Abstract
Ewing sarcoma (ES) is an uncommon mesenchymal neoplasm that typically develops as a bone mass, although up to 30% arise in extraskeletal sites. ES of the gastrointestinal (GI) and hepatobiliary tract is rare and may be misdiagnosed as other, more common neoplasms that occur in these sites. However, the correct classification of extraskeletal ES is important for timely clinical management and prognostication. We reviewed our experience of ES in the GI and hepatobiliary tract in order to further highlight the clinicopathologic features of these neoplasms and document the potential for misdiagnosis in this setting. The archives and consultation files of 6 academic institutions were retrospectively queried for cases of ES occurring in the GI and hepatobiliary tract. The histologic slides and ancillary studies were reviewed and clinical data were retrieved for each case through the electronic medical records, when available. Twenty-three patients with ES in the GI and/or hepatobiliary tract were identified from 2000 to 2022. Of these, 11 were women and 12 were men with a median age of 38 years (range, 2 to 64). Tumor locations included the pancreas (n=5), liver (n=2), stomach (n=3), colorectum (n=3), and small intestine (n=5), as well as tumors involving multiple organs, pelvis and retroperitoneum (n=5). Tumor size varied between 2 cm and 18 cm. Twenty were primary and 3 were metastases. Of the 23 cases, only 17% were initially diagnosed as ES. The most common misdiagnoses involved various forms of neuroendocrine neoplasia due to expression of synaptophysin and other neuroendocrine markers (22%). A wide variety of diagnoses including GI stromal tumor was considered due to aberrant CD117 expression (4%). The diagnosis of ES was ultimately confirmed by detection of the EWSR1 rearrangement in 22 cases. The remaining case was diagnosed using traditional immunohistochemistry. Follow-up information was available in 20 cases, with follow-up time varying between 2 and 256 months. Six patients with follow-up died of disease between 6 and 60 months following initial presentation. Our data indicate ES in the GI and hepatobiliary tract is commonly misdiagnosed leading to a delay in therapy. In light of the attendant therapeutic and prognostic implications, ES should be considered in the differential diagnosis of any GI or hepatobiliary tumor with epithelioid and/or small round cell morphology.
Key Words: Ewing sarcoma, gastrointestinal tract, liver, pancreas, hepatobiliary, neuroendocrine tumor, GIST
Ewing sarcoma (ES) is an uncommon sarcoma of uncertain phenotype with an incidence of approximately 3 cases per million per year in the United States.1 It is most prevalent in adolescents and young adults with a peak between 5 and 24 years. Although it is well known that approximately 30% of ES arise in extraskeletal sites,2,3 the appearance of ES in the gastrointestinal (GI) tract is uncommon and not well recognized.
Classical ES is characterized by sheets and nests of uniform small cells, typically 1-2x the size of lymphocytes, with round nuclei, small nucleoli, and scanty amphophilic to clear glycogenated cytoplasm. The nuclei contain inconspicuous nucleoli and finely stippled chromatin. In some cases, neuroectodermal differentiation (Homer-Wright pseudorosettes) is seen. Morphologic variants include atypical, clear cell, spindle cell, sclerosing, and adamantinoma-like. Atypical ES features nuclear enlargement, prominent nucleoli, and irregular nuclear contours, while the adamantinoma-like variant is composed of nests of basaloid cells with peripheral palisading and cording set within a prominent myxoid, fibromyxoid or hyalinized stroma. The nuclei are high grade but exhibit minimal pleomorphism.
ES is defined by balanced translocations between EWSR1 and genes encoding ETS family transcription factors (predominantly FLI1 and ERG). The most common chromosomal translocations are t(11;22)(q24;q12), resulting in EWSR1::FLI1 fusion (~85 to 90%) and t(21;22)(q22;q12), resulting in EWSR1::ERG fusion (~5 to 10%). Rare fusions involve ETV1 (7p22), ETV4 (17q21) and FEV (2q35-36) (< 1%). Other rare translocations include t(16;21)(p11;q22) FUS::ERG and t(2;16)(q35;p11) FUS::FEV translocation. The two major translocations are typically detected with reverse-transcription polymerase chain reaction (RT-PCR), fluorescence in situ hybridization, or next-generation sequencing (NGS).
In the GI and hepatobiliary tract, ES is rare and with 1 recent exception, most of the literature consists of case reports and a few case series.4–21 Since the differential diagnosis of ES includes a wide spectrum of neoplasms, accurate diagnosis can be challenging for the surgical pathologist. Here, we report the clinical, morphologic, immunohistochemical, and molecular features of 23 ES encountered in the GI and hepatobiliary tract in order to shed further light on the differential diagnostic problems that it presents in this setting and provide strategies to prevent misdiagnosis and delay in the implementation of appropriate therapy.
MATERIALS AND METHODS
We retrospectively queried the archives of 6 academic institutions, and the consultation files of 12 of the authors for cases of ES of the GI and hepatobiliary tract, in line with appropriate Institutional Research Board approval. The histologic slides and ancillary studies, including immunohistochemistry and molecular studies were reviewed. Clinical data, including demographic information, clinical presentation, imaging studies, endoscopic and/or gross appearance, involvement of other parts of the GI tract, treatment and clinical follow-up were retrieved for each case through the electronic medical records, when available.
