Abstract
Background
Antimicrobial-resistant (AMR) bacterial infection is a significant global threat to the healthcare systems. Pseudomonas aeruginosa, the leading infectious agent in the healthcare setting is now one of the major threats due to AMR. A comprehensive understanding of the magnitude of AMR, particularly highly public health important pathogens such as P. aeruginosa, is necessary for the management of infections based on local information.
Objective
This systematic review and meta-analysis aimed to determine the country-wide AMR of P. aeruginosa.
Methods
Systematic searches were performed to retrieve articles from PubMed, Scopus, Web of Science, ScienceDirect electronic databases, Google Scholar search engine, and repository registrars from 2015 to 31st December 2023. Twenty-three studies that provided important data on AMR in P. aeruginosa were systematically reviewed and analyzed to determine the country-wide magnitude of P. aeruginosa AMR profile from healthcare-associated infections. AMR of P. aeruginosa to 10 different antibiotics were extracted separately into Microsoft Excel and analyzed using STATA 17.0. Cohen’s kappa was computed to determine the agreement between reviewers, the Inverse of variance (I2) was used to evaluate heterogeneity across studies, and Egger’s test to identify publication bias. A random effect model was used to determine the pooled resistance to each antibiotic. Subgroup analysis was performed by infection type and year of publication.
Results
This systematic review and meta-analysis revealed that the pooled prevalence of P. aeruginosa in clinical specimens associated with HAI was 4.38%(95%CI: 3.00–5.76). The pooled prevalence of AMR in P. aeruginosa for different antibiotics varies, ranging from 20.9% (95%CI: 6.2–35.8) for amikacin to 98.72% (95%CI: 96.39–101.4) for ceftriaxone. The pooled resistance was higher for ceftriaxone (98.72%), Trimethoprim-sulfamethoxazole (75.41), and amoxicillin-clavulanic acid (91.2). In contrast relatively lower AMR were observed for amikacin (20.9%) and meropenem (28.64%). The pooled multi-drug resistance (MDR) in P. aeruginosa was 80.5% (95%CI: 66.25–93.84). Upon subgroup analysis by infection types and year of publication, P. aeruginosa isolated from healthcare-associated infections exhibited higher resistance to ceftazidime (94.72%) compared to isolates from mixed types of healthcare-associated infections (70.84%) and surgical site infections (57.84%). Antimicrobial resistance in gentamicin was higher during the periods of 2018–2020 (73.96%), while comparatively lower during 2021–2023 (42.69%) and 2015–2017 (29.82%)
Conclusions
Significantly high AMR and MDR were observed from this systematic review and meta-analysis. AMR obtained from this systematic review and meta-analysis urges the need for improved infection control, antimicrobial stewardship practices, and strengthened surveillance systems to control the spread of AMR and ensure effective treatment of P. aeruginosa infections.
Protocol registration
This systematic review and meta-analysis was registered on PROSPERO (Registration ID: CRD42024518145).
Introduction
Healthcare-associated infections (HAIs) are defined as infections that an individual acquires during medical treatment for other conditions [1]. Pseudomonas aeruginosa (P. aeruginosa) is a ubiquitous Gram-negative bacterium with simple nutritional requirements that exhibit the ability to thrive in various environments, including water, surfaces, medical devices, and hospital waste products [2]. P. aeruginosa is one of the prominent opportunistic pathogens, contributing to HAIs such as pneumonia, bloodstream infections, surgical site infections (SSI), urinary tract infections (UTI), burn wound infections, keratitis, and otitis media [3].
Infectious diseases resulting from antimicrobial-resistant (AMR) bacteria pose a significant global threat to healthcare systems, with an estimated 4.95 million global deaths associated with bacterial AMR in 2019, including 1.27 million deaths directly attributable to bacterial AMR [4]. P. aeruginosa was one of the six major bacterial pathogens (E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa) responsible for 18.8% of all deaths associated with AMR globally [4]. In the World Health Organization (WHO) African region, an estimated 1.05 million deaths were associated with bacterial AMR, from this, 250, 000 deaths were directly attributable to bacterial AMR in 2019 [5]. Apart from its impacts on mortality and disability, AMR also incurs substantial economic burdens. According to the estimates by the World Bank, AMR could lead to an extra US$1 trillion in healthcare expenses by 2050. Additionally, it could cause annual gross domestic product losses ranging from US$1 trillion to US$3.4 trillion by 2030 [6].
The misuse and overuse of antimicrobials in diverse sectors drive the rise of AMR pathogens, impacting nations of all income levels, especially worsening conditions in low- and middle-income countries (LMICs) [7]. Factors such as inappropriate antimicrobial use, easy access without prescription, lack of public awareness of proper usage, and inadequate surveillance systems exacerbate the prevalence of infections caused by AMR pathogens, particularly in developing nations [8].
Antimicrobial-resistant P. aeruginosa has been identified as a critical priority pathogen by the WHO [9]. P. aeruginosa has developed multi-drug resistance (MDR) by modifying outer membrane permeability, utilizing efflux pumps, producing antibiotic-inactivating enzymes, and facilitating the transfer of resistance genes or undergoing mutation, making the treatment of common infectious diseases challenging [10].
Understanding the extent and severity of AMR in P. aeruginosa is imperative, given its significance as a prominent opportunistic bacterium causing HAI. Previous studies conducted in Ethiopia have assessed the prevalence of AMR in P. aeruginosa against various antibiotics. However, the findings have been inconsistent, with reported resistance rates to different antibiotics ranging from zero [11] to one hundred percent [12–15] across different studies. In Ethiopia, there is a lack of systematic review and meta-analysis on P. aeruginosa, a predominant healthcare-associated pathogen. Therefore this systematic review and meta-analysis were undertaken to assess the comprehensive AMR profile of P. aeruginosa, which will provide crucial insights for guiding empirical therapy, infection control measures, and antibiotic stewardship efforts in Ethiopian healthcare settings. Furthermore, beyond its local significance, understanding the magnitude of AMR in P. aeruginosa will contribute to global health initiatives aimed at combating antimicrobial resistance, particularly against multidrug-resistant pathogens.
Methods
Protocol registration
This systematic review and meta-analysis have been registered on PROSPERO (International Prospective Register of Systematic Reviews) (registration ID: CRD42024518145).
