Abstract
Opioid use disorders (OUD) are a public health concern in the United States and worldwide. Current medications for OUD have severe side effects and are often heavily regulated. A novel treatment strategy to be used alone or in combination with existing medications is active immunization with anti-opioid vaccines, which stimulate production of opioid-specific antibodies that bind to the target drug and prevent its distribution to the brain. While anti-opioid vaccines have shown promising preclinical efficacy, prior clinical evaluation of vaccines targeting stimulants indicate that efficacy is limited to a subset of subjects that achieve optimal antibody responses. We have previously reported that depletion of interleukin-4 (IL-4) with a monoclonal antibody increased opioid-specific IgG2a and total IgG, and increased number of germinal centers and germinal center Tfh cells in response to anti-opioid vaccines via Type I IL-4 signaling. The current study further investigates the mechanisms associated with IL-4-mediated increases in efficacy, and whether IL-4 depletion affects specific processes involved in germinal center formation, including affinity maturation, class-switching, and plasma cell differentiation in mice. Additionally, results demonstrate that pre-immunization production of IL-4 after ex vivo whole blood stimulation predicted in vivo vaccine-induced antibody titers in outbred mice. Such mechanistic studies are critical for rational design of next-generation vaccine formulations, and support the use of IL-4 as a predictive biomarker in ongoing OUD vaccine clinical studies.
INTRODUCTION
Opioid use disorders (OUD) affect over 40 million individuals worldwide (1), which imposes an estimated annual economic burden of over 1 trillion dollars in the United States alone (2). Current medications for OUD target the mu opioid receptor (MOR) and include the MOR agonist methadone, partial agonist buprenorphine, and antagonist naltrexone. While effective, these medications are limited by poor accessibility and issues with patient compliance due to the potential for severe side effects. Active immunization with an anti-opioid conjugate vaccine is a novel therapeutic strategy that may be useful alone or in combination with currently available pharmacotherapies. Anti-opioid vaccines consist of an opioid-like hapten conjugated to a large immunogenic carrier protein such as subunit keyhole limpet hemocyanin (sKLH), tetanus toxoid (TT), or cross-reactive material (CRM197) and combined with adjuvants such as aluminum hydroxide to stimulate an immune response. Active immunization stimulates the production of opioid-specific polyclonal IgG antibodies which can bind to the target drug and prevent its distribution to brain, inhibiting centrally mediated opioid-induced effects [reviewed in (3)]. Proof of preclinical efficacy, selectivity, and safety has been shown for anti-opioid vaccines against oxycodone/hydrocodone, heroin/morphine, fentanyl, and fentanyl analogues [reviewed in (3-5)], and an oxycodone vaccine (OXY-sKLH adsorbed on aluminum salts) is now under investigation in a Phase Ia/Ib first-in-human clinical trial (NCT04458545).
To date, clinical trials for vaccines for substance use disorder (SUD) have had mixed success. While some Phase II trials for anti-nicotine vaccines have shown promising efficacy in increasing smoking cessation rates, several Phase III trials have failed to meet their primary efficacy endpoints [reviewed in (6)]. However, data from clinical trials for both anti-nicotine (7) and anti-cocaine (8) vaccines provided proof of efficacy in increasing drug abstinence among individuals who produced high titers of high affinity antibodies. For example, anti-cocaine vaccine clinical trials have reported that individuals with greater than 43 μg/mL serum drug-specific antibodies had >50% reduction in overall cocaine use and a greater incidence of cocaine-free urine, but only 38% of individuals attained this serum antibody concentration (8). These results highlight the importance of investigating strategies to increase OUD vaccine efficacy by increasing antibody production using novel adjuvants, vaccine delivery platforms, or identification of predictive biomarkers of vaccine efficacy.
Most first-generation SUD vaccine formulations, including most of those tested in clinical trials, used aluminum hydroxide (alum) as an adjuvant (4, 6). Alum is currently the most widely used adjuvant in FDA-approved vaccine formulations, and is often chosen due to its known efficacy and safety profile (9). However, since current clinical studies suggest that alum may not be sufficient to produce an adequate antibody response in all subjects, new adjuvants are being investigated with OUD vaccines to increase vaccine efficacy. In preclinical studies, OUD vaccines have been tested with MF59 (10), LTA1 and dmLT (11), CpG ODN (12), dsRNA (13), MPLA (14), Advax (13) complete Freund’s (14), and ALF (15) with varying degrees of success, although many of these adjuvants may not suitable for clinical translation due to cost, patent rights, or toxicity. As an alternative strategy to increase vaccine efficacy, and to provide mechanistic insights, our laboratory investigated whether the addition of immunomodulators, including monoclonal antibodies (mAb) to neutralize various cytokines, would increase the immune response to the candidate OXY-sKLH vaccine. It was found that a neutralizing mAb against interleukin-4 (IL-4) increased oxycodone-specific IgG titers, increased class switching from IgG1 to IgG2a, and increased efficacy of the vaccine after drug challenge (16).