RESULTS
Clinical Features
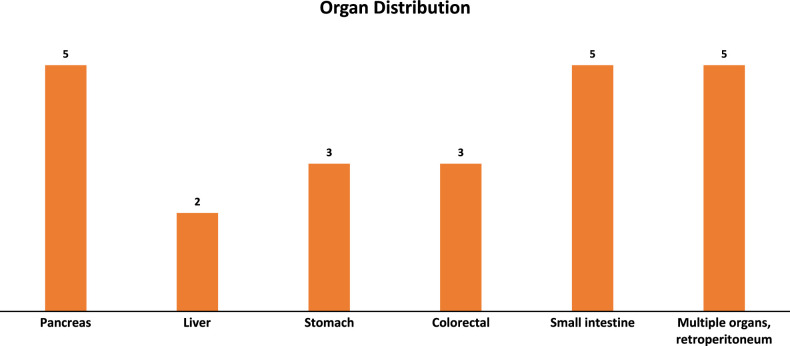
We identified 23 patients with ES of the GI and hepatobiliary tract from 2000 to 2022 (Table 1). An additional case diagnosed as ES in the liver was not included; this case exhibited typical morphology for ES and was positive for CD99 and FLI1, but was negative for EWSRI::ERG and EWSR1::FLI1 translocations on RT-PCR and negative for EWSR1 rearrangement on break-apart FISH. Of the 23 included cases, 11 were women and 12 were men with a median age of 36 years (range, 2 to 64). Tumor locations (Fig. 1) included the pancreas (n=5, 22%), liver (n=2, 9%), stomach (n=3, 13%), colorectum (n=3, 13%), and small intestine (n=5, 22%). In 5 cases (22%), there was involvement of multiple abdominal organs and/or the retroperitoneum. Twenty were primary and 3 were metastases. Most patients presented with symptoms related to the GI and/or hepatobiliary tract. The most common presentation was abdominal pain, followed by nausea and vomiting, weight loss, anemia, and obstructive jaundice. Imaging studies revealed an intra-abdominal mass with heterogeneous enhancement in 4 cases and an enlarging liver mass in 2 additional cases. One patient presented with necrotic rectal prolapse. Preliminary clinical diagnoses included gastrointestinal stromal tumor (GIST), carcinoma, lymphoma, pancreatic carcinoma, ovarian small cell carcinoma with hypercalcemia, hepatic adenoma, and hepatocellular carcinoma. Seven of 23 (30%) patients presented with abdominopelvic disseminated disease; in 2 of these patients, a gynecological primary was suspected. ES was clinically suspected in only 1 patient, the sole pediatric patient in this series (Table 1, patient 1).
TABLE 1.
Clinicopathologic Features of Gastrointestinal and Hepatobiliary Ewing Sarcoma
| Case | Age (yr) | Sex | Location | Size (cm) | Initial Diagnosis | Follow-up (mos) | EWSR1 FISH | Molecular testing |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | F | “Pelvis”, bowel site not specified | 6 | Ewing sarcoma | NED (256) | N/A | N/A |
| 2 | 39 | M | “Retroperitoneum”, bowel site not specified | 9.7 | Ewing sarcoma | NED (60) | N/A | EWSR1::FLI1 |
| 3 | 31 | F | “Abdomen”, bowel site not specified | 18 | Desmoplastic small round cell tumor | LFU (24) | Rearrangement detected | N/A |
| 4 | 20 | F | Small and large intestines | 8 | Small cell malignant neoplasm with neuroendocrine differentiation | DOD (10) | N/A | EWSR1::FLI1 |
| 5 | 35 | M | Stomach, left psoas mass | 3.4 | Melanoma, small round blue cell tumor | DOD (60) | Rearrangement detected | N/A |
| 6 | 64 | M | Sigmoid colon | 13 | Epithelioid gastrointestinal stromal tumor | DOD (12) | Rearrangement detected | N/A |
| 7 | 54 | F | Rectum | 11.2 | Well-differentiated neuroendocrine tumor, grade 2 | DOD (6) | Rearrangement detected | N/A |
| 8 | 36 | M | Pancreas, tail | 2.9 | Poorly differentiated neuroendocrine carcinoma | NED (11) | Rearrangement detected | N/A |
| 9 | 35 | F | Ileum, mesentery | 10 | Poorly differentiated neuroendocrine carcinoma | AWD (34) | Rearrangement detected | N/A |
| 10 | 40 | F | Pancreas mass (body/tail) | 10 | Well-differentiated, neuroendocrine tumor, grade 3 | AWD (22) | N/A | EWSR1::FLI1 |
| 11 | 46 | M | Pancreas (body/tail), retroperitoneum | 2.7 | Small round blue cell tumor | AWD (4) | N/A | EWSR1::FLI1 |
| 12 | 38 | M | Rectum | 1.7 | N/A | NED (24) | N/A | EWSR1::FLI1 |
| 13 | 51 | M | Pancreas | 12 | Solid neoplasm | LFU (8) | N/A | EWSR1::FLI1 |
| 14 | 46 | F | Multiple sites (small bowel, sigmoid colon, mesentery, cecum) | 15 | Malignant neoplasm not further classifiable | AWD (7) | Rearrangement detected | N/A |
| 15 | 22 | F | Liver | 30 | Ewing Sarcoma | AWD (29) | Rearrangement detected | N/A |
| 16 | 20 | F | Liver* | 4.1 | Lymphoma; primary liver tumor | AWD (10) | Rearrangement detected | N/A |
| 17 | 62 | M | Jejunum* | 11 | Ewing Sarcoma | NED (25) | N/A | EWSR1::ERG |
| 18 | 41 | F | Pancreas and duodenum | N/A | Malignant epithelioid neoplasm | NED (3) | Rearrangement detected | N/A |
| 19 | 26 | M | Stomach | 6.5 | Small round blue cell tumor | AWD (48) | Rearrangement detected | N/A |
| 20 | 38 | F | Multiple sites (small intestine and omentum) | 3.1 | Poorly differentiated adenocarcinoma | AWD (2) | N/A | EWSR1::ERG |
| 21 | 20 | M | Jejunum, liver | 6.5 | Lymphoma | LFU (0) | Rearrangement detected | N/A |
| 22 | 35 | M | Stomach* | N/A | Carcinoma | DOD (52) | Rearrangement detected | N/A |
| 23 | 18 | M | Jejunum | Lymphoma | NED (23) | N/A | EWSR1::FEV |
Recurrent/metastatic ES.