Databases and search strategy
Systematic searches were conducted across various databases, including PubMed, Scopus, Web of Science, and ScienceDirect electronic databases, to retrieve published articles. Additionally, articles available on Google Scholar and online repository sites of different institutions were also retrieved as part of the search process. Appropriate MeSH (Medical Subject Headings) terms and keywords were employed to retrieve relevant articles published in the English language within the timeframe of January 1, 2015, to December 31, 2023, from the listed databases. The search terms were: (((Antimicrobial resistance [MeSH Terms]) OR (Antibiotic resistance [MeSH Terms]) OR (Microbial drug resistance [MeSH Terms])) AND Pseudomonas aeruginosa [MeSH Terms] AND (Nosocomial infection) OR (Hospital-acquired infection) OR (Healthcare-associated infection)) AND Ethiopia. The complete search strategy and searching strings for different databases are depicted in the (S1 Table in S1 File). Furthermore, we reviewed the reference lists of primary studies and review papers to identify grey literature.
Eligibility criteria
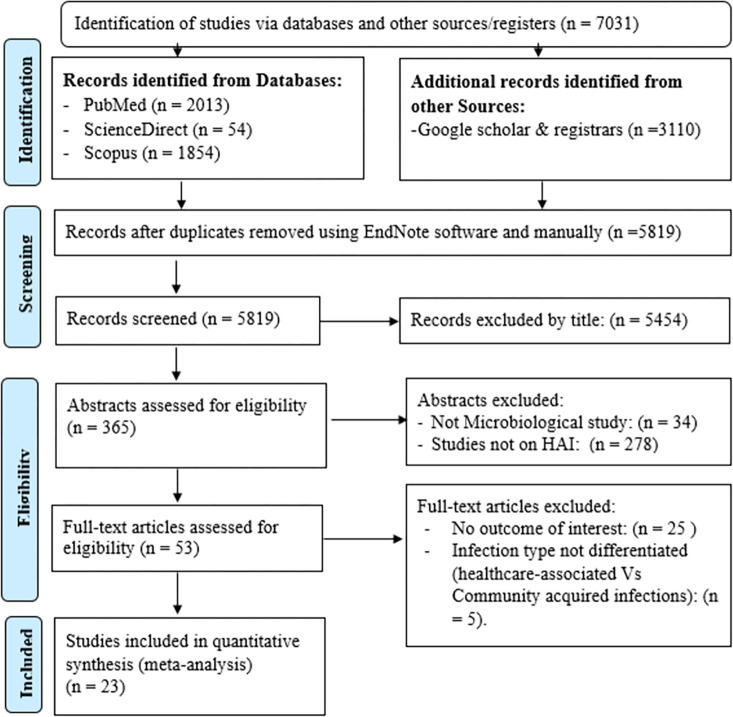
Studies obtained from the aforementioned databases were imported into EndNote X7 reference management software (Thomson Reuters, Toronto, Ontario, Canada), and following the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16] (Fig 1), duplicates were eliminated, and the remaining studies underwent initial screening by titles, followed by detailed abstract and full-text screening by two reviewers (ZA and EG). To identify eligible articles, we employed predetermined inclusion and exclusion criteria. The inclusion criteria comprised; (a) articles published exclusively in the English language, (b) studies that reported the proportion or percentage of AMR in P. aeruginosa utilizing appropriate phenotypic or molecular AMR detection methods, and (c) studies focused solely on HAI or clinical samples. Studies that did not adhere to the aforementioned inclusion criteria were excluded. Moreover, studies that presented combined AMR results in categories such as "Gram-negative bacteria" and "Others," lacked explicit information regarding whether the infection type was hospital-acquired or community-acquired, as well as studies reported both hospital-acquired and community-acquired infections without differentiating the types of isolates and their respective AMR patterns, studies that didn’t report the outcomes of interest were excluded.
Fig 1. PRISMA flow diagram to show the result of the search and the reason for exclusion.
Quality assessments
To assess the quality of each study, we utilized the Joanna Briggs Institute tool designed for prevalence and cohort studies [17]. Two impartial and independent reviewers (ZA and MAR) conducted a critical appraisal of each study. In instances where consensus between the two independent reviewers could not be achieved, a third reviewer (YG) was enlisted to resolve any disagreements and reach on consensus. Studies with a final quality score of 50% or higher were considered for inclusion in the systematic review and meta-analysis (S2 Table in S1 File).
Data extraction
A standardized data extraction form on Microsoft Excel 2016 was utilized to systematically gather or record relevant information from each included potential study. The extraction process covered various domains, including study characteristics, such as the name of the author(s), publication year, study design, geographic location, types of participants (patients undergo surgery, catheterized patients, all-age patients, pediatric patients, post-partum and post-abortion women) and the number of study participants, clinical data, such as infection type (Healthcare-associated urinary tract infection (HAUTI), SSI, mixed type of HAI, puerperal sepsis) and specimen type (swab/pus, cerebrospinal fluid, blood, urine), the total number of bacteria isolates, and the number of P. aeruginosa isolate. It covered details on the AMR profile of P. aeruginosa to various antibiotics, including the number of tested isolates, the count of AMR isolates (at least for one antibiotic), and the number of MDR P. aeruginosa isolates (Table 1).
Table 1. Summary of included studies in systematic review and meta-analysis on antimicrobial-resistant P. aeruginosa healthcare-associated infections.
