Abstract
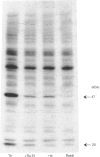
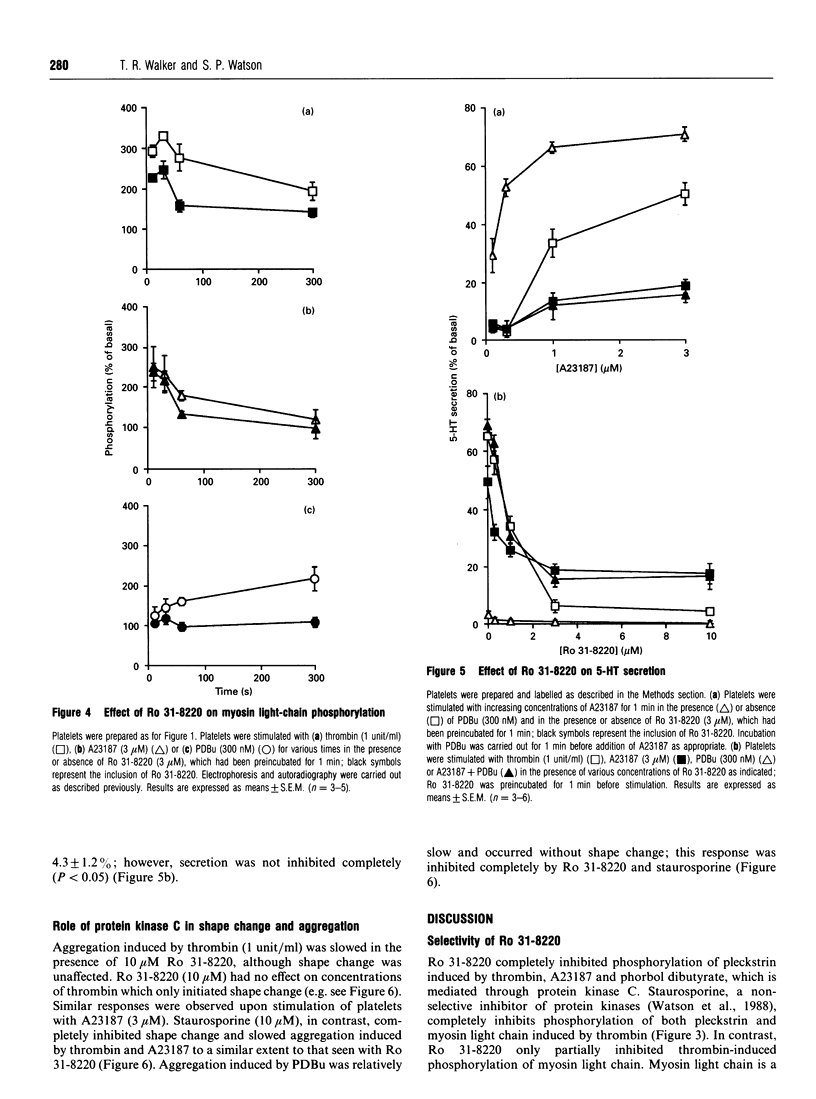
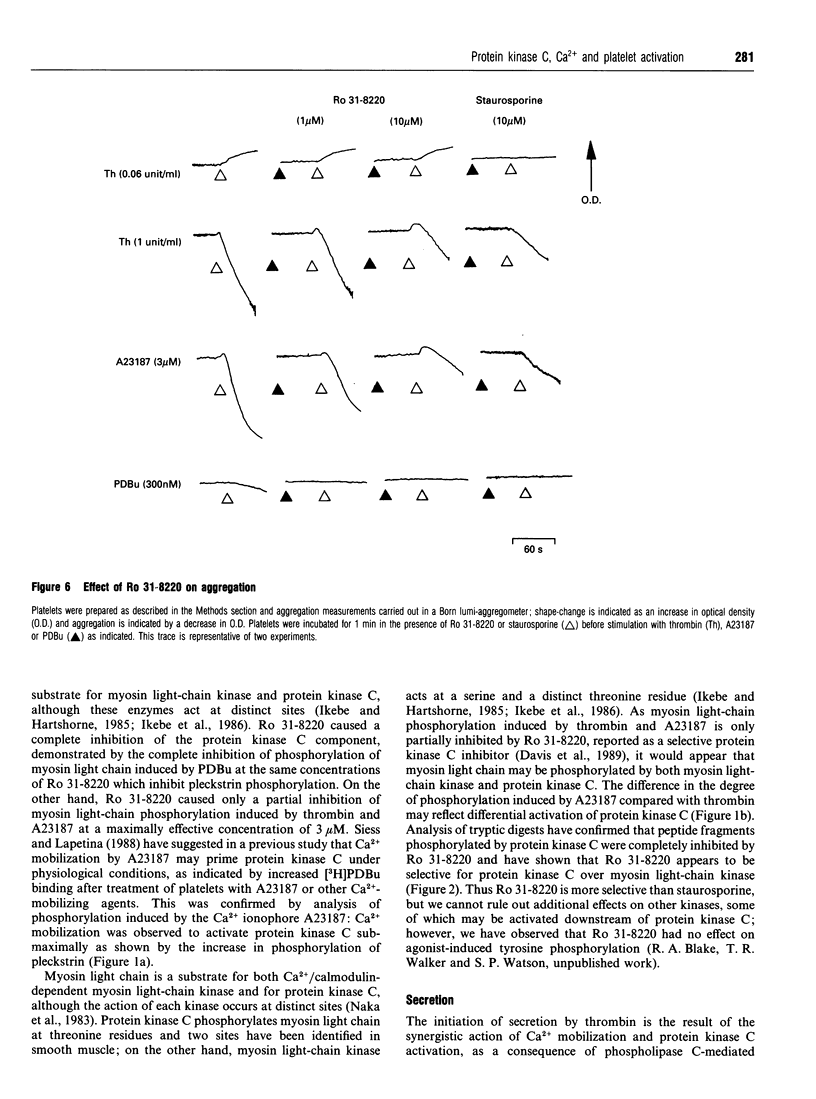
The aim of this study was to establish further the role of protein kinase C in aggregation and secretion of 5-hydroxytryptamine (5-HT) from human platelets by using the selective inhibitor Ro 31-8220. Ro 31-8220 (3 microM) inhibited completely phosphorylation of pleckstrin, the major protein kinase C substrate, induced by thrombin, A23187 or phorbol dibutyrate (PDBu). Myosin light-chain phosphorylation induced by PDBu was also inhibited completely, but that induced by thrombin or A23187 was only inhibited partially. As myosin light chain is a substrate for both myosin light-chain kinase and protein kinase C, these results suggest that Ro 31-8220 is inhibiting only the protein kinase C-induced phosphorylation and that Ro 31-8220 has a greater selectivity to protein kinase C than does its structural analogue staurosporine. The stimulation of secretion of 5-HT by maximally effective concentrations of thrombin and A23187 was decreased significantly by 3 microM Ro 31-8220, but not inhibited completely. These results indicate a major role for protein kinase C in the stimulation of secretion by agonist- and ionophore-induced activation. On its own, a maximal concentration of PDBu induced a small degree of secretion (3.3 +/- 1.0%), but potentiated markedly the response to a submaximal concentration of A23187 (300 nM) to a level greater than seen with a maximal concentration of A23187. A similar set of results was also seen with aggregation, but not with shape change. We interpret these results to mean that the signalling event for secretion and aggregation is Ca2+, and this is potentiated markedly by protein kinase C. In the case of secretion, it appears that it is the synergy which is the major determining factor in influencing the extent.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
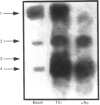