IL-4 is a pleiotropic cytokine that is produced by T cells, natural killer T (NKT) cells, mast cells, innate lymphoid cells, eosinophils, and basophils (17). IL-4 can signal through either the type I or type II IL-4 receptors (IL-4R). The type I receptor is a heterodimer of IL-4Rα and IL-2R common gamma chain (γc), and is found on most lymphocytes and myeloid cells. Downstream of this receptor are signal transducer and activator of transcription 6 (STAT6) and insulin receptor substrate 2 (IRS2). The type II receptor consists of the IL-4Rα and the IL-13Rα1, and can signal in response to either IL-4 or IL-13. The type II receptor is found on most myeloid and non-hematopoietic cells, and canonically signals downstream through STAT6 [reviewed in (18)]. IL-4 is extremely important for the protection against parasites and helminth worms (19-21), and has more recently been shown to be critical for germinal center (GC) formation in many contexts (22-30). Within the GC, IL-4 has been found to be secreted by NKT (25) and Tfh cells (31), and can affect specific processes such as GC B cell differentiation (28), class switching to IgG1 and IgE (27, 32), affinity maturation (28, 31) and the development of memory and plasma cells in activated B cells (22-24, 28). Immunization in IL-4 deficient mice (25) or in mice with STAT6 deficient B cells (26), antibody-based depletion of IL-4 (25, 30), or depletion of cells that produce IL-4 (25) led to smaller or misshapen GCs with less GC B cells and altered gene expression, which led to impaired antibody responses and decreased vaccine efficacy in a variety of viral infection models or after immunization with NP-OVA adsorbed on alum. On the other hand, there is some evidence that depletion of IL-4 can positively affect GC formation after secondary immunization with OVA in complete Freund’s adjuvant (33), and leads to increased vaccine efficacy after administration of an HIV vaccine (34) or after challenge with respiratory syncytial virus (35). These contradicting reports suggest that the role of IL-4 in the GC may be dependent on antigen and/or adjuvant context. This is supported by evidence that Th2-mediated responses are negatively affected by IL-4 depletion, while Th1-mediated responses are not (28). The complex role of IL-4 in the GC was further highlighted by a recent study showing that IL-4R can activate signaling in some cell populations, while also acting as a decoy to sequester IL-4 away from the GC in other cell populations, such as follicular dendritic cells (24).
Previously, we found that immunization with OXY-sKLH or a model peptide-carrier conjugate vaccine in conjunction with IL-4 depletion led to an increase in GC-Tfh cells (16), changed the T cell transcription profile, and increased the number and size of GCs (36). These changes were mediated through Type I IL-4 signaling, but not through STAT6. Therefore, we hypothesized that these changes must be mediated by a reduction in IRS2 signaling. NKT cells, a major source of IL-4 during the GC response, were not necessary for vaccine efficacy (36). The current study sought to further elucidate the molecular signaling following IL-4 depletion in the context of anti-opioid immunization, and its effect on GC processes such as affinity maturation, class switching, and plasma cell differentiation. Contrary to our original hypothesis, we found that the increase in efficacy after IL-4 depletion is not reproduced by ablation of IRS2 signaling. Additionally, IL-4 depletion did not change somatic hypermutation or affinity maturation in B cells after OXY-sKLH immunization. While we have previously reported that IL-4 depletion increases classing switching from IgG1 to IgG2a, we found that the antibody subclass itself did not affect the efficacy of an oxycodone-specific mAb in reducing oxycodone’s effects after drug challenge. IL-4 depletion increased the number of oxycodone-specific antibody secreting cells (ASCs) after 3 immunizations, but did not lead to an increase in long-lived plasma cells in bone marrow. Finally, we investigated the potential of IL-4 as a predictive biomarker of vaccine efficacy, and found that pre-immunization IL-4 derived from ex vivo T cell stimulation negatively correlates with oxycodone-specific antibody titers and vaccine efficacy after immunization. These studies further uncover mechanisms of vaccine efficacy that can be used as a blueprint for rational design of next generation vaccine formulations, and support the exploration of IL-4 as a putative predictive biomarker in clinical studies.
MATERIALS AND METHODS
Drugs and Immunomodulators.
Oxycodone HCl was obtained from Sigma Aldrich (St. Louis, MO). Anti-IL-4 (αIL-4) monoclonal antibody (rat anti-mouse IgG1, clone 11b11, Cat. No. BE0045) was obtained from Bio X cell (West Lebanon, NH). PHA-P was obtained from Invitrogen (Cat No. inh-phap) and lipopolysaccharides from Escherichia coli O55:B5 were obtained from Sigma Aldrich (Cat No. L2880-10MG).
Ethics Statement.
Animal studies were performed according to the Guide for the Care and Use of Laboratory Animals and the National Institute of Health. Protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Animals were euthanized by AALAC approved CO2 chambers, and all efforts were made to minimize suffering.
Mice.
Six- to ten- week old male wild-type Balb/c, C.129X1-Il4ratm1Tch/J (strain # 007680), or J:DO diversity outbred mice (strain #009376) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were group housed under a 14/10 hour light/dark cycle and had free access to food and water. All testing occurred during the light phase.
Hapten synthesis and conjugate vaccines.
An oxycodone-based hapten containing a tetraglycine linker at the C6 position (OXY) was synthesized and conjugated to either subunit keyhole limpet hemocyanin (sKLH, Biosyn, Carlsbad, CA) or chicken ovalbumin (OVA) as previously described (37, 38). 60 μg of unconjugated sKLH or conjugate vaccines were adsorbed on 300 μg aluminum adjuvant (Alhydrogel ‘85’, 2%, Brenntag Biosector, Denmark) diluted in PBS for immunization at a total volume of 60 μL.
Experimental Design.
For active immunization studies, mice were immunized on days 0, 14, and 28 with sKLH alone or OXY-sKLH intramuscularly (i.m.) in two sites (opposite legs) in the gastrocnemius muscle. For IL-4 depletion, 0.5 mg αIL-4 was administered intraperitoneally (i.p.) on days −2 and 1. Mice were bled via facial vein on day 34 for oxycodone-specific IgG analysis. J:DO mice were also bled on days −28 and −14 prior to study for in vitro stimulation of whole blood. On day 35, mice were challenged with 2.25 mg/kg oxycodone delivered subcutaneously (s.c.). Thirty minutes after injection, mice were euthanized and decapitated to collect brains and blood samples for LCMS/MS analysis. For passive immunization studies, Balb/c mice were immunized i.p. with 40 mg/kg mAb. Twenty-four hours later, mice were bled via facial vein to determine antibody concentration using biolayer interferometry. One hour later, mice were challenged with 2.25 mg/kg oxycodone, s.c. After 30 minutes, mice were euthanized and blood and brain were collected to determine oxycodone concentration via LCMS/MS analysis.
