAF >30 s | 24.5±8.7 days |
|
| ||||||||||
| Gladstone, 2014, Canada | RCT | 572 | 72.5±8.5 | 54% | 3 (range 2–6) | Cryptogenic ischaemic stroke or TIA within 6 months (≥55 years) | 30-day event-triggered recorder on wearable belt | Routine 24-h Holter | AF | 30 days |
|
| ||||||||||
| Kalani, 2015, USA | Retrospective cohort | 85 | 65.6±14.7 | 51% | 4 (IQR: 3–6) | Cryptogenic ischaemic stroke | 14–30 days Lifestar ACT ambulatory cardiac telemetry, Lifestar AF Express autodetect looping monitor and Cardiomedix cardiac event monitor | None | AF >30 s | 30 days median |
|
| ||||||||||
| Kass-Hout, 2018, USA | Retrospective cohort | 132 | 70±14.4 | 50% | NR | Cryptogenic ischaemic stroke or TIA | Mobile cardiac outpatient telemetry | None | AF >30s | Median 29 days |
|
| ||||||||||
| Khan, 2020, USA | Prospective cohort | 467 | 65.9±13 | 48% | NR | Cryptogenic stroke, TIA or syncope with unknown aetiology | 14 day ECG monitoring patch (Zio® Patch) | None | AF, VT | 12.1 days |
|
| ||||||||||
| Krathen, 2021 | Prospective cohort | 15 | 62±12.7 | 40% | NR | Cryptogenic stroke referred for ILR | MCOT | ILR | AF>6 min | 6±1.5 months |
|
| ||||||||||
| Kulach, 2019, Poland | Case series | 40 | 59±9 | 68% | NR | Cryptogenic stroke | 7-day Holter monitoring and handheld ECG recorder (CheckMe Pro) for ambulatory 30-day single-lead ECG recording | None | AF, SVT | 30±3 days |
|
| ||||||||||
| Lang, 2022, UK | Prospective trial | 218 | NR | NR | NR | Cryptogenic stroke and TIA | 14-day ECG monitoring patch (Zio® Patch) | Routine 24-h Holter | Median time to the patient having the monitor fitted | 14 days |
|
| ||||||||||
| Lumikari, 2019, Finland | Case series | 57 | 64.5±8.2 | 53% | 3.5±1.2 | Aged ≥50 with recent ESUS | One-lead ECG device capable to record continuous ECG for up to 4 weeks (Bittium Faros 180° device) | None | AF | 28 days (IQR: 26–30) |
|
| ||||||||||
| Lumikari, 2020, Finland | Prospective trial | 15 | 59.5±7.4 | 67% | NR | Aged ≥50 with recent ESUS | External electrode belt-based, one-lead ECG device (Beat2Phone) continuously for 2 weeks | 24-h or 48-h Holter | PAF, new TIA | 3 months |
|
| ||||||||||
| Magnusson, 2020, Sweden | Prospective trial | 100 | 67.6±10.8 | 60% | 4.4±1.9 | Ischaemic cryptogenic stroke | Chest and thumb ECG system Coala Heart Monitor for 28 days | None | AF or atrial flutter | 28 days |
|
| ||||||||||
| Martinez Tomas, 2021, Spain | Prospective cohort | 104 | 67 median | 48% | NR | ESUS | Nuubo™ and Nuubo Leonardo reading software for 21 days | None | AF | 21 days |
|
| ||||||||||
| Matsumura, 2020 | Retrospective cohort | 196 | 67 (range 24–96) | 50% | 5.5 | Cryptogenic stroke or TIA and negative inpatient telemetry | Wearable ambulatory ECG monitor for 14 days | None | AF ≥30 s of a single AF episode, or multiple episodes of AF of <30 s | 14 days |
|
| ||||||||||
| Miller, 2013, USA | Retrospective cohort | 156 | 68.5±11 | 50% | NR | Cryptogenic stroke or TIA within 6 months | CardioNet® MCOT | None | PAF | Median 21 days (range: 1–30) |
|
| ||||||||||
| Motolese, 2021 | Prospective cohort | 54 | 68.5 (IQR: 53.5–73) | 39% | NR | ESUS TIA or mild ischaemic stroke (NIHSS<3) | Smartwatch capable of reliably detecting AF through a single-lead ECG | None | AF, recurrent stroke | |
|
| ||||||||||
| Pagola, 2016, Spain | Prospective trial | 80 | NR | NR | NR | Cryptogenic stroke | Holter wearable (CryptoAF) device for 4 weeks | 72-h ECG telemetry + 1 week of ECG external registrator | AF | 28 days |
|
| ||||||||||
| Pagola, 2017, Spain | Prospective trial | 146 | 76 (IQR: 68–83) | 61% | 5 (IQR: 4–6) | Cryptogenic stroke | Textile wearable Holter with garment and recorder for 28 days | None | AF | 28 days |
|
| ||||||||||
| Pagola, 2021, Spain | Prospective cohort | 254 | NR | NR | NR | Cryptogenic stroke | Nuubo 90-day ECG monitoring | None | AF | 90 days |
|
| ||||||||||
| Patel, 2021, USA | Case report | 1 | 70 | 0 | NR | ESUS | Apple smartwatch | None | AF | NR |
|
| ||||||||||
| Pico, 2014, France | Case series | 171 | NR | NR | NR | Cryptogenic stroke or TIA | 21 days MCOT | None | AF | 21 days |
|
| ||||||||||