N/A, not assessed; NED, no evidence of disease; DOD, died of disease; AWD, alive with disease; LFU, lost to follow-up; yr, years; mo, months.
FIGURE 1.
Primary tumor location of intra-abdominal Ewing sarcoma.
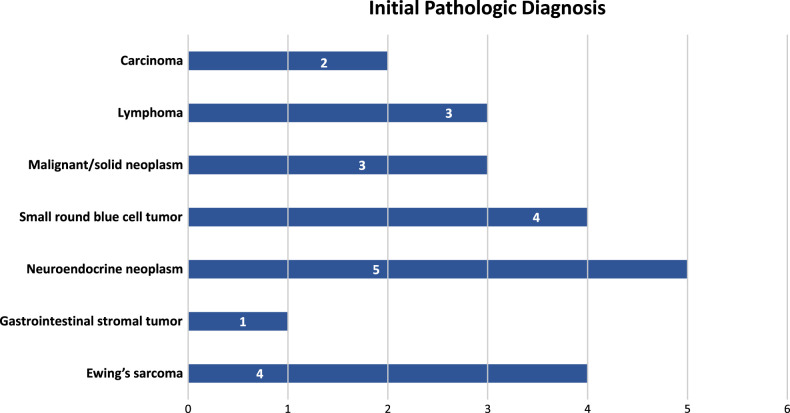
Initial pathologic diagnoses (Fig. 2) included GIST (n=1), poorly differentiated neuroendocrine carcinoma (n=2), well-differentiated neuroendocrine tumor grade 2 (n=1), well-differentiated neuroendocrine tumor grade 3 (n=1), small cell malignant neoplasm with neuroendocrine differentiation (n=1), melanoma (n=1), desmoplastic small round cell tumor (n=1), poorly differentiated carcinoma (n=2), and lymphoma (n=3). Other preliminary diagnoses included small round blue cell tumor (n=2), malignant epithelioid neoplasm (n=1), “solid” neoplasm (n=1), and malignant neoplasm not otherwise classifiable (n=1). Only 4 of the 23 (20%) cases were initially diagnosed as ES, one of which was a metastasis from a known previously diagnosed ES. The tumors from 8 patients were seen in consultation by 2 of the authors and initially diagnosed as GIST1 and neuroendocrine neoplasm1 with the remaining diagnosed descriptively.
FIGURE 2.
Initial pathologic diagnosis of intra-abdominal Ewing sarcoma.
Misdiagnosis led to delayed treatment in 16 of 23 patients in this series. Five patients experienced a prolonged delay in appropriate therapy due to implementation of treatment for the initial misdiagnosis. These misdiagnoses included neuroendocrine tumor/carcinoma (n=3), GIST (n=1), and ovarian small cell carcinoma with hypercalcemia (n=1). The misdiagnosis of neuroendocrine carcinoma in two of the ES was perpetuated when reviewed at a second institution, leading to further delay in diagnosis. Overall, the delay in treatment in these 5 cases ranged from 5 months to 2 years.
Pathologic Features
The tumors measured 2 to 18 cm (mean, 9.4 cm; median, 9.7 cm) in maximum diameter (Table 1). Initial diagnostic procedures were FNA (n=3), biopsy (n=12), FNA and biopsy (n=1), and resection (n=6). Two cases required re-biopsy due to extensive necrosis and/or insufficient tissue for diagnosis. The treatment naïve resection specimens demonstrated lobulated or multinodular masses that were friable and hemorrhagic. Two of the tumors were excised following neoadjuvant therapy and these showed fibrosis, necrosis, remote hemorrhage and small foci of residual tumor. Microscopically, the tumors exhibited solid sheets (Figs. 3 and 4) and nests of uniform small cells with scant cytoplasm, round nuclei and finely stippled chromatin (Fig. 5). Homer-Wright pseudorosettes were identified in 1 case. In 2 cases, the nests of small cells were embedded in dense, fibroblastic stroma suggestive of desmoplastic small round cell tumor. Mitotic figures were present in each case, ranging up to 42 per 10 high power fields.