Flow Cytometry.
Bone marrow collected from mouse femurs on day 100 post-immunization was analyzed for oxycodone-specific long-lived plasma cells. Bone marrow was processed to a single cell suspension and stained extracellularly for APC-eFluor780 CD90.2 (eBioscience), APC-eFluor780 F4/80 (eBioscience), APC-eFluor780 Ly-6G (eBioscience), and APC-eFluor780 CD11c (eBioscience) all as part of the DUMP gate, and with CD138 BV650 (eBioscience). Cells were permeabilized using a BD Cytofix/Cytoperm kit, and stained with PE-AF647 decoy, OXY-Biotin-Streptavidin-PE, and Pacific Orange F(ab)’2 (H+L) (Invitrogen). All fluorophores were diluted 1:100 for staining. Oxycodone-specific bait and decoy reagents were produced as previously described (16). Plasma cells were defined as DUMP−Decoy−Ig+CD138+, and oxycodone-specific plasma cells were defined as plasma cells that were Decoy−OXY+.
Antibody Analysis.
Total IgG and IgG subclass-specific titers were measured via ELISA as previously described (16). Briefly, 96 well Costar plates were coated with 5 ng/well OXY-OVA in carbonate coating buffer overnight at 4°C. The following day, plates were blocked for 1 hr with 1% porcine gelatin, and serum samples were serial diluted starting at 1:200 in PBS-T. Plates were incubated for 2 hours, followed by overnight incubation with secondary antibodies: goat-anti-mouse IgG HRP (1:30,000; Jackson ImmunoResearch, West Grove, PA), goat-anti-mouse IgG1 (1:35,000; Alpha Diagnostic International, Inc., Cat. No. 40126-GAF-BLK) or goat-anti-mouse IgG2a (1:7500; Alpha Diagnostic International, Cat. No. 40127-GAF-BLK). For IgE ELISAs, plates were coated with 5 ng/well OXY-OVA or IgE capture antibody (Biotechne, Cat. No. MAB9935-100). Serum was diluted starting at 1:50, and primary antibodies were incubated with 1:5000 anti-IgE antibody conjugated to HRP (VWR, Cat. No. 100242-786). Purified mouse IgE (Biolegend, Cat. No. 401701) was used to obtain a concentration curve. The following day, plates were developed using SIGMAFAST OPD substrate (Sigma-Aldrich, St. Louis, MO).
BCR Sequencing.
BCR sequencing of previously characterized hybridomas generated from mice immunized with OXY-sKLH or OXY-sKLH+αIL-4 (39) was carried out as previously described (40). Briefly, 1-5 million cells were pelleted in a microfuge tube via centrifugation at 2,000 rpm. Cells were washed with 1 mL PBS, pelleted, and frozen at −80°C until processed. To extract RNA, pellets were thawed, and RNA was extracted using RNeasy Mini kit (Qiagen, Hilden, Germany). RNA was reverse transcribed with Maxima First Strand cDNA synthesis kit (Thermo Fisher, Waltham. MA). cDNA was amplified via PCR using Q5 High-Fidelity DNA polymerase. PCR product was then purified using a QIAquick PCR purification kit (Qiagen, Hilden, Germany). Amplified DNA was sent for Sanger sequencing at the University of Minnesota Genomics Center. Heavy chain sequences were aligned to germline mouse heavy chain sequences using IgBlast, and mutations in CDR1 and CDR2 regions were quantified.
Expression of oxycodone-specific monoclonal antibodies.
Oxycodone-binding mAb (clone HY1-3G8) (39) VH and VL sequences were cloned into pcDNA3.4 mammalian expression vectors (Genscript). The CMV promoter driven pcDNA3.4 expression vector was modified to contain a Kozak consensus sequence preceding an open-reading frame (ORF) with a murine IGHV signal peptide (MGWSCIILFLVATATGVHS), or a murine IGKV signal peptide (METDTLLLWVLLLWVPGSTG) for the antibody heavy and light chain expression vector, respectively. The heavy chain ORF terminates with a murine IgG1 or IgG2a constant region (Accession # P01868.1 and P01863.1, www.uniprot.org), and the light chain vector ORF terminates with a murine IgK constant region (Accession # P01837.2, www.uniprot.org). The IgG2a sequence was mutated using site-directed mutagenesis to introduce L234A, L235A, and P329G mutations (IgG2a-LALA-PG) to block FcγRI-IV binding as previously described (41). Oxycodone-specific mAbs were produced via transient expression in the Expi293 or ExpiCHO expression system (ThermoFisher Catalog # A14635 and A29133) according to manufacturer instructions. Transfections were performed using a 2.5:1 ratio of LC vector:HC vector, with 1 μg of total vector DNA/mL of culture volume. Cell culture supernatant was harvested 7-10 days following transfection, and mAb was purified from filtered cell culture supernatant via liquid chromatography on an ÄKTA pure with a HiTrap Protein G HP column (Cytiva Product # 29048581), and buffer exchanged into PBS, pH 7.4.
ELISPOT.