| Sampaio, 2018, Brazil | Prospective trial | 26 | 70.7±10.5 | 52% | 3.3±1.2 | Cryptogenic stroke within 15 days | 7-day new ambulatory monitoring system with mobile data transmission (PoIP) | Routine 24-h Holter | AF, AT, VT, VE | 148.8±20.8 h |
|
| ||||||||||
| Tayal, 2008, USA | Case series | 56 | 66±11 | 52% | NsR | Cryptogenic TIA/stroke | CardioNet® up to 21 days | None | AF | 21 (IQR: 5–21) days |
|
| ||||||||||
| Wouters, 2022, Belgium | Case report | 1 | 59 | 100% | NR | Cryptogenic stroke | Smartphone monitoring based on PPG, two 1-min spot checks using FibriCheck® | ICM | AF | NR |
|
| ||||||||||
| Wouters, 2022, Belgium | RCT | 39 | 63.0±12.6 | 69% | 4 (IQR: 3–5) | Cryptogenic stroke and TIA | Smartphone monitoring based on PPG, two 1-min spot checks using FibriCheck® for 180 days | Smartwatch/ILR | AF, pause, tachyarrhythmia, AT | 180 days |
AF: atrial fibrillation, AT: atrial tachycardia, AVB: atrioventricular block, ECG: electrocardiogram, ESUS: embolic stroke with undetermined source, ILR: implantable loop recorder, IQR: interquartile range, NIHSS: National Institutes of Health Stroke Scale, NR: not reported, NSVT: non-sustained ventricular tachycardia, PAF: paroxysmal atrial fibrillation, PPG: photoplethysmography, RCT: randomised controlled trial, SVT: supraventricular tachycardia, TIA: transient ischaemic attack, VE: ventricular ectopics, VT: ventricular tachycardia, MCOT: Mobile Cardiac Outpatient Telemetry, CHADsVasc: congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category score, ICM: implantable cardiac mobitor
Table 2.
Details of wearable devices used in post-cryptogenic stroke monitoring.
| Device name | Type | Description | Approval | Studies |
|---|---|---|---|---|
| Nuubo System, Smart Solutions Technologies, Spain | External wearable | Two-lead ECG monitoring using textile electrode vest, wearable for up to 30 days. Optional inclusion of automatic AF and event detection algorithm | FDA approval, CE certification | Castrejon, 2022; Finocchi, 2019; Martinez Tomas, 2021, Spain; Pagola, 2021, Spain |
|
| ||||
| ER910AF Cardiac Event Monitor, Braemar, USA and Cardiac BioSystems | External wearable | Three-channel event-triggered recorder, worn on the chest with dry electrode non-adhesive belt | FDA approval, CE certification | Gladstone, 2014, Canada |
|
| ||||
| Beat2Phone, VitalSignum Oy, Finland | External wearable belt | ECG sensor attached to chest with flexible belt, mobile application and cloud service | Lumikari, 2020, Finland | |
|
| ||||
| CheckMe Pro, Viatom Technology Co., China | Handheld | Handheld recorder, cable-free recording of one-lead ECG | FDA approval, CE certification | Kulach, 2019, Poland |
|
| ||||
| Coala Heart Monitor, Coala Life, Sweden | Handheld | Smartphone powered, two-lead chest and thumb ECG system and stethoscope, algorithm-based real-time remote analysis | FDA approval, CE certification | Magnusson, 2018, Sweden |
|
| ||||
| Vitaphone, Vitaphone GmbH, Germany | MCT | One- or three-lead ECG monitoring with wireless transmission | FDA approval, CE certification | Bulkova, 2019 |
|
| ||||
| PocketECG, Medicalalgorithmics, Poland | MCT | Three-lead ECG monitoring with continuous online streaming of ECG for analysis | FDA approval, CE certification | Bulkova, 2019 |
|
| ||||
| Bittium Faros, Bittium Corporation, Finland | MCT | One- or three-lead ECG monitoring using ultra-small lightweight recorder | FDA approval, CE certification | Bulkova, 2019; Lumikari, 2019, Finland |
|
| ||||
| Policardiógrafo IP®, PoIP, Brazil | MCT | Ambulatory monitoring system with mobile data transmission | Sampaio, 2018, Brazil | |
|
| ||||
| CardioPAL SAVI, Medicomp, Inc, USA | MCT | 30-day auto-triggering event monitor, analysis via algorithm | FDA approval, CE certification | Flint, 2012, USA |
|
| ||||
| Lifestar ACT ambulatory cardiac telemetry, Lifestar AF Express autodetect looping monitor, Lifewatch, Switzerland | MCT | One-lead automatic event-triggered recorder up to 30 days, automatic data transmission via smartphone | FDA approval | Kalani, 2015, USA |
|
| ||||
| Cardiomedix cardiac event monitor, Cardiomedix, USA | MCT | Digital loop recorder with detection algorithms for automatic rhythm detection, automatic transmission wirelessly or transtelephonically | FDA approval | Kalani, 2015, USA |