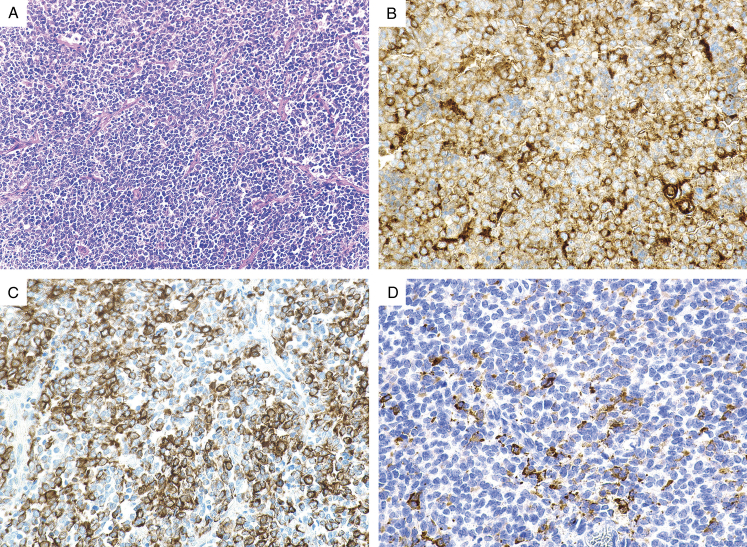
FIGURE 3.
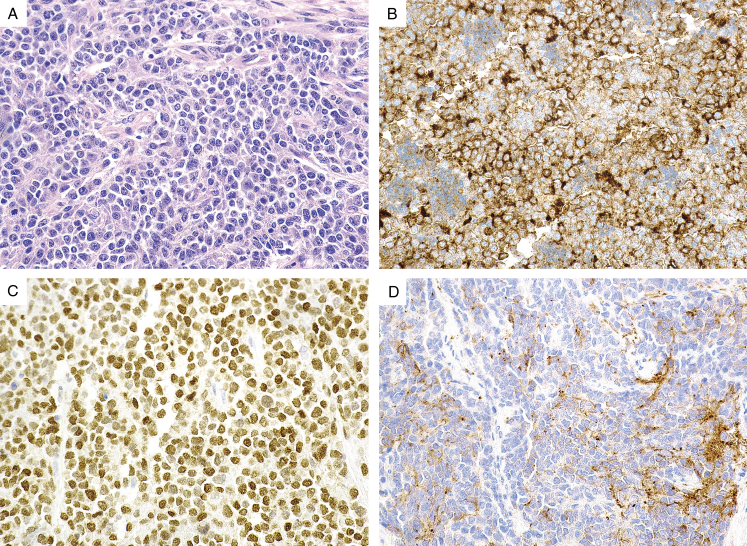
(A) Ewing sarcoma arising in the rectum exhibits a nested epithelioid morphology. The tumor cells demonstrate (B) diffuse positive CD117 expression, (C) strong cytokeratin expression, and (D) patchy synaptophysin expression. The tumor cells also expressed strong CD99 expression (not shown).
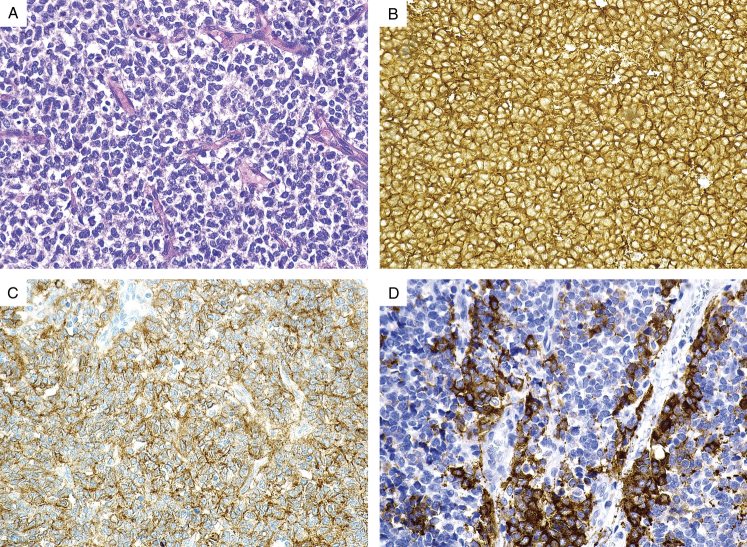
FIGURE 4.
(A) Gastric Ewing sarcoma exhibits a uniform epithelioid morphology. The tumor cells demonstrate (B) diffuse positive CD99 expression, (C) diffuse positive CD117 expression, and (D) patchy synaptophysin expression.
FIGURE 5.
(A) Ewing sarcoma arising in jejunum. The nuclei have a stippled chromatin pattern, suggestive of a neuroendocrine neoplasm. The tumor cells demonstrate (B) diffuse positive CD99 expression, (C) diffuse positive PAX7 expression, and (D) patchy synaptophysin expression.