ELISPOT was performed using Mabtech Mouse IgG ELISpotBASIC kit (HRP). PVDF membrane ELISPOT plates were pretreated with 35% EtOH for 1 minute and then washed 5x with sterile water. Plates were then coated with 5 μg/mL OXY-OVA or 15 μg/mL IgG capture antibody overnight for detection of oxycodone-specific IgG secreting cells or total IgG secreting cells, respectively. The following day, plate was washed 5x with PBS and blocked with DMEM+10% FBS. Meanwhile, spleens and lymph nodes from immunized mice were collected and processed to a single cell suspension. Cells were washed 3 times with ClonaCell Medium A (StemCell). Cells were counted and plated in triplicate in ClonaCell Medium A at a density of 200,000 cells/well for OXY-OVA coated wells and 50,000 cells/well for IgG coated wells. Plates were incubated overnight at 37°C+5% CO2 and spots were visualized using TMB substrate according to the manufacturer’s instructions. Images were acquired with an CTL BioSpot S5 Core Analyzer and analyzed using ImmunoSpot 7 software (Cell Technology Limited, Shaker Heights, OH).
Biolayer Interferometry.
Antibody avidity assays were performed on an Octet Red 96e instrument (Sartorius). Serum samples from immunized mice on day 35 (analyte) and biotinylated antigen, OXY-Biotin, (ligand) were diluted in PBS. Assays were performed by loading OXY-Biotin onto pre-hydrated streptavidin sensors at 0.1 μg/ml (loading step 60 sec) followed by 60 sec baseline. Sensors were then moved into analyte for 180 sec for association, followed by a 300 sec dissociation step. All steps were performed at room temperature with shaking at 1000 rpm. Serum samples were run at 1:200 dilution in PBS. Dissociation rate (Kdiss) and response values were calculated using Sartorius HT analysis software version 11.1.3.50. All data were inspected for quality of fit to the calculated curve (R2>0.95). Monoclonal serum antibody concentrations were calculated by fitting response values to a standard curve produced with an oxycodone-specific monoclonal antibody.
In vitro whole blood stimulation.
In vitro stimulation of whole blood samples was adapted from a previously described protocol (42). Facial blood samples were collected in EDTA coated tubes (Sai Infusion Technologies, Lake Villa, IL). Blood was mixed with 200 μL RPMI and plated in a 96 well plate. Phytohemagglutinin (PHA-P) was added to a final concentration of 10 μg/mL, while lipopolysaccharide (LPS) was added to a final concentration of 1 ng/mL. Samples were incubated at 37°C with 5% CO2 overnight. The following day, supernatants were collected and quantified using an IL-4 ELISA (Biolegend, San Diego, CA) using the manufacturer’s instructions.
LCMS/MS analysis of oxycodone concentrations.
Blood and brain samples were processed and analyzed on an Agilent G6470A triple quadrupole LCMS/MS system as previously described (43). Data acquisition and peak integration were analyzed using Mass Hunter software (Tokyo, Japan).
Statistical Analysis.
Statistical analyses were performed using Prism version 9.1.2 (GraphPad, LaJolla, CA). Mean antibody titers and concentration, IgG2a:IgG1 ratios, and drug concentrations were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons post hoc test. Kdiss measurements and number of heavy chain mutations were analyzed using Mann-Whitney U test. Total IgG+ spots, ratio of OXY+/IgG+ spots, total plasma cells, and OXY+ plasma cells were analyzed using Student’s T test with or without Welch’s correction, depending on whether the samples had equal variances as indicated by an F test. The relationship between oxycodone-specific antibodies, serum and brain oxycodone concentrations, and LPS- or PHA- induced IL-4 concentrations were analyzed via two-tailed Pearson correlation after determination of normality using D’Agostino-Pearson’s test.
RESULTS
Ablation of IL-4R mediated IRS2 signaling does not increase vaccine efficacy.
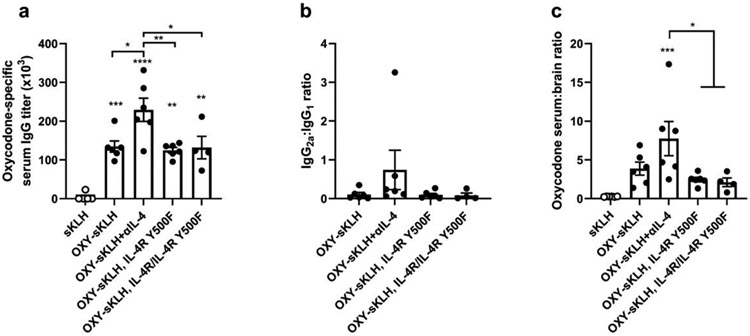
We previously hypothesized that the increase in vaccine efficacy after IL-4 depletion was due to loss of IRS2 signaling downstream of the IL-4 receptor (36). To test this, WT Balb/c mice or mice with a Y500F mutation in the IL-4R that prevents IRS2 phosphorylation downstream of the IL-4R (IL-4R Y500F) were immunized with OXY-sKLH. Heterozygous littermates (IL-4R/IL-4R Y500F) were also immunized to test whether reduced IRS2 signaling would produce an equivalent increase in vaccine efficacy. A group of WT Balb/c mice was immunized with OXY-sKLH with antibody-based IL-4 depletion as a positive control. After 3 immunizations, IL-4R Y500F and IL4R/IL4R Y500F mice did not show increased oxycodone-specific IgG antibody titers (Figure 1A) nor an increased IgG2a:IgG1 ratio (Figure 1B) compared to WT. 30 min after oxycodone challenge, serum:brain ratio of oxycodone in IL4R Y500F and IL4R/IL4R Y500F mice was comparable to WT mice without IL-4 depletion (Figure 1C). These data suggest that deletion of IRS2 signaling downstream of the IL-4R does not increase vaccine efficacy.