| S.N | Author/s | Year of publication | Study region | Study design | Sample size | No of total bacterial isolates | No of P. aeruginosa isolates and tested for AMR | No of P. aeruginosa resistance to at least one antibiotic | Reports |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abayneh et al | 2022 | SNNPR | CH | 262 | 41 | 5 | 4 | AMR |
| 2 | Abosse et al | 2021 | AM | CS | 165 | 115 | 26 | 22 | AMR, MDR |
| 3 | Adugna et al | 2021 | AM | CS | 422 | 53 | 3 | 2 | AMR |
| 4 | Dagninet et al | 2022 | SNNPR | CS | 245 | 72 | 10 | 7 | AMR, MDR |
| 5 | Alemayehu et al | 2019 | SNNPR | CS | 384 | 47 | 4 | 4 | AMR, MDR |
| 6 | Ali et al | 2023 | AM | CS | 338 | 48 | 2 | 2 | AMR, MDR |
| 7 | Asmare et al | 2023 | AM | CS | 211 | 52 | 6 | 6 | AMR, MDR |
| 8 | Asres et al | 2017 | AA | CS | 197 | 168 | 9 | 8 | AMR, MDR |
| 9 | Awoke et al | 2019 | OR | CS | 143 | 60 | 3 | 3 | AMR |
| 10 | Bekele et al | 2015 | OR | CS | 73 | 36 | 36 | 0 | AMR |
| 11 | Bizuayehu et al | 2022 | AA | CS | 220 | 79 | 12 | 10 | AMR, MDR |
| 12 | Dessie et al | 2016 | AA | CS | 107 | 104 | 6 | 6 | AMR, MDR |
| 13 | Gashaw et al | 2018 | OR | CS | 240 | 126 | 9 | 9 | AMR, MDR |
| 14 | Gebissa et al | 2021 | OR | CS | 150 | 147 | 18 | 16 | AMR |
| 15 | Mekonen et al | 2021 | AM | CS | 254 | 34 | 18 | 15 | AMR, MDR |
| 16 | Melaku et al | 2023 | DD | CS | 188 | 120 | 9 | 9 | AMR |
| 17 | Misha et al | 2021 | OR | CH | 251 | 38 | 8 | 8 | AMR |
| 18 | Motbinor et al | 2020 | AM | CS | 238 | 20 | 11 | 11 | AMR, MDR |
| 19 | Sahile et al | 2016 | OR | CS | 200 | 111 | 8 | 7 | AMR, MDR |
| 20 | Tilahun et al | 2022 | AM | CS | 384 | 343 | 31 | 18 | AMR, MDR |
| 21 | Tilahun et al | 2022 | AM) | CS | 423 | 75 | 46 | 25 | AMR, MDR |
| 22 | Tolera et al | 2018 | HR | CS | 394 | 54 | 6 | 5 | AMR |
| 23 | Worku et al | 2023 | Mixed | CS | 752 | 494 | 18 | 12 | AMR, MDR |
Abbreviation: AM: Amhara; AA: Addis Ababa, OR: Oromia; SNNPR: South Nation and Nationality and Peoples Regions; HR: Harari; DD: Dire Dawa: AMR: Antimicrobial resistance; MDR: Multidrug resistance; CH: cohort; CS: cross-sectional
Statistical analysis
The data was initially entered into a prepared Microsoft Excel sheet, and subsequently, it was exported to STATA 17.0 software (StataCorp, Texas, USA) for final analysis. The inverse variance (I2) test was used to assess the heterogeneity across studies with interpretations assigned to I2 values: 0% (no heterogeneity), 0–25% (low heterogeneity), 25–50% (medium heterogeneity), and >75% (high heterogeneity) [18]. A subgroup analysis based on various categories was performed for studies that exhibited high heterogeneity. The Egger’s test was employed to evaluate the presence of publication bias with a significance threshold of p < 0.05, and a trim-and-fill analysis was conducted to address and manage potential bias. A random effect model for meta-analysis was used to estimate the pooled prevalence of P. aeruginosa in clinical specimens associated with HAI, and the pooled prevalence of AMR and MDR P. aeruginosa. The aggregate prevalence of HAI associated with P. aeruginosa was determined by assessing the proportion of P. aeruginosa cases among the total number of specimens. To calculate the pooled prevalence of AMR and MDR continuity correction was made for zero and one-hundred percent AMR values which resulted in zero standard error [19]. Finally, the pooled prevalence of AMR was calculated separately for each antibiotic tested.
Results
A descriptive summary of included studies
This systematic review and meta-analysis encompassed 23 studies that provided important data on the microbiologically confirmed prevalence, AMR, and MDR profile of P. aeruginosa isolates. In this review, 6,212 study participants suspected of hospital-acquired infections were assessed for bacterial infections, yielding a total of 2,437 hospital-acquired infections. From these studies, 304 isolates of P. aeruginosa were obtained and tested for resistance to a maximum of ten different antibiotics. All the studies included followed Clinical Laboratory Standard Institute (CLSI) guidelines to report AMR resistances and considered bacterial isolates as MDR based on the published guideline by Magiorakos, et al. [20]. In these studies, the prevalence of P. aeruginosa associated with HAI ranged from 0.59–15.76% [11–15, 21–38] (Table 1).
The pooled prevalence of P. aeruginosa in healthcare-associated infections
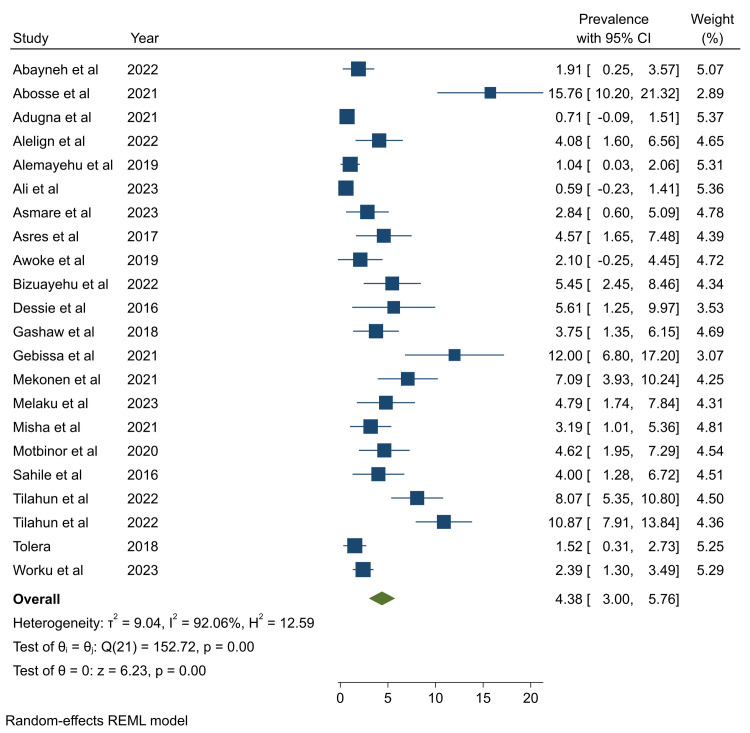
In this systematic review and meta-analysis, the pooled prevalence of P. aeruginosa associated with HAI was determined to be 4.38% (95%CI: 3.00–5.76) (Fig 2). Since Egger’s test revealed the presence of publication bias (P <0.001), to correct the publication bias, the trim-and-fill analysis was performed, and the pooled prevalence was found to be 4.61% (95%CI: 3.23–6.00) (S3 Table in S1 File). Even if high heterogeneity (I2 = 92.06) was observed across studies, subgroup analysis by types of infection and year of publication showed no significant variation in the prevalence of P. aeruginosa associated HAI (S10 and S11 Figs in S1 File).
Fig 2. Forest plot showing the prevalence of P. aeruginosa associated with healthcare-associated infections.