Ancillary Studies
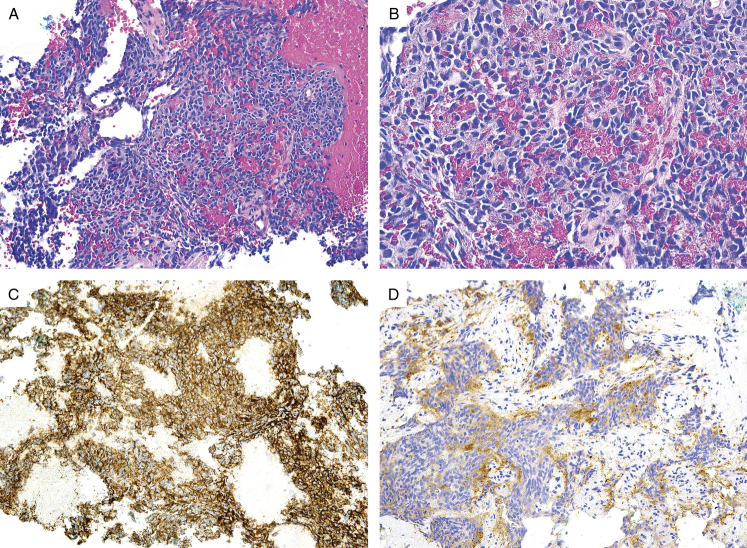
All of the tumors tested showed diffuse and strong membrane staining of CD99 (23/23, 100%) (Fig. 1-4) and 5 tumors displayed diffuse and strong nuclear positivity for FLI1 (5/5, 100%) (Table 2). They were also variably positive for CD117 (5/6, 83%) (Figs. 3 and 4), synaptophysin (10/15, 67%), and pancytokeratin (9/23, 39%) (Figs. 3–6). Rare cases were positive for S100 protein (1/11, 9%), chromogranin (1/14, 8%), and desmin (1/15, 7%). The ES cases were negative for all other markers, including CD45 (0/11), myogenin (0/9), WT1 (0/9), DOG1 (0/5), beta-catenin (0/4), HMB45 (0/4), inhibin (0/3), and CDX2 (0/3).
TABLE 2.
Immunohistochemical Features of Gastrointestinal and Hepatobiliary Ewing Sarcoma
| Immunohistochemical stain | Number positive* (%) |
|---|---|
| Pancytokeratin | 9/23 (33) |
| CD99 | 23/23 (100) |
| FLI1 | 5/5 (100) |
| NKX2.2 | 6/6 (100) |
| S100 | 2/13 (15) |
| CD117 | 5/6 (83) |
| DOG1 | 0/5 |
| Synaptophysin | 9/15 (67) |
| Chromogranin | 1/14 (8) |
| NSE | 2/2 (100) |
| INSM-1 | 2/2 (100) |
| CD45 | 0/11 |
| Desmin | 1/15 (7) |
| Myogenin | 0/9 |
| Beta-catenin | 0/4 |
| HMB45 | 0/4 |
| WT1 | 0/9 |
| CDX2 | 0/3 |
| Inhibin | 0/3 |
Tumor cells show at least focal expression.
FIGURE 6.
(A) Fine needle aspiration of Ewing sarcoma arising in the abdomen. (B) The constituent cells have scant cytoplasm and uniform, ovoid nuclei that are hyperchromatic, suggestive of a neuroendocrine carcinoma. The tumor cells demonstrate (C) diffuse positive CD99 expression and (D) patchy synaptophysin expression.
The diagnosis of ES was confirmed by detection of the EWSR1 rearrangement in 22 cases. Nine were confirmed by next-generation sequencing and 15 by EWSR1 break-apart FISH. Six cases exhibited EWSRI::FLI1 fusion, 2 cases showed EWSR1::ERG fusion and one case had a EWSR1::FEV fusion. The remaining case was diagnosed using traditional morphology and immunohistochemistry.
Clinical Treatment and Patient Follow-Up
Limited treatment and follow-up information was available, with follow-up time varying between 2 months and 256 months (mean, 31.7 months; median, 22 months). Five had complete resection. Four patients had abdominopelvic dissemination and 5 patients had metastatic disease at the time of diagnosis. Four received adjuvant chemotherapy using vincristine, doxorubicin, and cyclophosphamide alternating with ifosfamide and etoposide (VDC+IE), and 2 patients received adjuvant radiotherapy and chemotherapy (VDC+IE). Five patients received neoadjuvant chemotherapy consisting of VDC+IE. Three patients were initially treated with platinum-etoposide due to the diagnosis of neuroendocrine carcinoma, 1 patient was initially treated with imatinib due to the diagnosis of GIST, and 1 patient was initially treated with carboplatin and etoposide following a presumptive clinical diagnosis of ovarian small cell carcinoma, hypercalcemic type. Six patients are alive with no evidence of disease 3, 11, 23, 24, 25, 60, and 256 months after initial diagnosis. Eight are alive with disease 1 to 48 months after initial diagnosis. Five patients died of disease 6 to 60 months after initial diagnosis.
DISCUSSION
ES was first described in 1921 by James Ewing, who reported a series of bone tumors distinct from osteosarcoma that were composed of broad sheets of small cells with pale cytoplasm, small hyperchromatic nuclei, and well-defined cell borders in the absence of any intercellular material.22 Although initially regarded as “diffuse endothelioma of bone”, it is now believed that ES arises from an undefined mesenchymal stem cell.23 ES most frequently arises in patients under 20 years of age and often involves the diaphysis and metaphyseal-diaphyseal regions of the lower limbs and pelvis. Less typical locations include the spine, ribs and skull. Extraskeletal ES occurs in slightly older patients (>30 years) with a wide anatomic distribution. Intrabdominal and retroperitoneal ES are well documented, but involvement of the GI and hepatobiliary tract is not well recognized.