|
| ||||
| CardioNet®, CardioNet, USA | MCT | Continuous ambulatory ECG monitoring, data sent via wireless transmission to smartphone, real-time transmission to CardioNet laboratory for analysis | FDA approval, CE certification | Miller, 2013, USA; Tayal, 2008, USA |
|
| ||||
| Zio® Patch, iRhythm Technologies, USA | Patch | Single-use patch monitor for up to 14 days, analysed by machine learning algorithm | FDA approval, CE certification | Lang, 2022, UK; Khan, 2020, USA |
|
| ||||
| FibriCheck®, Belgium | PPG | Smartphone application that utilises PPG to detect signs of AF using phone camera | FDA approval, CE certification | Wouters, 2022, Belgium |
|
| ||||
| Apple Watch | Smartwatch | Automatic algorithm to detect irregularities in pulse using optical sensor PPG | FDA approval, CE certification | Patel, 2021, USA |
AF: atrial fibrillation, CE: Conformité Européene, ECG: electrocardiogram, FDA: US Food and Drug Administration, MCT: mobile cardiac telemetry, PPG: photoplethysmography
AF detection and anticoagulation use
We identified four studies (one RCT, two prospective trials, one prospective cohort study) that compared AF detection on wearable technology versus routine Holter monitoring or ILR. In the meta-analysis of 354 patients in the wearables group and 365 in the Holter monitoring/ILR group, wearable technology was not significantly associated with increased AF detection (odds ratio [OR] 2.35, 95% confidence interval [CI] 0.74–7.48), with significant heterogeneity (I2 = 70%, P = 0.02) [Figure 1a]. Three studies with 689 patients in total compared wearable belt, vest and MCT with mobile data transmission versus 24–72 h Holter monitoring,[4,17,18] and subgroup analysis of these studies resulted in an OR of 3.20 (95% CI 0.91–11.28) with significant heterogeneity (I2 = 73%, P = 0.03) [Figure 1b].
Figure 1.
Forest plots of atrial fibrillation (AF) detection on wearable devices versus (a) Holter monitor or implantable loop recorder (ILR) and (b) Holter monitor only. CI: confidence interval, MH: Mantel-Haenszel
Most patients with newly detected AF were started on anticoagulation, resulting in a change in clinical management. In the RCT by Gladstone et al.[4] comparing 30-day event-triggered recorder on wearable belt and 24-h Holter monitoring, wearable use led to greater anticoagulation use (18.6% vs. 11.1%, P = 0.01) and switch from antiplatelet therapy to anticoagulation (13.6% vs. 4.7%, P < 0.001).
External wearable ECG-based device
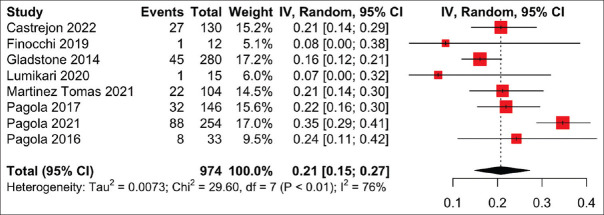
External wearable devices include wearable vests and belts embedded with electrodes. In a meta-analysis of eight studies on 974 patients monitored with external wearable devices, AF was detected in 20.7% (95% CI 14.9%–27.2%) of patients, with significant heterogeneity (I2 = 76%, P < 0.01) [Figure 2]. Five studies used the Nuubo wearable vest,[16,20,22,30,31] two studies used wearable belts (one of which was Beat2Phone)[4,14] and one study did not specify the type of external wearable device.[17] The duration of monitoring ranged from 21 to 90 days.
Figure 2.
Forest plot of the proportion of atrial fibrillation (AF) detection on external wearable devices. CI: confidence interval, IV: inverse variance
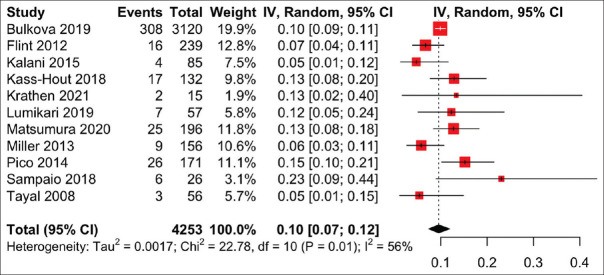
Mobile cardiac telemetry
Mobile cardiac telemetry with real-time continuous ECG monitoring via a small portable sensor is increasingly being used clinically, and 11 articles investigated its use in patients with cryptogenic stroke or ESUS. Overall, meta-analysis showed that MCT detected new AF in 9.6% (95% CI 7.4%–11.9%) of patients, with significant heterogeneity (I2 = 56%, P = 0.01) [Figure 3]. The MCT systems studied included Vitaphone Vitaphone (Vitaphone GmbH, Mannheim, Germany), PocketECG (Medicalalgorithmics, Warsaw, Poland), Bittium Faros (Bittium, Oulu, Finland), PoIP (eMaster, Minas Gerais, Brazil), CardioPAL SAVI (Medicomp, Washington, DC, USA), Lifestar ACT and Lifestar AF Express (Lifewatch, Zug, Switzerland), Cardiomedix (Cardiomedix, Evanston, IL, USA) and CardioNet (CardioNet, Malvern, PA, USA), which are all FDA-approved devices. The monitoring time ranged from 7 to 30 days.
Figure 3.