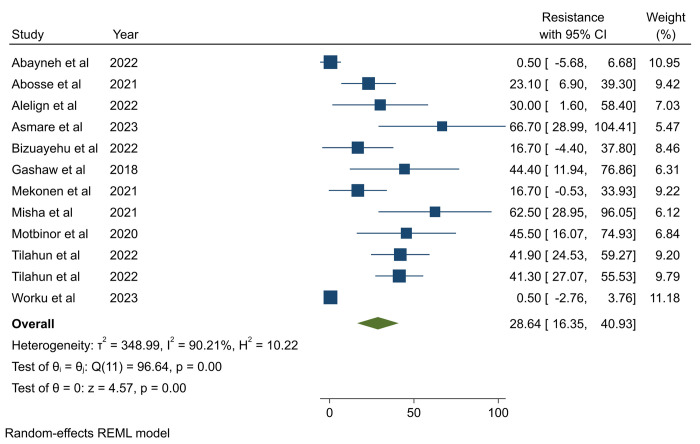
Antimicrobial resistance in P. aeruginosa. The pooled prevalence of AMR in P. aeruginosa was calculated based on a maximum of 22 studies for gentamicin [11–15, 21–24, 26, 27, 29–38] and ciprofloxacin [11–15, 21–24, 26, 27, 29–38] and a minimum of 7 studies for trimethoprime-sulfamethoxazole [13–15, 26–28, 33], with a total of 10 antibiotics (amikacin, amoxicillin-clavulanic acid, ampicillin, ceftazidime, ceftriaxone, chloramphenicol, ciprofloxacin, trimethoprim-sulfamethoxazole, gentamicin, and meropenem) being pooled separately (Table 2). The pooled prevalence of AMR in P. aeruginosa for the listed antibiotics varies, ranging from 20.9% (95%CI: 6.2–35.8) for amikacin to 98.72% (95%CI: 96.39–101.4) for ceftriaxone (Table 2). From this systematic review and meta-analysis, it was found that the pooled AMR of P. aeruginosa to third-generation cephalosporins was higher, ranging from 66.8% for ceftazidime to 98.72% for ceftriaxone. In contrast, relatively lower levels of AMR were observed for amikacin (20.9%) and the last resort antibiotics, carbapenem/meropenem (28.64%) (95%CI: 16.35–40.93) (Fig 3). The Egger’s test showed that there was publication bias across studies used to estimate the pooled resistance of meropenem, ceftriaxone, amoxicillin-clavulanic acid, and trimethoprim-sulfamethoxazole (Table 2). To address the publication bias trim-and-fill analysis was computed and resulted in a significant change in AMR of meropenem, ceftriaxone, and trimethoprime-sulfamethoxazole (S4-S6 Tables in S1 File). For amoxicillin-clavulanic acid, there was no effect on the AMR of antibiotics after trim-and-fill analysis.
Table 2. The pooled prevalence of Pseudomonas aeruginosa to ten different antibiotics.
| Antibiotics | No of studies | Pooled resistance (95% CI) | Pooled resistance after trim-and-fill analysis | Heterogeneity (I2) (p-value) | (Egger’s test) p-value |
|---|---|---|---|---|---|
| Amikacin | 8 | 20.98 (6.2–35.8) | 92.17% (<0.01) | 0.166 | |
| Amoxicillin-clavulanic acid | 8** | 91.2 (80.6–101.8) | No change | 92.48% (<0.01) | <0.001 |
| Ampicillin | 9 | 79.66 (56.6–102.8) | 99.07% (<0.01) | 0.210 | |
| Ceftazidime | 17 | 66.85 (54.6–79.1) | 91.04% (<0.01) | 0. 209 | |
| Ceftriaxone | 13 (3*) | 98.72 (96.39–101.04) | 99.1 (96.8–101.4) | 0.01% (0.13) | <0.001 |
| Chloramphenicol | 9 | 69.2 (52.8–85.6) | 82.52% (<0.01) | 0.122 | |
| Ciprofloxacin | 22 | 46.5 (35.3–57.7) | 90.75% (<0.01) | 0.434 | |
| Trimethoprim-Sulfamethoxazole | 11 (4*) | 75.41 (58.39–92.43) | 92.1 (72.9–111.3) | 70.52% (<0.01) | <0.001 |
| Gentamicin | 22 | 47.4 (35.3–59.5) | 93.46% (<0.01) | 0.317 | |
| Meropenem | 14 (2*) | 28.64 (16.35–40.93) | 24.1 (11.5–36.7) | 90.21% (<0.01) | <0.001 |
* The number of imputed studies during Trim-and-fill analysis
** No effect on the pooled prevalence after Trim-and-fill analysis; CI: Confidence Interval; I2: Inverse of Variance. Forest plots of the pooled resistance of P. aeruginosa for each antibiotic are available in (S1-S9 Figs in S1 File)
Fig 3. Pooled antimicrobial resistance of P. aeruginosa to meropenem.
Inverse of variance (I2) statistics showed greater than 70.0% heterogeneity among studies for all antibiotics except studies pooled to estimate the resistance of ceftriaxone (Table 2). To identify the possible source of heterogeneity, subgroup analysis was performed for each antibiotic by year of publication and type of infection (S12-S27 Figs in S1 File). Subgroup analysis based on study year and infection type revealed noteworthy disparities in the AMR of ceftazidime and gentamicin. Particularly, when examining infection types, it was evident that P. aeruginosa isolated from HAUTI exhibited higher resistance to ceftazidime (94.72%) compared to isolates from mixed types of HAI (70.84%) and SSI (57.84%) (S15 Fig in S1 File). Notable fluctuations in gentamicin AMR were observed across different years, with resistance rates being higher during the periods of 2018–2020 (73.96%), while comparatively lower during 2021–2023 (42.69%) and 2015–2017 (29.82%) (S24 Fig in S1 File).