Figure 1. Ablation of IL-4 dependent IRS2 signaling does not increase vaccine efficacy after OXY-sKLH immunization.
WT mice (n=6/group) were immunized on days 0, 14, and 28 with OXY-sKLH, with or without IL-4 depletion with a αIL-4 mAb and compared to IL-4R Y500F mice (n=6) or their heterozygous littermates (n=4). Blood was collected via facial vein on day 34 to measure A) oxycodone-specific serum IgG titers, and B) ratio of IgG2a vs. IgG1 subclass titers. On day 35, mice were challenged with 2.25 mg/kg oxycodone. Thirty minutes later, blood and brain were collected to measure concentration of oxycodone in each, expressed as C) serum:brain oxycodone ratio. Data are mean±SEM. Statistical analysis performed via one way ANOVA with Tukey’s multiple comparisons post hoc test. Data are from one independent experiment. Stars over columns indicate significance compared to the control or as indicated by brackets. Statistical symbols: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
IL-4 depletion does not change somatic hypermutation or affinity maturation.
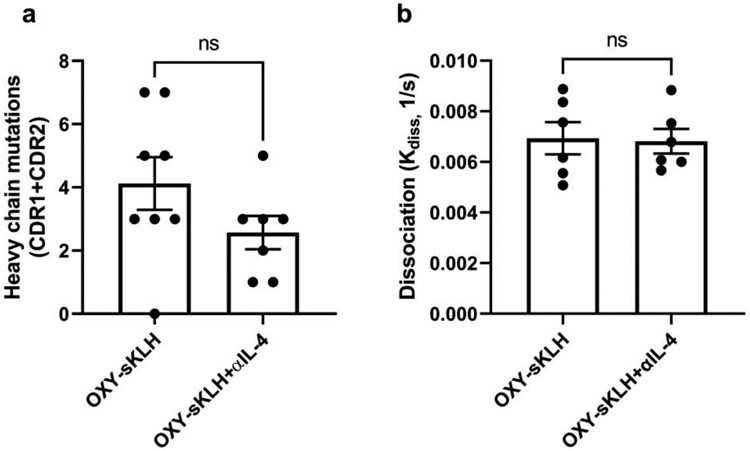
IL-4 is involved in somatic hypermutation and affinity maturation of B cells in the GC to produce high affinity antibodies (28, 31). To determine whether depletion of IL-4 during anti-opioid immunization affects these processes, BCRs were sequenced from hybridomas previously isolated from mice that were immunized with OXY-sKLH with or without an IL-4 depleting antibody. BCR heavy chain sequences were compared to germline sequences using IgBlast, and mutations in complementary determining regions (CDR) 1 and 2 were quantified. There was no significant difference in the number of mutations after anti-oxycodone immunization with or without IL-4 depletion, indicating that IL-4 depletion had no effect on somatic hypermutation (Figure 2A). To further test this hypothesis, the dissociation rate (kdiss) of polyclonal antibodies from WT mice immunized with or without IL-4 depletion from Figure 1 was measured using biolayer interferometry as a correlate of antibody avidity. There were no differences in antibody avidity between the two groups (Figure 2B), suggesting that affinity maturation is unaffected by IL-4 depletion during anti-opioid immunization.
Figure 2. IL-4 depletion does not influence somatic hypermutation or affinity maturation.
The heavy chain B cell receptor sequence from hybridomas (n=7-8/group) generated by immunization with OXY-sKLH or OXY-sKLH with IL-4 depletion were analyzed against germline heavy chain sequences using IgBlast. A) Comparison of heavy chain CDR1 and CDR2 mutations in oxycodone-specific antibodies after OXY-sKLH immunization with or without IL-4 depletion. B) Sera from OXY-sKLH and OXY-sKLH+αIL-4 immunized mice from Figure 1 (n=6/group) were analyzed for avidity via biolayer interferometry. Data are mean±SEM. Statistical analysis performed via Mann Whitney U test. Data are from one independent experiment.
Oxycodone-specific IgE was not detected in mice immunized with OXY-sKLH.
Since IL-4 is known to induce class switching to IgE after immunization (27, 32), we hypothesized that IL-4 produced after vaccination with OXY-sKLH may induce a subset of opioid-specific IgE antibodies which is prevented by addition of an IL-4 depleting antibody. Without the influence of IL-4, these B cells would instead class-switch to IgG, which would lead to the increased IgG titers seen in mice with depleted IL-4. Mice were immunized with OXY-sKLH with or without IL-4 depletion on days 0, 14, and 28, and then serum was collected on day 34 for analysis oxycodone-specific and total IgE concentration. While total IgE was detected and did decrease after IL-4 depletion, no oxycodone-specific IgE was detected in any groups (Supplementary Figure 1), indicating that oxycodone-specific IgE is not produced in response to OXY-sKLH immunization, and that the reduction in IgE class-switching is not the cause of the IL-4 mediated increase in vaccine efficacy.
IgG2a antibodies display equivalent efficacy to IgG1 antibodies against drug challenge in mice.