Forest plot of the proportion of atrial fibrillation (AF) detection on mobile cardiac telemetry. CI: confidence interval, IV: inverse variance
Patch ECG-based monitoring
Two prospective studies investigated the use of Zio® Patch (iRhythm Technologies, San Francisco, CA, USA) to detect AF post-cryptogenic stroke. Zio® Patch is a single-use, 2-by-5 inch adhesive monitor without visible electrodes that is worn on the chest and used for up to 14 days. Data from the patch are analysed automatically using deep learning algorithms, and it is FDA approved and Conformité Européene (CE) marked. Lang et al.[6] reported that the median time to having the monitor fitted was lower in the Zio® Patch group than in the 24-h Holter group. In a cohort of 467 patients with cryptogenic stroke, transient ischaemic attack (TIA) or syncope with unknown aetiology from the stroke clinic, 3.9% had at least one episode of paroxysmal AF sustained for more than 30 s and ventricular tachycardia occurred in 13.3% of patients.[19]
Handheld ECG-based device
ECG monitoring is also performed using small handheld devices such as the Coala Heart Monitor (Coala Life, Uppsala, Sweden Viatom Technology, Guangdong, China), which are both FDA approved and CE marked. Kulach et al.[32] found that in a case series of 40 patients with cryptogenic stroke, 7-day Holter detected AF in 12.5% of patients, but 30-day patient-activated handheld ECG recording did not diagnose any additional AF cases. In the prospective study by Magnusson et al,[38] the Coala Heart Monitor, which records ECG from the chest and thumb, detected AF in 9% of patients after 28 days.[38]
Smartwatch
Automatic monitoring of AF with smartwatches was investigated in a prospective cohort study by Motolese et al.,[21] where patients were instructed to use the ECG app to record at least two ECGs per day. The study reported that AF was detected in six out of 54 (11.3%) patients with ESUS TIA or mild ischaemic stroke (National Institutes of Health Stroke Score <3). Patel and Tarakji reported on a patient with ESUS and no AF detected on 30-day ambulatory cardiac monitor, who was monitored with Apple Watch. The patient had more than one episode of paroxysmal AF on recordings of excellent quality; therefore, no further monitoring was necessary to diagnose AF and start anticoagulation, thus avoiding the need for ILR.[36]
Photoplethysmography-based devices
Photoplethysmography-based rhythm monitoring is broadly divided into wearable devices such as smartwatches and handheld devices such as mobile apps that use smartphone cameras as the emission light point for PPG. The two FDA-approved handheld PPG-based technologies are Fibricheck (Fibricheck, Hasselt, Belgium) and Preventicus Heartbeats (Preventicus, Jena, Germany). Wouters et al.[13] performed an RCT comparing smartphone (Fibricheck, Belgium) and smartwatch PPG monitoring in 39 cryptogenic stroke patients and found that insufficient signal quality was more common in smartwatch recordings than smartphone recordings. However the, smartwatch resulted in significantly more 12-h periods, with at least one recording of sufficient quality. One case of AF was identified on ILR, the gold standard for AF monitoring, which was also confirmed on smartphone monitoring. In the case report of the AF patient in this RCT,[37] a head-to-head comparison of AF detection on smartwatch and ILR showed similar time until AF detection and AF burden.
DISCUSSION
Cardiac monitoring post-cryptogenic stroke or ESUS has been feasibly performed using wearable devices such as wearable vests, belts, patches, MCT, and PPG-based smartphone and smartwatch technology. Our meta-analysis did not demonstrate a definitive overall increase in AF detection with wearable devices compared to Holter monitoring and ILR, and further RCTs are needed in the setting of cryptogenic stroke and ESUS.
Wearable devices with automatic analysis using algorithms have generally good accuracy for rhythm detection. A systematic review of 208 studies on AF detection using mobile health solutions reported the sensitivity and specificity for ECG-based devices as follows: patches: 93.4%–97.0% and 95.6%–98.5%; belts: 96.3% and 98.2%; and handheld devices: 94.0%–98.0% and 76.0%–95.0%, respectively.[39] In a meta-analysis of 28 studies comparing smartphone PPG versus ECG for AF detection, the sensitivity (94%, 95% CI 92–95) and specificity (97%, 95% CI 96–98) for AF detection were found to be high.[40] However, the studies were of low quality and had high risk of selection and publication bias, with high inter-study heterogeneity. In the post-ischaemic stroke setting, the sensitivity and specificity of KardiaMobile (AliveCor, Mountain View, CA, USA) for AF detection were 100% and 98.3%, respectively.[41] Further setting-specific investigation of AF detection accuracy is needed, especially in patients with cryptogenic stroke or ESUS who are at high risk of paroxysmal AF and recurrent stroke.[2]
The current consensus for diagnosis of AF is >30 s on cardiac monitoring or 10 s on 12-lead ECG, and unless contraindicated, these patients should be anticoagulated to reduce the risk of further ischaemic strokes.[8] Despite the lack of clear guidelines on the duration, timing and use of device for cardiac monitoring post-cryptogenic stroke, there is strong evidence that prolonged cardiac monitoring increases detection of subclinical AF and ILR is considered the gold standard.[42] In a meta-analysis of 47 studies, increasing the duration of monitoring on ILR also increased AF detection from 12.2% at 3 months to 28.5% at 36 months.[43] We found that on monitoring of 21–90 days using external wearable devices, 21% had new AF detected, and 7–30 days of MCT revealed AF in 10% of cryptogenic stroke and ESUS patients. This is consistent with the data on ILR, further supporting the feasibility of wearable technology in cryptogenic stroke.