Multi-drug resistance profile of P. aeruginosa
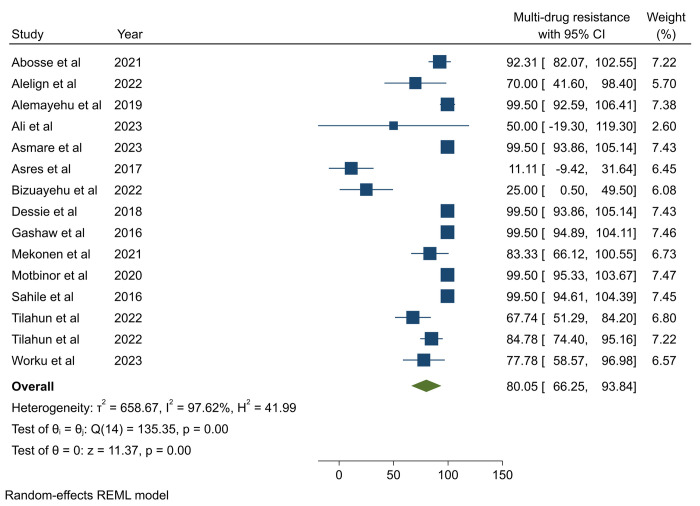
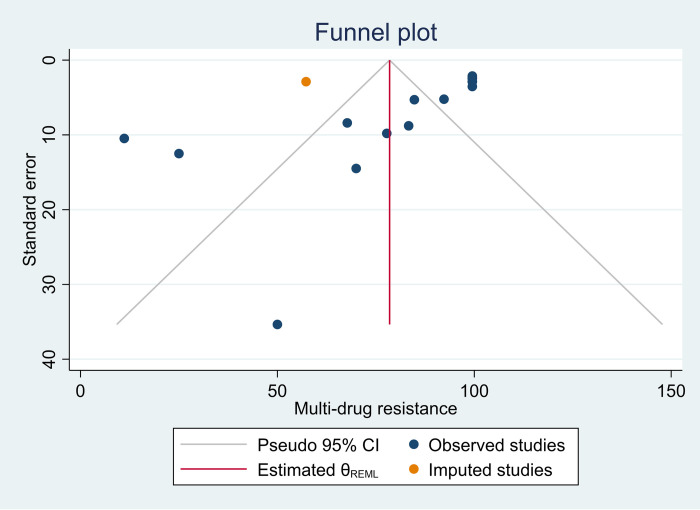
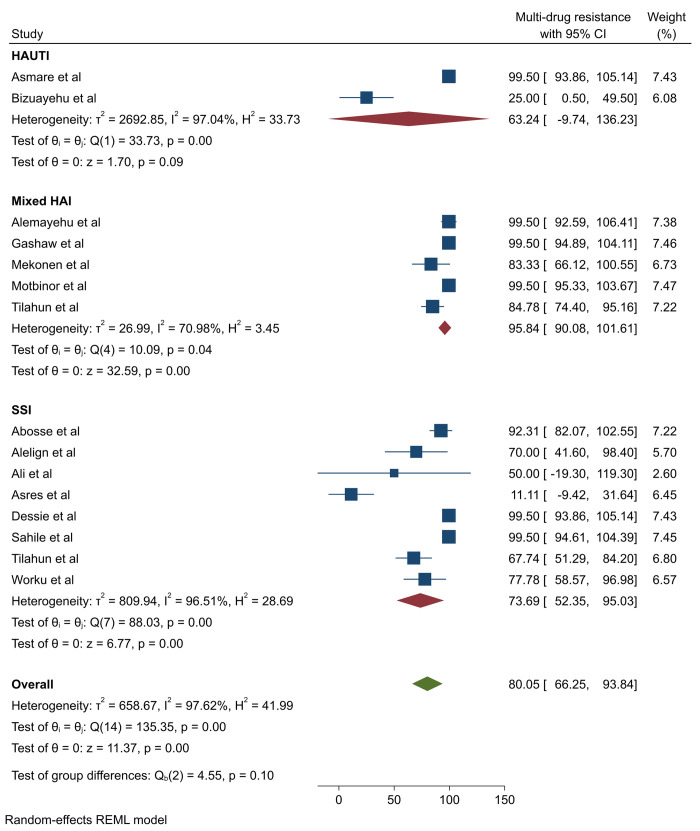
The pooled prevalence of MDR in P. aeruginosa was 80.05% (95%CI: 66.25–93.84) (Fig 4). However, Egger’s test revealed the presence of publication bias and was subjected to trim-and-fill analysis, and the pooled MDR of P. aeruginosa was adjusted to be 78.49% (95%: CI 65.27–91.72) (S7 Table in S1 File and Fig 5). High heterogeneity, indicated by an I2 value of 97.62%, was noted across studies. Subsequently, subgroup analysis was conducted based on infection types and publication years. The analysis revealed a higher prevalence of MDR cases among mixed types of HAI (95.84%) compared to SSI (73.69%) and HAUTI (63.24%) (Fig 6). However, subgroup analysis based on years of publication did not demonstrate significant variation (S28 Fig in S1 File).
Fig 4. Pooled multi-drug resistance profile of P. aeruginosa isolated from healthcare-associated infections.
Fig 5. Funnel plot for multi-drug resistance of P. aeruginosa after trim-and-fill analysis.
Fig 6. Subgroup analysis of multi-drug resistant P. aeruginosa isolated from healthcare-associated infections by infection type.
Discussion
The findings of this systematic review and meta-analysis provide a comprehensive insight into the alarming rates of AMR observed in P. aeruginosa isolates from HAIs within Ethiopian healthcare settings. Our analysis reveals a concerning burden, with AMR prevalence ranging from 20.9% to 98.72% across ten different antibiotics analyzed. This wide spectrum of resistance underscores the complexity and severity of the AMR crisis facing healthcare facilities in Ethiopia. Moreover, the high prevalence of MDR P. aeruginosa (80.0%), poses a substantial challenge to the effective management and treatment of HAIs. Notably, our findings also indicate that 4.38% of HAIs in Ethiopian hospitals can be attributed to P. aeruginosa. These results highlight the urgent need for targeted interventions and strengthened antimicrobial stewardship programs to combat the spread of AMR and mitigate its impact on patient outcomes and healthcare delivery in Ethiopia.
In this thorough systematic review and meta-analysis conducted in Ethiopia, P. aeruginosa was identified as responsible for 4.38% of HAIs, a prevalence rate consistent with findings reported from China (6.53%) [39]. However, this prevalence is lower than the reported rate from Egypt (19.9%) [40]. Variations in prevalence rates of P. aeruginosa HAIs across regions can be attributed to differences in healthcare practices, antibiotic usage, environmental factors, healthcare infrastructure, and population characteristics.
The AMR pattern of P. aeruginosa for aminoglycoside antibiotics reveals varying resistance rates. The amikacin resistance rate of 20.9% in this study aligns with rates in Turkey (17.8%) [41], China (20.8%, 22.2%) [42, 43], and Somalia (20%) [44], while higher rates are seen in India (80%) [45] and Nepal (37.5%) [46]. Additionally, the gentamicin resistance rate of 47.4% in this study is in line with resistance rates in China (42.4%) [43] and Somalia (45.5%) [44], but exceeds rates in Turkey (28.2%) [41] and China (29.7%) [42]. Furthermore, lower resistance rates than in India (88%) [45] and Nepal (62.5%) [46] were observed. Factors including overuse of antibiotics, inadequate infection control practices, prolonged hospitalization, and limited surveillance in developing countries might be the possible reasons for this increased magnitude of AMR [47, 48].