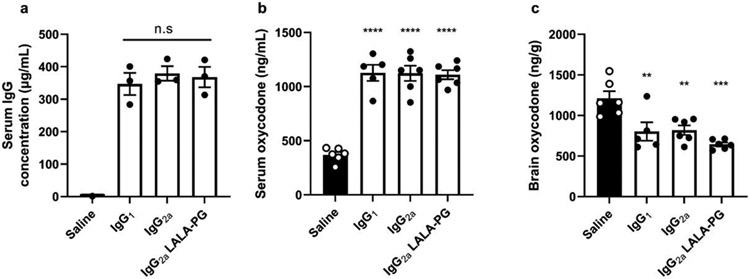
We have reported that depletion of IL-4 increases class switching to IgG2a after anti-opioid immunization and increases vaccine efficacy after drug challenge (16, 36). To directly assess whether the IgG2a subclass itself is responsible for the increase in vaccine efficacy, an anti-oxycodone mAb (39) was expressed recombinantly as murine IgG1, IgG2a, or IgG2a LALA-PG, the latter of which contains mutations to remove FcγRI-IV binding (41). Mice were passively immunized with recombinant mAb and then challenged with oxycodone 24 hours later. Blood was collected immediately before challenge, and no differences in antibody concentration were observed between groups (Figure 3A). Similarly, there were no differences in oxycodone concentration in the serum (Figure 3B) or brain (Figure 3C) between the passively immunized groups 30 minutes post-drug challenge. These data suggest that an increase in IgG2a alone is not sufficient to explain the increase in efficacy seen after depletion of IL-4 during anti-opioid immunization. Because no differences were found between the wild-type IgG2a and the IgG2a LALA-PG displaying mutated FcγRI-IV binding sites, this study further supports previous reports that antibody mediated effector functions do not play a role in opioid vaccine efficacy (44).
Figure 3. IgG subclass and antibody mediated effector functions do not influence vaccine efficacy.
Mice (n=6/group) were passively immunized with recombinant mAb expressed as IgG1, IgG2a, or IgG2a LALA-PG (mutation which prevents binding to FcγRI-IV). One day later, serum was collected to measure A) antibody concentration via biolayer interferometry. Mice were then challenged with 2.25 mg/kg oxycodone and B) blood and C) brain were collected 30 minutes later to determine opioid concentration by LC-MS/MS. Data are mean±SEM. Statistical analysis performed via one-way ANOVA with Tukey’s multiple comparisons post hoc test. Two independent experiments were performed, data shown are representative from one experiment. Stars directly over columns indicate significance compared to control. Statistical symbols: ** p<0.01, *** p<0.001, **** p<0.0001
IL-4 depletion increases early antibody secreting B cells but not long-term plasma cells.
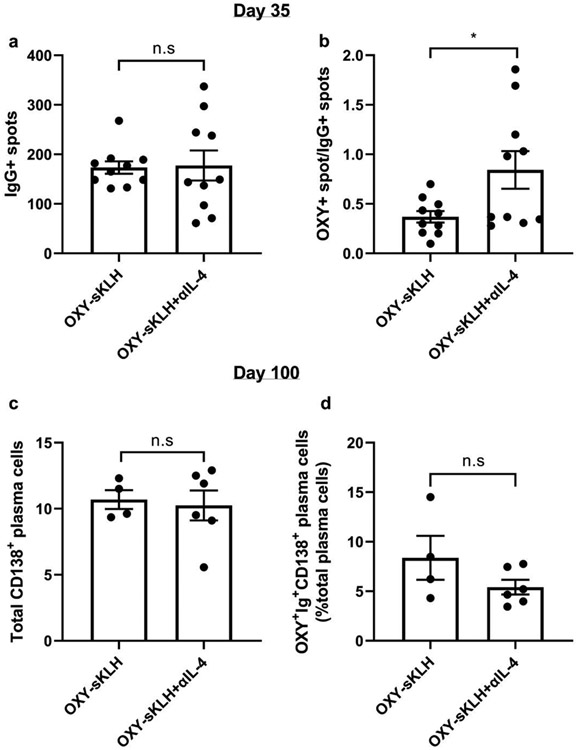
Previous literature has suggested that IL-4 is involved in the memory cell vs. plasma cell choice in the GC (22-24, 28). To assess whether depletion of IL-4 increases the number of antibody secreting cells after anti-opioid vaccination, the same mice from Supplementary Figure 1 were sacrificed on day 35, and then spleens and lymph nodes were harvested to perform antigen-specific ELISPOT. There were no differences in total IgG secreting cells between mice immunized with OXY-sKLH and OXY-sKLH with an IL-4 depleting antibody (Figure 4A). Conversely, there was a significant increase in oxycodone-specific IgG secreting cells when normalized to the total IgG secreting cells (Figure 4B). A second cohort of mice was then immunized in a similar fashion and left until day 100 to assess whether this increase in early antibody secreting cells led to an increase in oxycodone-specific long-lived plasma cells (LLPCs) in the bone marrow (Gating strategy provided in Supplementary Figure 2). Analysis of bone marrow showed there was no significant difference in total or oxycodone-specific LLPCs in mice immunized with or without IL-4 depletion (Figure 4C and D). These data suggest that the depletion of IL-4 may increase plasmablast formation at day 35; however, these plasmablasts are short-lived and do not home to the bone marrow.
Figure 4. IL-4 depletion increases early oxycodone-specific antibody secreting B cells.
Mice (n=10/group, combined from two independent experiments) were immunized with OXY-sKLH with or without IL-4 depletion. After 3 immunizations, spleens and lymph nodes were collected and processed into a single cell suspension. Total and oxycodone-specific antibody secreting cells (ASCs) were measured by ELISPOT. A) Number of total IgG ASCs and B) oxycodone-specific ASCs normalized to total IgG ASCs. A second cohort of mice (n=4-6/group, from one independent experiment) were similarly immunized and bone marrow was collected on day 100. C) Total plasma cells in the bone marrow and D) oxycodone-specific plasma cells as a percent of total plasma cells were identified via flow cytometry. Data are mean±SEM. Statistical analysis performed via Student’s t test (A, C, D) or Welch’s t test (B). Statistical symbols: * p<0.05.