Although our meta-analysis did not show a statistically significant difference in AF detection in cryptogenic stroke between wearable devices in conventional Holter monitoring or ILR, other RCTs in the general stroke setting have shown promising results. In the EPACS open-label RCT, Zio® patch was associated with higher incidence of AF at 90 days in patients with ischaemic stroke or TIA in the past 72 h compared to 24-h Holter monitoring.[44] A multicentre, open-label RCT on patients aged ≥55 years with ischaemic stroke or TIA in the past 12 months found that 30-day smartphone ECG monitoring with KardiaMobile (AliveCor) showed an absolute difference of 7.5% in AF detection (P = 0.024) compared to 24-h Holter monitoring.[45] The lack of significant finding in our meta-analysis may be due to the paucity of studies and the overall small sample sizes of the included studies (thus they may be underpowered to detect any differences in AF detection). Ongoing RCTs will provide further evidence for the use of wearable technology after cryptogenic stroke. For example, the CANDLE-AF study, a multicentre, prospective RCT, aims to compare an adhesive single-lead ECG patch (mobiCARE-MC100; Seers Technology, Gyeonggi-do, Republic of Korea) with a single-lead handheld event recorder (KardiaMobile system; AliveCor) and Holter monitoring (cris.nih.go.kr KCT0005592).[46] The MOBILE-AF international multicentre RCT tests the use of KardiaMobile device and app (AliveCor`) against 7-day Holter monitor in cryptogenic stroke (NCT02507986).[47] While the preliminary results from the REMOTE trial suggested that PPG-based smartphone monitoring may be feasible,[13] the final results are awaited with a planned completion date in August 2023 (NCT05006105). The results of these RCTs would be important in informing the efficacy and usability of wearable devices for cardiac monitoring post-cryptogenic stroke.
Overall, wearable technologies have good usability and patient acceptability in stroke patients. Regarding the Zio® patch, >80% of cryptogenic stroke and TIA patients found it easy to use, and comfortable to wear, were able to perform normal activity and would wear the patch again.[6] The usability of Beat2Phone assessed with Systems Usability Scale with Likert rating scale revealed an average score of 81.4 out of 100.[14] However, a few patients reported challenges with device charging and use of mobile phone application.[14] The Pulsewatch study (NCT03761394) compared a smartwatch–smartphone app (Samsung/Android) to a patch monitor (Cardea SOLO™ ECG System) among older post-ischaemic stroke adults aged >50 years.[5] These patients preferred the smartwatch over the patch monitor and traditional cardiac monitoring, but patients who required assistive device (e.g., wheelchair) and those with history of anxiety or depression were more likely to report anxiety with smartwatch use.[5] Usability may be improved with in-person training, simpler device interface and longer battery life, which should be considered in future device development and clinical implementation. Wearable technology adoption has been increasing year on year, and is estimated to reach more than 1 billion users in 2022,[48] which may increase the acceptability and cost-effectiveness of wearable devices for cardiac monitoring of patients.
The cost-effectiveness of using mobile health in AF detection has been studied in the population screening and post-stroke setting. In six European countries, screening for AF using Preventicus Heartbeats, a handheld PPG-based tool, is modelled to lower the cost per case in countries with relatively high healthcare costs, for example, Switzerland (€75) and UK (€7), but increase the costs in countries with moderate or low healthcare costs, for example, Poland (€20) and Greece (€6).[49] Handheld ECG-based screening with MyDiagnostick® (MyDiagnostick Medical, Maastricht, Netherlands) in all patients >65 years of age who attended seasonal influenza vaccination in the Netherlands was modelled to decrease the cost by €764 and increase quality-adjusted life-years (QALY) by 0.27 years per patient.[50] Monitoring of post-stroke patients using smartphone-based handheld ECG devices during hospital stay was associated with marginally higher costs but greater QALY due to higher cost of the device and anticoagulants, with an incremental cost-effectiveness ratio of AUD 3103/QALY over 20 years.[51] This nurse-led, handheld ECG monitoring is recommended in post-stroke AF monitoring by the 2021 Asia Pacific Heart Rhythm Society guidelines.[52] The EPACS RCT reported that the use of Zio® patch in patients with ischaemic stroke and TIA would avoid 10.8 more strokes per year compared to Holter monitoring, and this is associated with a yearly saving of £113,630–£162,491 over 5 years. In cryptogenic stroke, initial 30-day MCT monitoring followed by ILR compared to ILR only was associated with significant cost savings of USD 4083 per 1000 patients, and the cost per AF patient was significantly lower with MCT (USD 29,598 vs. USD 228,507).[53] Therefore, wearable devices are generally cost-effective, but this needs to be tested in different healthcare settings and health systems.