Pseudomonas aeruginosa showed varying resistance rates to penicillin and cephalosporin antibiotics, which are among the most frequently prescribed antibiotics in Ethiopia. Specifically amoxicillin-clavulanic acid and ceftriaxone exhibited extremely high resistance levels (91.2%) and (98.72%), respectively. The amoxicillin-clavulanic acid resistance rate aligns with a report from Somalia (88.9%) [44] but surpasses rates reported in Iran (50.6%) [49]. Similarly, the ceftriaxone resistance rate exceeds rates reported from China (78.6%) [42]. Additionally, the ceftazidime resistance rate of 66.8%, although consistent with India (70%) [45] and Iran (57.75%) [50], surpasses rates in Turkey (38.6%) [41] and China (34.3%) [43], as well as a report from Somalia (53.8%) [44] and Spain (20.3%) [51]. However, lower ceftazidime resistance rates than in China (94.1%) and Nepal (91.6%) [46].
In this systematic review and meta-analysis, P. aeruginosa exhibited a noteworthy 28.64% resistance rate to meropenem, a critical last-resort antibiotic, indicating significant antimicrobial resistance against carbapenems. This resistance rate is comparable with resistance reported from Turkey (30.1%) [41], China (35.7%) [43], and Spain (14.1%) [51], although it exceeds a lower rate from China (7.7%) [42]. It is notably lower than reported resistance in Iran (40%) [49], Somalia (50%) [44], India (80%) [45], and Nepal (62.5%) [46].
The ciprofloxacin resistance rate of P. aeruginosa in this study, at 46.5%, aligns closely with rates reported in Iran (47.3%) [49] and Spain (38.4%) [51]. However, it surpasses rates in Turkey (30.7%) [41] and China (21.2% and 35%) [42, 43], as well as Somalia (14.3%) [44], though remaining lower than in India (96%) [45] and Nepal (95.8%) [46]. On the other hand, the Trimethoprim-Sulfamethoxazole resistance rate of 75.41% in this study indicated alarmingly high levels of resistance requiring immediate attention. This was consistent with the resistance rate in Somalia (89%) [44]. The variability of AMR rates of P. aeruginosa observed in our systematic review compared to studies abroad might be attributed to diverse local epidemiological factors, differences in antibiotic usage practices, variations in healthcare settings, and implementation of antibiotic stewardship programs [52, 53].
The MDR profile of P. aeruginosa isolated from HAIs in this comprehensive systematic review and meta-analysis was found to be 80.0%. This percentage aligns closely with MDR rates reported from Somalia (68%) [44] and Nepal (83.3%) [46]. However, it surpasses the rate reported from India (50%) [45], Spain (26.2%) [51], and Iran (58%) [49]. The difference in MDR P. aeruginosa across countries can be attributed to variations in antibiotic prescribing practices, AMR patterns, healthcare infrastructure, infection control measures, antibiotic stewardship programs, surveillance systems, and population characteristics such as prevalence of comorbidities and immunocompromised individuals.
Overall in this systematic review and meta-analysis, there was a significantly increased AMR in P. aeruginosa to different antibiotics which is an indicator of a lack of antimicrobial stewardship programs, surveillance of AMR, and infection prevention and control practices [48]. Clinicians in Ethiopian healthcare settings should reconsider empirical antibiotic therapy for healthcare-associated infections caused by P. aeruginosa due to high levels of antimicrobial resistance, necessitating adjustments in treatment protocols to optimize patient outcomes. Urgent implementation of antimicrobial stewardship programs is underscored by the study findings, which can promote judicious antibiotic use, optimize treatment regimens, and mitigate the spread of antimicrobial resistance among P. aeruginosa isolates. Strengthening infection control practices, including improved hand hygiene, environmental disinfection, and patient isolation protocols, is crucial to prevent and contain the spread of antimicrobial-resistant P. aeruginosa within Ethiopian healthcare settings. Moreover, the study provides valuable data for informing policy decisions aimed at addressing antimicrobial resistance in Ethiopia and facilitating the development of evidence-based strategies for antimicrobial stewardship, infection prevention, surveillance, and resource allocation to combat the growing threat of antimicrobial resistance in healthcare settings.
Future research could focus on investigating novel therapeutic strategies, exploring the molecular mechanisms underlying antimicrobial resistance in P. aeruginosa, assessing the impact of local epidemiological factors, evaluating interventions to reduce resistance prevalence, and assessing the impact of socioeconomic factors on antimicrobial resistance dynamics within healthcare settings to improve patient care.
Strength and limitations
This systematic review and meta-analysis have strengths such as employing a predefined protocol for search strategy and data extraction, alongside internationally recognized tools for critical appraisal to evaluate study quality. However, limitations were observed due to the inclusion criteria restricting studies solely published in English and selection bias. The included studies varied in quality, methodologies, and outcomes, contributing to heterogeneity in the results, despite our efforts to address this statistically. Publication bias is also a concern, as studies with positive findings are more likely to be published. Errors or inconsistencies in data extraction and analysis, although minimized, cannot be entirely eliminated. Additionally, inconsistencies in reporting specific data points across studies limited some subgroup analyses.
Conclusion and recommendations
The findings of this systematic review and meta-analysis concerning the AMR profile of P. aeruginosa isolated from HAIs in Ethiopia revealed a significant prevalence of MDR, indicating a substantial challenge in managing infections caused by this pathogen in healthcare settings. The observed increase in AMR and MDR underscores the urgent need for enhanced infection control measures, careful antimicrobial stewardship practices, and strengthened surveillance systems to curb the spread of resistant strains and ensure effective treatment of P. aeruginosa infections in Ethiopia. Additionally, collaborative efforts at local, national, and international levels are warranted to address the multifaceted factors contributing to AMR and mitigate its impact on public health. Based on the finding from this comprehensive systematic review and meta-analysis we would like to recommend all stakeholders such as governmental, non-governmental organizations, health department officers, policy makers and researchers to work collaboratively to enhance infection prevention control and antimicrobial stewardship practices.