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Crabos M., Imber R., Woodtli T., Fabbro D., Erne P. Different translocation of three distinct PKC isoforms with tumor-promoting phorbol ester in human platelets. Biochem Biophys Res Commun. 1991 Aug 15;178(3):878–883. doi: 10.1016/0006-291x(91)90973-b. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Adelstein R. S. Isolation and properties of platelet myosin light chain kinase. Biochemistry. 1976 Jun 1;15(11):2370–2377. doi: 10.1021/bi00656a019. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Rigmaiden M., Stewart G. Evidence for a role of myosin phosphorylation in the initiation of the platelet shape change response. J Biol Chem. 1984 Aug 10;259(15):9826–9831. [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J., Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem. 1986 Jan 5;261(1):36–39. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem. 1985 Aug 25;260(18):10027–10031. [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nunn D. L., Watson S. P. A diacylglycerol kinase inhibitor, R59022, potentiates secretion by and aggregation of thrombin-stimulated human platelets. Biochem J. 1987 May 1;243(3):809–813. doi: 10.1042/bj2430809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Ca2+ mobilization primes protein kinase C in human platelets. Ca2+ and phorbol esters stimulate platelet aggregation and secretion synergistically through protein kinase C. Biochem J. 1988 Oct 1;255(1):309–318. [PMC free article] [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989 Jan;69(1):58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Stabel S., Parker P. J. Protein kinase C. Pharmacol Ther. 1991;51(1):71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tyers M., Rachubinski R. A., Stewart M. I., Varrichio A. M., Shorr R. G., Haslam R. J., Harley C. B. Molecular cloning and expression of the major protein kinase C substrate of platelets. Nature. 1988 Jun 2;333(6172):470–473. doi: 10.1038/333470a0. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Hambleton S. Phosphorylation-dependent and -independent pathways of platelet aggregation. Biochem J. 1989 Mar 1;258(2):479–485. doi: 10.1042/bj2580479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McNally J., Shipman L. J., Godfrey P. P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem J. 1988 Jan 15;249(2):345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Ruggiero M., Abrahams S. L., Lapetina E. G. Inositol 1,4,5-trisphosphate induces aggregation and release of 5-hydroxytryptamine from saponin-permeabilized human platelets. J Biol Chem. 1986 Apr 25;261(12):5368–5372. [PubMed] [Google Scholar]