There are several limitations to this study. Our meta-analysis of AF detection on wearable devices versus Holter monitor or ILR consisted of very few studies, and there was high heterogeneity, limiting the reliability of the pooled estimate. This may be due to the varying types of wearable device and duration of monitoring, and differences in cryptogenic stroke population. There were only two RCTs in the systematic review, and non-randomised studies are susceptible to confounding factors. All the studies were not blinded due to the nature of the intervention, thus increasing the risks of observation, reporting and performance bias. The long-term outcomes of monitoring with wearable devices versus routine clinical care after cryptogenic stroke are unknown and require further study. Furthermore, many wearable devices require manual triggering of recording on their devices, which requires symptomatic presentation of AF and may miss silent AF, an important cause of cryptogenic stroke or ESUS. This would also rely on patients regularly checking their heart rhythm multiple times daily, which may not be feasible or realistic in many patient populations. Development of future devices should focus on automatic AF detection on baseline ECG or PPG, without requiring manual input. Lastly, there is a lack of data published on the comparison of wearable devices with gold standard ILR or multi-day Holter monitoring; therefore, we were unable to estimate the false-positive or false-negative rates reliably. At present, there is also no evidence to suggest that AF detected by wearable devices results in increase in recurrent stroke, and RCTs such as the LOOP study showed that increased AF detection and anticoagulation rates on ILR did not translate to a reduction in the risk of stroke and systemic embolism.[54] Therefore, the significance of AF detected on wearable devices requires further investigation.
In conclusion, wearable devices that are ECG or PPG based have comparable rates of paroxysmal AF detection after cryptogenic stroke and ESUS to conventional Holter monitors and ILR, but further investigations on the reliability and significance of AF detected on wearable devices are needed. There is a range for devices currently approved by FDA and CE marked for use on patients, and ongoing clinical trials would provide further evidence on their efficacy, safety and acceptability to patients.
Financial support and sponsorship
Sia CH was supported by the National University of Singapore Yong Loo Lin School of Medicine’s Junior Academic Fellowship Scheme.
Conflicts of interest
Tan BYQ, Poh KK and Sia CH are members of the SMJ Editorial Board, and were thus not involved in the peer review and publication decisions of this article.
Supplemental digital content
Appendix at http://links.lww.com/SGMJ/A92
Digital abstract
PRISMA flow diagram
REFERENCES
- 1.Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic stroke. Circ Res. 2017;120:527–40. doi: 10.1161/CIRCRESAHA.116.308447. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source. Stroke. 2017;48:867–72. doi: 10.1161/STROKEAHA.116.016414. [DOI] [PubMed] [Google Scholar]
- 3.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 5.Ding EY, CastañedaAvila M, Tran KV, Mehawej J, Filippaios A, Paul T, et al. Usability of a smartwatch for atrial fibrillation detection in older adults after stroke. Cardiovasc Digit Health J. 2022;3:126–35. doi: 10.1016/j.cvdhj.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang A, Basyal C, Benger M, Bhalla A, Edwards F, Farag M, et al. Improving stroke pathways using an adhesive ambulatory ECG patch: Reducing time for patients to ECGs and subsequent results. Future Healthc J. 2022;9:64–6. doi: 10.7861/fhj.2021-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biersteker TE, Schalij MJ, Treskes RW. Impact of mobile health devices for the detection of atrial fibrillation: Systematic review. JMIR Mhealth Uhealth. 2021;9:e26161. doi: 10.2196/26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-Up: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS);in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and Approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Heart Rhythm. 2007;4:816–61. [Google Scholar]
- 9.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123–8. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [[Last accessed on 29 Oct 2022]]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 11.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Wouters F, Gruwez H, Vranken J, Vanhaen D, Daelman B, Ernon L, et al. The potential and limitations of mobile health and insertable cardiac monitors in the detection of atrial fibrillation in cryptogenic stroke patients: Preliminary results from the REMOTE trial. Front Cardiovasc Med. 2022;9:848914. doi: 10.3389/fcvm.2022.848914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumikari TJ, Pirinen J, Putaala J, Sibolt G, Kerola A, Pakarinen S, et al. Prolonged ECG with a novel recorder utilizing electrode belt and mobile device in patients with recent embolic stroke of undetermined source: A pilot study. Ann Noninvasive Electrocardiol. 2020;25:e12802. doi: 10.1111/anec.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnusson P, Koyi H, Mattsson G. A protocol for a prospective observational study using chest and thumb ECG: Transient ECG assessment in stroke evaluation (TEASE) in Sweden. BMJ Open. 2018;8:e019933. doi: 10.1136/bmjopen-2017-019933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagola J, Juega J, Francisco-Pascual J, Moya A, Sanchis M, Bustamante A, et al. Yield of atrial fibrillation detection with textile wearable holter from the acute phase of stroke: Pilot study of crypto-AF registry. Int J Cardiol. 2018;251:45–50. doi: 10.1016/j.ijcard.2017.10.063. [DOI] [PubMed] [Google Scholar]