The findings in our series largely confirm and support those of prior reports of extraskeletal ES, with some caveats. The age range (range, 2 to 64 years; median age: 34 years) of ES in the GI and hepatobiliary tract is similar to that reported in a recently published series of GI ES (range, 9 to 59 years; median age, 38 years), but is slightly older than that reported for extraskeletal soft tissue ES (range, 20 months to 63 years; median age, 20 years).22 Although GI ES has been previously reported to show a predilection for the small bowel,20 ES cases were distributed throughout the tubular GI tract in our series, with the pancreas and small bowel equally exhibiting the most common sites of involvement. Pancreatic ES has been previously documented.14 The prognosis of ES for our patients appears similar to previously reported series in the GI tract, with instances of local recurrence and distant metastases. Several large series of extraskeletal ES suggest the prognosis is similar to the more common skeletal subtype, although patients with extraskeletal ES may have a significantly higher risk for local recurrence.24–26 The current treatment recommendation by the National Comprehensive Cancer Network (NCCN) is local treatment (surgery and/or radiotherapy) plus VDC-IE chemotherapy for extraskeletal ES.2 Extraskeletal ES is considered to be a potentially curable disease and has the best prognosis in young patients who are treated with early resection in conjunction with chemotherapy.24,25,27
Seventeen of our 23 patients were initially diagnosed with another entity; the primary diagnosis for 5 cases was a neuroendocrine tumor and in another 1 case the original diagnosis was a GIST. Other preliminary diagnoses included a wide variety of neoplasms typically encountered in the differential diagnosis for tumors with small round cell morphology. Metastases were included in our series, as initial misdiagnosis occurred in one of these tumors as well. Eight cases were referred for consultation for a definitive diagnosis, suggesting that due to its rarity, ES is particularly problematic to diagnose in the gastrointestinal and hepatobiliary tract.
The differential diagnosis of ES in the GI and hepatobiliary tract is broad and includes neuroendocrine neoplasms, epithelioid GI tumor, poorly differentiated carcinoma, lymphoma, desmoplastic small round cell tumor, melanoma, poorly differentiated synovial sarcoma, malignant GI neuroectodermal tumor, and capicua transcriptional repressor (CIC)-rearranged sarcoma, among others. Misdiagnosis of ES as GIST due to the expression of aberrant CD117 has been previously documented.20 However, the propensity to misdiagnose ES as a neuroendocrine neoplasm in the GI and hepatobiliary tract has not been sufficiently emphasized. In this series, 25% of ES were initially diagnosed as neuroendocrine tumors, in large part due to immunohistochemical expression of neuroendocrine markers (n=5) and cytokeratin (n=3). Although unexpected, this misdiagnosis is understandable, given that ES and neuroendocrine tumors are both composed of uniform small round blue cells with round nuclei and stippled chromatin. The presence of small dyshesive cells in conjunction with limited sampling, unusual location, and on occasion, suboptimal tissue preservation further contributes to the misdiagnosis of a neuroendocrine neoplasm in the GI and hepatobiliary tract. However, this diagnostic pitfall can be avoided by including CD99 in the immunohistochemical panel for neuroendocrine neoplasms and, if positive, pursuing reflex testing for EWSR1 rearrangement. It is important to point out that NKX2.2 is not useful in this distinction due to the expression of this marker in well-differentiated neuroendocrine tumors and neuroendocrine carcinomas.28–30
With some important caveats, utilization of select immunohistochemical panels can help in differentiating ES from these as well as other more common entities that occur in the GI tract. Strong and diffuse membranous expression of CD99 is seen in greater than 95% of ES, but some degree of CD99 expression can also be seen poorly differentiated synovial sarcoma, CIC-rearranged sarcoma, lymphoblastic lymphoma, and myeloid sarcoma. Since CD99 lacks specificity in this differential diagnosis, a diagnosis of ES should not rely on this marker alone, in absence of other corroborative immunohistochemical or molecular data. Although nearly all cases of ES show strong nuclear expression of NKX2.2, its utility is limited by expression in other small round cell tumors, including neuroblastoma, small cell carcinoma, poorly differentiated synovial sarcoma, rhabdomyoblastoma, and lymphoblastic lymphoma in addition to neuroendocrine neoplasms.30–33 Similarly, PAX7 immunoreactivity alone is not completely specific for ES, although diagnostic specificity improves when combined with CD99 and NKX2.2.34,35 The diagnosis of poorly differentiated synovial sarcoma can be confirmed by diffuse and strong expression of SS18::SSX fusion-specific antibody (E9X9V) and evidence of SS18::SSX gene fusion on molecular testing,36 while WT1 expression and evidence of CIC gene rearrangement can help to confirm CIC-rearranged sarcoma.37 CD45, TdT, myeloperoxidase, and other hematolymphoid markers can be utilized in the differential diagnosis of lymphoma and myeloid sarcoma. Similarly, as indicated above, a variety of markers utilized in the identification of neuroendocrine tumors, including synaptophysin, CD56, neuron-specific enolase (NSE), chromogranin, and INSM-1 can be seen in ES. ES is reported to show immunoreactivity with antibodies to cytokeratin in up to 25% of cases (33% in our series), which not only leads to diagnostic confusion with neuroendocrine tumors but also poorly differentiated carcinoma. However, CD99 expression is unusual in carcinomas. More importantly, poorly differentiated carcinomas usually show more nuclear pleomorphism, consisting of hyperchromatic nuclei with irregular nuclear membranes, whereas ES is composed of a monomorphic population of cells lacking nuclear pleomorphism or significant cytologic atypia. When tested, a significant number of ES in our series expressed CD117 (5/6, 83%), which led to misdiagnosis as GIST and delayed treatment in at least one case. This can be prevented with incorporation of DOG1, CD34 and PDGFRA38 as supplemental immunohistochemical stains, as ES is essentially negative for these markers. Mutational analysis can confirm the diagnosis of suspected GISTs lacking both CD117 and DOG1. Desmoplastic small round cell tumor can exhibit an overlapping immunohistochemical profile with ES, but it is also characterized by a unique perinuclear dot pattern of desmin, which is not seen in ES, and membranous CD99 expression is not as strong and diffuse. Since the t(11;22)(p13;q12) translocation in this tumor results in an EWSR1::WT1 fusion transcript, caution should be exercised in the interpretation of EWSR1 rearrangement by FISH in this setting.