Supporting information
(DOCX)
(DOCX)
Abbreviations
- AMR
Antimicrobial Resistance
- HAI
Healthcare-associated Infection
- HAUTI
Healthcare-associated Urinary Tract Infection
- MDR
Multi-drug Resistance
- SSI
Surgical Site Infection
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Paitoonpong L, Wong CKB, Perl TM. Healthcare-associated infections. Infectious disease epidemiology theory and practice. 2013:369–466. [Google Scholar]
- 2.Remold S.K, Brown C.K, Farris J.E, Hundley T.C, Perpich J.A, Purdy M.A. Differential habitat use and niche partitioning by Pseudomonas species in human homes. Microb Ecol. 2011;62:505–17. [DOI] [PubMed] [Google Scholar]
- 3.Qin Shugang, Xiao Wen, Zhou Chuanmin, Pu Qinqin, Deng Xin, Lan Lefu, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduction and Targeted Therapy. 2022;7(199). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartorius B, Gray AP, Weaver ND, Aguilar GR, Swetschinski LR, Ikuta KS, et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. The Lancet Global Health. 2024;12(2):e201–e16. doi: 10.1016/S2214-109X(23)00539-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World_Bank. Drug-Resistant Infections: A Threat to Our Economic Future. Washington, DC: World Bank; 2017. [Google Scholar]
- 7.WHO. Global action plan on antimicrobial resistance. 2015. [Google Scholar]
- 8.WHO. Antimicrobial resistance. 2023. [Google Scholar]
- 9.WHO. Global priority list of antibiotic-resistant bacteria. 2017. [Google Scholar]
- 10.Breidenstein E.B., de la Fuente-Nunez C, Hancock R.E. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–26. [DOI] [PubMed] [Google Scholar]
- 11.Bekele T, Tesfaye A, Sewunet T, Waktola HD. Pseudomonas aeruginosa isolates and their antimicrobial susceptibility pattern among catheterized patients at Jimma University Teaching Hospital, Jimma, Ethiopia. BMC Res Notes. 2015;8(1):1–4. doi: 10.1186/s13104-015-1497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dessie W, Mulugeta G, Fentaw S, Mihret A, Hassen M, Abebe E. Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals, Addis Ababa, Ethiopia. International journal of microbiology. 2016;2016. doi: 10.1155/2016/2418902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gashaw M, Berhane M, Bekele S, Kibru G, Teshager L, Yilma Y, et al. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: a cross sectional study. Antimicrobial Resistance & Infection Control. 2018;7:1–8. doi: 10.1186/s13756-018-0431-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melaku TM, Yimer RM, Abdinasir MM, Alemu MK. Antibiotics Resistance Pattern of Aerobic Bacteria Causing Surgical Site Wound Infection and its Associated Factors in Public Hospital, Dire Dawa-Eastern Ethiopia. International Journal of Clinical Infectious Diseases. 2023;2(3). [Google Scholar]
- 15.Motbainor H, Bereded F, Mulu W. Multi-drug resistance of blood stream, urinary tract and surgical site nosocomial infections of Acinetobacter baumannii and Pseudomonas aeruginosa among patients hospitalized at Felegehiwot referral hospital, Northwest Ethiopia: a cross-sectional study. BMC infectious diseases. 2020;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed). 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn Z, Moola S, Lis K, Riitano D, C T. Systematic reviews of prevalence and incidence. In: Aromataris E Z. M, editors. JBI Manual for Evidence Synthesis: JBI; 2020. [Google Scholar]
- 18.Borenstein M, Cooper H, Hedges L, Valentine J. Heterogeneity in meta-analysis. The handbook of research synthesis and meta-analysis. 2019;3:453–70. [Google Scholar]
- 19.Sweeting M J., Sutton A J., Lambert P C. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in medicine. 2004;23(9):1351–75. doi: 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- 20.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas M, Giske C, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 21.Abayneh M, Asnake M, Muleta D, Simieneh A. Assessment of Bacterial Profiles and Antimicrobial Susceptibility Pattern of Isolates Among Patients Diagnosed with Surgical Site Infections at Mizan-Tepi University Teaching Hospital, Southwest Ethiopia: A Prospective Observational Cohort Study. Infection and Drug Resistance. 2022;15:1807–19. doi: 10.2147/IDR.S357704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abosse S, Genet C, Derbie A. Antimicrobial Resistance Profile of Bacterial Isolates Identified from Surgical Site Infections at a Referral Hospital, Northwest Ethiopia. Ethiopian Journal of Health Sciences. 2021;31(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adugna B, Sharew B, Jemal M. Bacterial profile, antimicrobial susceptibility pattern, and associated factors of community-and hospital-acquired urinary tract infection at Dessie Referral Hospital, Dessie, Northeast Ethiopia. International journal of microbiology. 2021;2021:1–14. doi: 10.1155/2021/5553356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alemayehu T, Tadesse E, Ayalew S, Nigusse B, Yeshitila B, Amsalu A, et al. High burden of Nosocomial infections caused by multi-drug Re-sistant pathogens in pediatric patients at Hawassa university comprehensive specialized hospital. Ethiopian Medical Journal. 2019;58. [Google Scholar]
- 25.Lakoh S, Yi L, Russell L JB, Zhang J, Sevalie S, Yongkun Z, et al. High incidence of catheter-associated urinary tract infections and related antibiotic resistance in two hospitals of different geographic regions of Sierra Leone: a prospective cohort study. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asmare Z, Awoke T, Genet C, Admas A, Melese A, Mulu W. Incidence of catheter-associated urinary tract infections by Gram-negative bacilli and their ESBL and carbapenemase production in specialized hospitals of Bahir Dar, northwest Ethiopia. Antimicrobial Resistance & Infection Control. 2024;13(1):1–12. doi: 10.1186/s13756-024-01368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asres G, Legese M, Woldearegay G. Prevalence of multidrug resistant Bacteria in postoperative wound infections at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Arch Med. 2017;9(4):12. [Google Scholar]
- 28.Awoke Netsanet, Kassa Tesfaye, Teshager L. Magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia. PLoS One. 2019;14(12):1–9. doi: 10.1371/journal.pone.0226140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bizuayehu H, Bitew A, Abdeta A, Ebrahim S. Catheter-associated urinary tract infections in adult intensive care units at a selected tertiary hospital, Addis Ababa, Ethiopia. Plos one. 2022;17(3):e0265102. doi: 10.1371/journal.pone.0265102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alelign Dagninet, Tena Teshome, Tadesse Dagimawie, Tessema Moges, Seid Mohamed, Oumer Yisiak, et al. Bacteriological Profiles, Antimicrobial Susceptibility Patterns, and Associated Factors in Patients Undergoing Orthopedic Surgery with Suspicion of Surgical Site Infection at Arba Minch General Hospital in Southern Ethiopia. Int J Microbiol. 2022;15:2427–43. doi: 10.2147/IDR.S367510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebissa T, Bude B, Yasir M, Mekit S, Noorulla K. Bacterial isolates and their antibiotic sensitivity pattern of surgical site infections among the surgical ward patients of Asella Referral and Teaching Hospital. Future Journal of Pharmaceutical Sciences. 2021;7(1):100. [Google Scholar]