- 17.Pagola J, Pascual F, Juega J, Bustamante A, Muchada M, Boned S, et al. The role of holter wearable in atrial fibrillation detection in cryptogenic strokes, [in English. Eur Stroke J. 2016;1((Suppl 1)):17–8. [Google Scholar]
- 18.Sampaio RF, Gomes IC, Sternick EB. Cryptogenic acute ischemic stroke: Assessment of the performance of a new continuous long-term monitoring system in the detection of atrial fibrillation. Arq Bras Cardiol. 2018;111:122–31. doi: 10.5935/abc.20180112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A, Abedi V, Ishaq F, Sadighi A, Adibuzzaman M, Matsumura M, et al. Fast-track long term continuous heart monitoring in a stroke clinic: A feasibility study. Front Neurol. 2020;10:1400. doi: 10.3389/fneur.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez Tomas C, Rodriguez Lavado I, Munoz Ruiz T, Toledo T, Bustamante Toledo R, Serrano Castro P. Prolonged ECG monitoring for 21 days in ESUS. Is it enough time?, [in English] Eur J Neurol. 2021;28((Suppl 1)):674. [Google Scholar]
- 21.Motolese F, Capone F, Pilato F, Magliozzi A, Vico C, Di Lazzaro V. The potential value of new generation smartphone electrocardiogram for detecting atrial fibrillation after an ischemic stroke. J Neurol Sci. 2021;429:117654. [Google Scholar]
- 22.Pagola J, Juega J, Francisco J, Rodriguez M, Cabezas J, De Lera M, et al. High detection of atrial fibrillation by 90 days textil holter monitoring in patients with cryptogenic stroke, [in English. Stroke. 2021;52((Suppl 1)) doi:10.1161/str.52.suppl-1. P624. [Google Scholar]
- 23.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: The stroke and monitoring for PAF in real time (SMART) registry. Stroke. 2012;43:2788–90. doi: 10.1161/STROKEAHA.112.665844. [DOI] [PubMed] [Google Scholar]
- 24.Krathen J, Tan JL, Essilfie G, Ortman M, Andriulli J, Russo A. Should mobile telemetry monitoring or implantable loop recorders be utilized for early detection of silent atrial arrhythmias in patients with cryptogenic stroke?, [in English] J Am Coll Cardiol. 2021;77((Suppl 1)):415. [Google Scholar]
- 25.Kalani R, Bernstein R, Passman R, Curran Y, Ruff I, Prabhakaran S. Low yield of mobile cardiac outpatient telemetry after cryptogenic stroke in patients with extensive cardiac imaging. J Stroke Cerebrovasc Dis. 2015;24:2069–73. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Kass-Hout O, Kass-Hout T, Parikh A, Hoskins M, Clements SD, Jr, Rangaraju S, et al. Atrial fibrillation predictors on mobile cardiac telemetry in cryptogenic ischemic stroke. Neurohospitalist. 2018;8:7–11. doi: 10.1177/1941874417711761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller DJ, Khan MA, Schultz LR, Simpson JR, Katramados AM, Russman AN, et al. Outpatient cardiac telemetry detects a high rate of atrial fibrillation in cryptogenic stroke. J Neurol Sci. 2013;324:57–61. doi: 10.1016/j.jns.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura ME, Amin H, Khalil Y, Subzposh F, Martin B. Utility of 14 day ambulatory ecg in the diagnosis of atrial fibrillation following cryptogenic stroke, [in English] J Am Coll Cardiol. 2020;75:415. [Google Scholar]
- 29.Bulkova V, Sanak D, Tomek A, Brozman M, Gandalovicova J, Kovarikova D, et al. Atrial fibrillation detection by integrated trans-telephonic ECG monitoring after cryptogenic stroke in the years |y2016-2018 in the Czech and Slovak Republics, [in English] Eur Heart J. 2019;40((Suppl 1)):763. [Google Scholar]
- 30.Castrejon Castrejon S, Ruiz-Ares G, Martinez Cossiani M, Rigual R, Gutierrez Zuniga R, Alonso De Lecinana M, et al. Incidence and type of arrhythmias recorded by one-month continuous ECG monitoring in stroke patients, [in English] Europace. 2021;23((Suppl 3)):iii31. [Google Scholar]
- 31.Finocchi C, Canessa A, Balestrino M, Schenone A, Serrati C, Gandolfo C, et al. Evaluation of incidence of paroxysmal atrial fibrillation (Va. Lin Fa study) in subjects with recent cryptogenic ischemic stroke, [in English] Eur Stroke J. 2019;4((Suppl 1)):298. [Google Scholar]
- 32.Kulach A, Dewerenda M, Majewski M, Balys M, Puz P, Lasek-Bal A, et al. Efficacy of 72-hour Holter monitoring, 7-day Holter monitoring and 30-day intermittent patient-activated heart rhythm recording in detecting arrhythmias in cryptogenic stroke patients. Kardiol Pol. 2019;77((Supplement 1)):24–5. doi: 10.1515/med-2020-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pico F, Sudacevschi V, Bertrand C, Tarnaud C, Messikh Z, Chadenat M, et al. Paroxysmal atrial fibrillation is detected in 15% of 171 patients with cryptogenic stroke and TIA with 21 days mobile cardiac outpatient telemetry, [in English. Cerebrovasc Dis. 2014;37((Suppl 1)):148. [Google Scholar]
- 34.Tayal AH, Tian M, Kelly KM, Jones SC, Wright DG, Singh D, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008;71:1696–701. doi: 10.1212/01.wnl.0000325059.86313.31. [DOI] [PubMed] [Google Scholar]