The diagnosis for most of the neoplasms in our series (22 of 23) was confirmed with molecular testing, either by next-generation sequencing or by break-apart FISH for EWSR1 rearrangement. Although FISH for EWSR1 rearrangement is not specific for ES, most investigators consider the demonstration of an EWSR1 rearrangement in the appropriate context to be sufficient to confirm the diagnosis. One case was diagnosed based on classic morphology, including Homer-Wright-type rosettes and intracytoplasmic glycogen, and a panel of more traditional immunohistochemical stains.
In summary, we present the clinicopathologic features of GI and hepatobiliary ES and provide several strategies to avoid misdiagnosis when encountered in this unusual location. Since early surgical removal and implementation of adjuvant therapy has a significant impact on the survival rate of these patients, ES should be considered in the differential diagnosis of any GI or hepatobiliary tumor with epithelioid and/or small round cell morphology, particularly in limited samplings.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Oyewale Shiyanbola, Email: oos267@stanford.edu;shiyanb14@hotmail.com.
Recep Nigdelioglu, Email: recep.nigdelioglu@sanfordhealth.org.
Deepti Dhall, Email: ddhall@uabmc.edu.
Iván A. González, Email: iagonzal@iu.edu.
Laura M. Warmke, Email: lwarmke@iu.edu.
Shula Schechter, Email: sschecht@med.umich.edu.
Won-Tak Choi, Email: won-tak.choi@ucsf.edu.
Shaomin Hu, Email: hus2@ccf.org.
Lysandra Voltaggio, Email: lvoltag1@jhmi.edu.
Yujie Zhang, Email: yzhan665@jh.edu.
Tom Z. Liang, Email: tliang8@jh.edu.
Huaibin M. Ko, Email: hmk2102@cumc.columbia.edu.
Greg W. Charville, Email: gwc@stanford.edu.
Teri A. Longacre, Email: longacre@stanford.edu.
REFERENCES
- 1. Esiashvili N, Goodman M, Marcus RB, Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–430. [DOI] [PubMed] [Google Scholar]
- 2. Abboud A, Masrouha K, Saliba M, et al. Extraskeletal Ewing sarcoma: Diagnosis, management and prognosis. Oncol Lett. 2021;21:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi EY, Gardner JM, Lucas DR, et al. Ewing sarcoma. Semin Diagn Pathol. 2014;31:39–47. [DOI] [PubMed] [Google Scholar]
- 4. Hallon K, Mansour S, Damouny M, et al. Primary Ewing Sarcoma of the Stomach. World J Oncol. 2021;12:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassan R, Meng LV, Ngee KT, et al. Extraskeletal Ewing sarcoma of the duodenum presenting as duodenojejunal intussusception. Lancet. 2022;399:1265. [DOI] [PubMed] [Google Scholar]
- 6. Inoue M, Wakai T, Korita PV, et al. Gastric Ewing sarcoma/primitive neuroectodermal tumor: A case report. Oncol Lett. 2011;2:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalid H, Hussain N, Shamshad R. Esophageal extraskeletal neoplasm Ewing’s sarcoma: Case report. Int J Surg Case Rep. 2022;97:107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khuri S, Gilshtein H, Sayidaa S, et al. Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Stomach. Case Rep Oncol. 2016;9:666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HS, Kim S, Min YD, et al. Ewing’s Sarcoma of the Stomach; Rare Case of Ewing’s Sarcoma and Suggestion of New Treatment Strategy. J Gastric Cancer. 2012;12:258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolosov A, Dulskas A, Pauza K, et al. Primary Ewing’s sarcoma in a small intestine - a case report and review of the literature. BMC Surg. 2020;20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liquidano-Perez E, Martinez-Vazquez R, Ponce-Cruz JG, et al. Intestinal Ewing sarcoma: An unusual presentation in the pediatric age. Bol Med Hosp Infant Mex. 2022;79:199–202. [DOI] [PubMed] [Google Scholar]
- 12. Maxwell AW, Wood S, Dupuy DE. Primary extraskeletal Ewing sarcoma of the stomach: a rare disease in an uncommon location. Clin Imaging. 2016;40:843–845. [DOI] [PubMed] [Google Scholar]
- 13. Nissim L, Mandell G. Gastric Ewing Sarcoma identified on a Meckel’s scan. Radiol Case Rep. 2020;15:1235–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel D, Nandu NS, Reddy A. Extraosseus Ewing’s Sarcoma in Pancreas: A Review. Cureus. 2020;12:e7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rafailidis S, Ballas K, Psarras K, et al. Primary Ewing sarcoma of the stomach--a newly described entity. Eur Surg Res. 2009;42:17–20. [DOI] [PubMed] [Google Scholar]