- 32.Mekonnen H, Seid A, Molla Fenta G, Gebrecherkos T. Antimicrobial resistance profiles and associated factors of Acinetobacter and Pseudomonas aeruginosa nosocomial infection among patients admitted at Dessie comprehensive specialized Hospital, North-East Ethiopia. A cross-sectional study. PLoS One. 2021;16(11):e0257272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misha G, Chelkeba L, Melaku T. Bacterial profile and antimicrobial susceptibility patterns of isolates among patients diagnosed with surgical site infection at a tertiary teaching hospital in Ethiopia: a prospective cohort study. Annals of Clinical Microbiology and Antimicrobials. 2021;20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahile T, Esseye S, Beyene G, Ali S. Post-surgical infection and antibiotic susceptibility patterns of bacteria isolated from admitted patients with signs of infection at Jimma University specialized hospital, Jimma, Ethiopia. International Journal of TROPICAL DISEASE & Health. 2016;17(4):1–12. [Google Scholar]
- 35.Tilahun M. Multi-Drug Resistance Profile, Prevalence of Extended-Spectrum Beta-Lactamase and Carbapenemase-Producing Gram Negative Bacilli Among Admitted Patients After Surgery with Suspected of Surgical Site Nosocomial Infection North East Ethiopia. Infection and Drug Resistance. 2022;15:3949–65. doi: 10.2147/IDR.S376622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilahun M, Gedefie A, Bisetegn H, Debash H. Emergence of high prevalence of extended-spectrum beta-lactamase and carbapenemase producing Acinetobacter species and pseudomonas aeruginosa among hospitalized patients at Dessie comprehensive specialized Hospital, North-East Ethiopia. Infection and Drug Resistance. 2022;15:895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolera M, Abate D, Dheresa M, Marami D. Bacterial Nosocomial Infections and Antimicrobial Susceptibility Pattern among Patients Admitted at Hiwot Fana Specialized University Hospital, Eastern Ethiopia. Advances in Medicine. 2018;2018. doi: 10.1155/2018/2127814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worku S, Abebe T, Alemu A, Seyoum B, Swedberg G, Abdissa A, et al. Bacterial profile of surgical site infection and antimicrobial resistance patterns in Ethiopia: a multicentre prospective cross-sectional study. Annals of Clinical Microbiology and Antimicrobials. 2023;22(1):96. doi: 10.1186/s12941-023-00643-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Liu F, Tartari E, Huang J, Harbarth S, Pittet D, et al. The prevalence of healthcare-associated infections in mainland China: a systematic review and meta-analysis. infection control & hospital epidemiology. 2018;39(6):701–9. [DOI] [PubMed] [Google Scholar]
- 40.Mahmoud AB, Zahran WA, Hindawi GR, Labib AZ, Galal R. Prevalence of multidrug-resistant Pseudomonas aeruginosa in patients with nosocomial infections at a university hospital in Egypt, with special reference to typing methods. J Virol Microbiol. 2013;13:165–59. [Google Scholar]
- 41.Acar A, Karaahmetoğlu G, Akalın H, Altay AF. Pooled prevalence and trends of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates over the past 10 years in Turkey: A meta-analysis. Journal of Global Antimicrobial Resistance. 2019;18:64–70. doi: 10.1016/j.jgar.2019.01.032 [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Zhang L, Wang J, Chen Z, Tong L, Wang Z, et al., editors. Proportions of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa among Patients with Surgical Site Infections in China: A Systematic Review and Meta-Analysis. Open Forum Infectious Diseases; 2023: Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding C, Yang Z, Wang J, Liu X, Cao Y, Pan Y, et al. Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: a systematic review and meta-analysis. International Journal of Infectious Diseases. 2016;49:119–28. doi: 10.1016/j.ijid.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 44.Mohamed AH, Sheikh Omar NM, Osman MM, Mohamud HA, Eraslan A, Gur M. Antimicrobial resistance and predisposing factors associated with catheter-associated UTI caused by uropathogens exhibiting multidrug-resistant patterns: a 3-year retrospective study at a tertiary Hospital in Mogadishu, Somalia. Tropical Medicine Infectious Diseases. 2022;7(3):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill J, Arora S, Khanna S, Kumar KH. Prevalence of multidrug-resistant, extensively drug-resistant, and pandrug-resistant Pseudomonas aeruginosa from a tertiary level intensive care unit. Journal of global infectious diseases. 2016;8(4):155. doi: 10.4103/0974-777X.192962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parajuli NP, Acharya SP, Mishra SK, Parajuli K, Rijal BP, Pokhrel BM. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrobial Resistance & Infection Control. 2017;6(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens and global health. 2015;109(7):309–18. doi: 10.1179/2047773215Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sulis G, Sayood S, Gandra S. Antimicrobial resistance in low-and middle-income countries: current status and future directions. Expert review of anti-infective therapy. 2022;20(2):147–60. doi: 10.1080/14787210.2021.1951705 [DOI] [PubMed] [Google Scholar]
- 49.Vaez H, Salehi-Abargouei A, Ghalehnoo ZR, Khademi F. Multidrug resistant Pseudomonas aeruginosa in Iran: A systematic review and metaanalysis. Journal of global infectious diseases. 2018;10(4):212. doi: 10.4103/jgid.jgid_113_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masoudifar M, Gouya MM, Pezeshki Z, Eshrati B, Afhami S, Farzami MR, et al. Health care-associated infections, including device-associated infections, and antimicrobial resistance in Iran: The national update for 2018. Journal of Preventive Medicine and Hygiene. 2021;62(4):E943. doi: 10.15167/2421-4248/jpmh2021.62.4.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.del Barrio-Tofiño E, Zamorano L, Cortes-Lara S, López-Causapé C, Sánchez-Diener I, Cabot G, et al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. Journal of Antimicrobial Chemotherapy. 2019;74(7):1825–35. doi: 10.1093/jac/dkz147 [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Li D, Beiersmann C, Neuhann F, Moazen B, Lu G, et al. Risk factors for antibiotic resistance development in healthcare settings in China: a systematic review. Epidemiology & Infection. 2021;149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrobial Resistance & Infection Control. 2018;7:1–14. doi: 10.1186/s13756-018-0370-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.