T-cell secreted IL-4 is a predictive biomarker of oxycodone vaccine efficacy in mice.
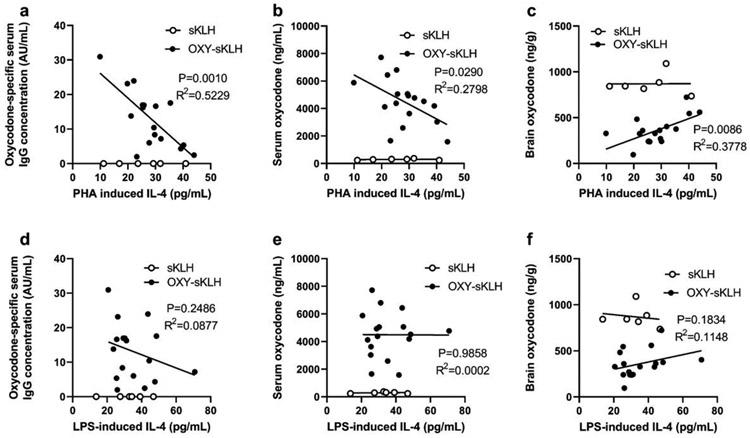
Since depletion of IL-4 increases antibody titers and vaccine efficacy after OXY-sKLH immunization, we hypothesized that pre-immunization production of IL-4 would predict post-immunization antibody titers and vaccine efficacy. To assess IL-4 as a predictive biomarker, blood was collected from genetically diverse outbred mice and stimulated ex vivo with either lipopolysaccharide (LPS, for innate immune cell stimulation) or phytohemagglutinin (PHA, for non-specific T cell stimulation). After 24 hours, supernatant was collected and analyzed for IL-4. Mice were then immunized with an anti-oxycodone vaccine on days 0, 14, and 28, and blood was collected again on day 34 to assess oxycodone-specific antibody titers. The next day, mice were challenged with 2.25 mg/kg oxycodone, and blood and brain were collected 30 minutes post-challenge to analyze the pharmacokinetics of oxycodone. Serum oxycodone-specific IgG concentration significantly correlated with serum and brain oxycodone concentration, as expected (Supplementary Figure 3). Additionally, pre-immunization concentration of IL-4 produced from T cells after ex vivo PHA stimulation showed a significant negative correlation with oxycodone-specific antibody concentration (Figure 5A), a significant negative correlation with serum oxycodone concentration (Figure 5B), and a significant positive correlation with brain oxycodone concentration after challenge (Figure 5C). On the other hand, these metrics did not correlate with pre-immunization IL-4 produced by innate immune cells through stimulation with LPS (Figure 5D-F). These data suggest that pre-immunization T cell derived IL-4 produced through non-specific ex vivo stimulations can be used as a biomarker to predict anti-oxycodone vaccine efficacy.
Figure 5. Pre-immunization IL-4 concentration correlates with vaccine efficacy in mice.
Jackson outbred diversity mice (J:DO) mice were bled pre-immunization, and whole blood was plated for in vitro stimulation with either PHA (to stimulate T cells) or LPS (to stimulate innate immune cells). After 24 hours, supernatant was collected, and IL-4 was measured via ELISA. Mice (n=18 active immunization, 6 control immunization) were then immunized 3 times and blood was collected 7 days after the final immunization. Oxycodone-specific IgG concentration was measured by ELISA. Mice were subsequently challenged with oxycodone (2.25 mg/kg, s.c.), and blood and brain were collected 30 minutes post-challenge to measure concentration of oxycodone via LC-MS/MS. Linear correlation between A) oxycodone-specific IgG concentration and PHA-induced IL-4, B) post-challenge serum oxycodone concentration and PHA induced IL-4, and C) post-challenge brain oxycodone concentration and PHA induced IL-4. There was no linear correlation between D) oxycodone-specific IgG concentration and LPS-induced IL-4, E) post-challenge serum oxycodone concentration and LPS-induced IL-4, or F) post-challenge brain oxycodone concentration and LPS-induced IL-4. Linear associations determined via Pearson correlation. Data are from one independent experiment.
DISCUSSION
As testing of anti-opioid vaccine in clinical settings begins, it is imperative to optimize vaccine efficacy to maximize their chance of success. The data from previous clinical trials of SUD vaccines indicate that generation of high concentrations of high affinity antibodies is important to achieve significant vaccine efficacy (7, 8); however, the immunological mechanisms underlying optimal antibody generation are not well characterized. Our previous studies indicate that depletion of IL-4 increased the efficacy of OUD vaccines for oxycodone and fentanyl by increasing total IgG titers and increasing class switching from IgG1 to IgG2a (16, 36). The role of IL-4 in GC formation appears to be specific to antigen/adjuvant context, with it being critical for GC formation in some studies, while in other studies GCs are enhanced in its absence (16, 24-26, 28, 33-36). We have previously shown that in the context of an OXY-sKLH vaccine, IL-4 depletion increases GC formation (36), increases antigen-specific GC-Tfh cells (16), and modulates T cell transcription via signaling through the Type I IL-4 receptor, but not through STAT6 (36). Additionally, we have found that depletion of IL-4 increases the efficacy of other anti-opioid vaccines including a lead fentanyl vaccine (F-CRM) (36), suggesting that these findings may be applicable to additional OUD or SUD conjugate vaccines. To extend our previous findings, we tested whether this increase in efficacy could be recapitulated by ablation of IRS2 signaling downstream of the IL-4 receptor. Further, we tested the effect of IL-4 depletion on specific GC processes that IL-4 has been shown to regulate, including somatic hypermutation and affinity maturation, class switching to IgG1 and IgE, and plasma cell generation. Finally, we tested whether the relative inherent ability of T cells or innate immune cells to produce IL-4 could be used as a predictive biomarker of vaccine efficacy.