- 16. Shek TW, Chan GC, Khong PL, et al. Ewing sarcoma of the small intestine. J Pediatr Hematol Oncol. 2001;23:530–532. [DOI] [PubMed] [Google Scholar]
- 17. Shu Q, Luo JN, Liu XL, et al. Extraskeletal Ewing sarcoma of the stomach: A rare case report. World J Clin Cases. 2023;11:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang S, Zhu W, Zhang H, et al. Extraosseous Ewing Sarcoma of the Cervical Esophagus: Case Report and Literature Review. Ear Nose Throat J. 2022;101:NP203–NP208. [DOI] [PubMed] [Google Scholar]
- 19. Ye Y, Qiu X, Mei J, et al. Primary gastric Ewing sarcoma/primitive neuroectodermal tumor. J Int Med Res. 2021;49:300060520986681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yin T, Shao M, Sun M, et al. Gastrointestinal Ewing Sarcoma: A Clinicopathological and Molecular Genetic Analysis of 25 Cases. Am J Surg Pathol. 2024;48:275–283. [DOI] [PubMed] [Google Scholar]
- 21. Young R, Dishop M, Eshun F, et al. Gastric Ewing Sarcoma: An Unexpected Endoscopic Discovery in a Girl With Abdominal Pain and Iron Deficiency Anemia. J Pediatr Gastroenterol Nutr. 2022;74:e16. [DOI] [PubMed] [Google Scholar]
- 22. Angervall L, Enzinger FM. Extraskeletal neoplasm resembling Ewing’s sarcoma. Cancer. 1975;36:240–251. [DOI] [PubMed] [Google Scholar]
- 23. Tirode F, Laud-Duval K, Prieur A, et al. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. [DOI] [PubMed] [Google Scholar]
- 24. Ahmad R, Mayol BR, Davis M, et al. Extraskeletal Ewing’s sarcoma. Cancer. 1999;85:725–731. [PubMed] [Google Scholar]
- 25. Rud NP, Reiman HM, Pritchard DJ, et al. Extraosseous Ewing’s sarcoma. A study of 42 cases. Cancer. 1989;64:1548–1553. [DOI] [PubMed] [Google Scholar]
- 26. Salah S, Abuhijla F, Ismail T, et al. Outcomes of extraskeletal vs. skeletal Ewing sarcoma patients treated with standard chemotherapy protocol. Clin Transl Oncol. 2020;22:878–883. [DOI] [PubMed] [Google Scholar]
- 27. Raney RB, Asmar L, Newton WA, Jr, et al. Ewing’s sarcoma of soft tissues in childhood: a report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J Clin Oncol. 1997;15:574–582. [DOI] [PubMed] [Google Scholar]
- 28. Ishida M, Sekine S, Fukagawa T, et al. Neuroendocrine carcinoma of the stomach: morphologic and immunohistochemical characteristics and prognosis. Am J Surg Pathol. 2013;37:949–959. [DOI] [PubMed] [Google Scholar]
- 29. Yang MX, Coates RF, Ambaye A, et al. NKX2.2, PDX-1 and CDX-2 as potential biomarkers to differentiate well-differentiated neuroendocrine tumors. Biomark Res. 2018;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russell-Goldman E, Hornick JL, Qian X, et al. NKX2.2 immunohistochemistry in the distinction of Ewing sarcoma from cytomorphologic mimics: Diagnostic utility and pitfalls. Cancer Cytopathol. 2018;126:942–949. [DOI] [PubMed] [Google Scholar]
- 31. Yoshida A, Sekine S, Tsuta K, et al. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012;36:993–999. [DOI] [PubMed] [Google Scholar]
- 32. Hung YP, Fletcher CD, Hornick JL. Evaluation of NKX2-2 expression in round cell sarcomas and other tumors with EWSR1 rearrangement: imperfect specificity for Ewing sarcoma. Mod Pathol. 2016;29:370–380. [DOI] [PubMed] [Google Scholar]
- 33. Saeed SM, Hassan U, Hussain M, et al. Expression of NKX2.2 in Non-Ewing Tumors With Round Cell Morphology. Cureus. 2023;15:e50704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Charville GW, Varma S, Forgo E, et al. PAX7 Expression in Rhabdomyosarcoma, Related Soft Tissue Tumors, and Small Round Blue Cell Neoplasms. Am J Surg Pathol. 2016;40:1305–1315. [DOI] [PubMed] [Google Scholar]
- 35. Machado I, Charville GW, Yoshida A, et al. Does PAX7 and NKX2.2 immunoreactivity in Ewing sarcoma have prognostic significance? Virchows Arch. 2022;480:909–917. [DOI] [PubMed] [Google Scholar]
- 36. Ortiz Requena D, Longacre TA, Rosenberg AE, et al. Synovial Sarcoma of the Gastrointestinal Tract. Mod Pathol. 2024;37:100383. [DOI] [PubMed] [Google Scholar]
- 37. Specht K, Sung YS, Zhang L, et al. Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumors compared to EWSR1-rearranged Ewing sarcomas: further evidence toward distinct pathologic entities. Genes Chromosomes Cancer. 2014;53:622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papke DJ, Jr, Forgo E, Charville GW, et al. PDGFRA Immunohistochemistry Predicts PDGFRA Mutations in Gastrointestinal Stromal Tumors. Am J Surg Pathol. 2022;46:3–10. [DOI] [PubMed] [Google Scholar]