- 35.Lumikari TJ, Putaala J, Kerola A, Sibolt G, Pirinen J, Pakarinen S, et al. Continuous 4-week ECG monitoring with adhesive electrodes reveals AF in patients with recent embolic stroke of undetermined source. Ann Noninvasive Electrocardiol. 2019;24:e12649. doi: 10.1111/anec.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel D, Tarakji KG. Smartwatch diagnosis of atrial fibrillation in patient with embolic stroke of unknown source: A case report. Cardiovasc Digit Health J. 2021;2:84–7. doi: 10.1016/j.cvdhj.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wouters F, Gruwez H, Vranken J, Ernon L, Mesotten D, Vandervoort P, et al. Will smartphone applications replace the insertable cardiac monitor in the detection of atrial fibrillation? The first comparison in a case report of a cryptogenic stroke patient. Front Cardiovasc Med. 2022;9:839853. doi: 10.3389/fcvm.2022.839853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnusson P, Lyren A, Mattsson G. Diagnostic yield of chest and thumb ECG after cryptogenic stroke, Transient ECG Assessment in Stroke Evaluation (TEASE): An observational trial. BMJ Open. 2020;10:e037573. doi: 10.1136/bmjopen-2020-037573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermans ANL, Gawalko M, Dohmen L, van der Velden RMJ, Betz K, Duncker D, et al. Mobile health solutions for atrial fibrillation detection and management: A systematic review. Clin Res Cardiol. 2022;111:479–91. doi: 10.1007/s00392-021-01941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill S, Bunting KV, Sartini C, Cardoso VR, Ghoreishi N, Uh HW, et al. Smartphone detection of atrial fibrillation using photoplethysmography: A systematic review and meta-analysis. Heart. 2022;108:1600–7. doi: 10.1136/heartjnl-2021-320417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leńska-Mieciek M, Kuls-Oszmaniec A, Dociak N, Kowalewski M, Sarwiński K, Osiecki A, et al. Mobile single-lead electrocardiogram technology for atrial fibrillation detection in acute ischemic stroke patients. J Clin Med. 2022;11:665. doi: 10.3390/jcm11030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanna T. Long-term monitoring to detect atrial fibrillation with the indwelling implantable cardiac monitors. Int J Stroke. 2018;13:893–904. doi: 10.1177/1747493018790023. [DOI] [PubMed] [Google Scholar]
- 43.Noubiap JJ, Agbaedeng TA, Kamtchum-Tatuene J, Fitzgerald JL, Middeldorp ME, Kleinig T, et al. Rhythm monitoring strategies for atrial fibrillation detection in patients with cryptogenic stroke: A systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2021;34:100780. doi: 10.1016/j.ijcha.2021.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaura AK, Sztriha L, Chan FK, Aeron-Thomas J, Gall N, Piechowski-Jozwiak, et al. Early prolongedambulatory cardiacmonitoringin stroke (EPACS)-an open-label randomised controlled trial, [in English. Europace. 2017;19((Suppl 1)):i6. [Google Scholar]
- 45.Koh KT, Law WC, Zaw WM, Foo DHP, Tan CT, Steven A, et al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: A multicentre randomized controlled trial. Europace. 2021;23:1016–23. doi: 10.1093/europace/euab036. [DOI] [PubMed] [Google Scholar]
- 46.Jung S, Lee HA, Kang IS, Shin SH, Chang Y, Woo Shin D, et al. Clinical implications of atrial fibrillation detection using wearable devices in patients with cryptogenic stroke (CANDLE-AF) trial: Design and rationale. Front Cardiovasc Med. 2022;9:837958. doi: 10.3389/fcvm.2022.837958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treskes RW, Gielen W, Wermer MJ, Grauss RW, van Alem AP, Dehnavi RA, et al. Mobile phones in cryptogenic strOke patients Bringing sIngle Lead ECGs for Atrial Fibrillation detection (MOBILE-AF): Study protocol for a randomised controlled trial. Trials. 2017;18:402. doi: 10.1186/s13063-017-2131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vijayan V, Connolly JP, Condell J, McKelvey N, Gardiner P. Review of wearable devices and data collection considerations for connected health. Sensors (Basel) 2021;21:5589. doi: 10.3390/s21165589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahler S, Birkemeyer R, Alexopoulos D, Siudak Z, Müller A, von der Schulenburg JM. Cost-effectiveness of a photopethysmographic procedure for screening for atrial fibrillation in 6 European countries. Health Econ Rev. 2022;12:17. doi: 10.1186/s13561-022-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs MS, Kaasenbrood F, Postma MJ, van Hulst M, Tieleman RG. Cost-effectiveness of screening for atrial fibrillation in primary care with a handheld, single-lead electrocardiogram device in the Netherlands. Europace. 2018;20:12–8. doi: 10.1093/europace/euw285. [DOI] [PubMed] [Google Scholar]
- 51.Gao L, Moodie M, Freedman B, Lam C, Tu H, Swift C, et al. Cost-effectiveness of monitoring patients post-stroke with mobile ECG during the hospital stay. J Am Heart Assoc. 2022;11:e022735. doi: 10.1161/JAHA.121.022735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan NY, Orchard J, Agbayani MJ, Boddington D, Chao TF, Johar S, et al. 2021 Asia Pacific Heart Rhythm Society (APHRS) practice guidance on atrial fibrillation screening. J Arrhythm. 2021;38:31–49. doi: 10.1002/joa3.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medic G, Kotsopoulos N, Connolly MP, Lavelle J, Norlock V, Wadhwa M, et al. Mobile cardiac outpatient telemetry patch vs implantable loop recorder in cryptogenic stroke patients in the US –cost-minimization model, [in English. Med Devices Evid Res. 2021;14:445–58. doi: 10.2147/MDER.S337142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet. 2021;398:1507–16. doi: 10.1016/S0140-6736(21)01698-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Digital abstract
PRISMA flow diagram