Previous studies have shown that depletion of IL-13 during active immunization against oxycodone does not produce an increase in vaccine efficacy equivalent to IL-4 depletion, indicating that the increase in efficacy is mediated by a reduction in Type I signaling (36). Furthermore, while genetic deletion of IL-4 in mice recapitulates the increase in vaccine efficacy seen after antibody-based depletion, deletion of IL-4R or STAT6 does not (36). Similarly, in the current study, mice bearing a Y500F mutation in the IL-4Ra chain to eliminate downstream signaling through phosphorylation of IRS2 did not show an increase in vaccine efficacy compared to WT mice. Taken together, these results were not able to implicate IL-13, IL-4R, STAT6, or IRS2, which are the main components of the canonical IL-4 signaling pathway, in the mechanism of the increase in vaccine efficacy following IL-4 depletion. Some literature suggests that IL-4 may signal through STAT5 in certain contexts (45), making this a possible contributor to the increase in efficacy seen after IL-4 depletion; however, testing this hypothesis may be challenging due to the lack of highly specific STAT5 pharmacological inhibitors and STAT5’s role downstream of other signaling pathways including IL-2 family receptors (IL-2, IL-7, IL-9, and IL-15), GM-CSF, erythropoietin, thrombopoietin, epidermal growth factor, and platelet-derived growth factor (46). Another potential hypothesis is that the effect is mediated through inhibition of IL-4 signaling through a non-IL-4R based pathway, although there is no evidence to date of IL-4 signaling through a non-canonical receptor. One caveat to these studies is the use of full genetic deletions to test the functions of IL-4 signaling. Not only can genetic manipulations lead to uncharacterized immune system deficiencies, but the elucidation of the increasingly complex and often opposing role of IL-4 signaling in different cell types (24, 28) makes one speculate that deletion of IL-4 signaling in all cell types may be hiding phenotypes specific to certain cell populations that would require more nuanced approaches to assess, such as conditional knockouts or adoptive transfer.
IL-4 has been shown to be important for somatic hypermutation and affinity maturation in some contexts (28, 31). Studies in IL-4 deficient animals have shown a significant reduction in activation induced cytidine deaminase (AID) (28) and a reduction in affinity of antibodies to the target antigen. These changes were more pronounced in IgG1 secreting B cells compared to IgG2a secreting B cells (31). In the current study, no differences were seen in the number of mutations in heavy chain CDR1 and CDR2 in anti-oxycodone mAbs isolated from vaccination with or without IL-4 depletion. A limitation of this method is that mAb selection using hybridoma technology may be biased towards high affinity clones, and a limited number of sequences were obtained to compare between groups. Therefore, as a follow-up experiment, antibody avidity in polyclonal sera was measured by BLI, and no difference was found in antibody avidity between groups with or without IL-4 depletion. Since the polyclonal antibody response consisted of both IgG1 and IgG2a antibodies, it is possible that there were differences in affinity maturation in the IgG1 subset which were not detected due to compensation in affinity from the IgG2a subset. Regardless, any changes in affinity that may be present in a subset of cells did not prevent an increase in efficacy after drug challenge, indicating that antibody affinity is not necessary for the increased efficacy in this specific context.
We have long reported that increases in IgG2a, along with increases in IgG1, have correlated with increases in vaccine efficacy in a variety of adjuvant contexts (16, 36). Here, we directly tested whether IgG2a antibodies have inherently greater capacity than IgG1 to protect against oxycodone after challenge by passively immunizing mice with an anti-oxycodone mAb expressed as IgG1, IgG2a, and the FcγR-silent IgG2a-LALA-PG variant. This study showed no difference between the mAbs in their efficacy in preventing oxycodone distribution to the brain, indicating that an individual IgG subclass is not inherently sufficient or responsible for increasing vaccine efficacy at the time of drug challenge. We hypothesize that activation of multiple IgG subclasses is required for achieving higher overall IgG antibody titers (i.e., IgG1+IgG2a+IgG2b) and a more effective response against opioids. The lack of noticeable effects of the FcγR-silent IgG2a-LALA-PG variant further reinforces previous reports that antibody mediated effector functions are not necessary for vaccine efficacy after drug challenge (44). One alternative hypothesis for the correlation between IgG2a production and increased vaccine efficacy is that an early IgG2a response during immunization increases Fc mediated antigen presentation of immune complexes, which in turn increases overall antibody production. Another plausible explanation is that rather than needing a Th1 polarized response, adjuvants that can stimulate both Th1 and Th2 responses can engage non-overlapping populations of B and T cells, leading to an overall increase in antibody production. These results will be important for future vaccine design and adjuvant selection to pair Th1 and Th2 inducing adjuvants.
During GC formation, IL-4 has been implicated in the differentiation of antibody-secreting plasma cells and in the plasma vs. memory cell choice (24, 28). Since IL-4 depletion increases GC-Tfh cells in the GC which results in increased antibody titers, we hypothesized that the increased number of GC-Tfh cells leads to increased interactions with GC B cells, increasing survival and differentiation into plasma cells. We found that immunization with OXY-sKLH and a depleting IL-4 mAb increased the number of oxycodone-specific antibody secreting cells at day 35, visualized via ELISPOT. When determining whether the increase in plasmablast formation translated into an increase in long-lived plasma cells in the bone marrow, we found no significant difference in oxycodone-specific plasma cells at day 100. This suggests that the increase in plasmablasts caused by IL-4 depletion is transient, and these cells likely die during the contraction of the immune response, or alternatively they may become tissue-resident plasma cells in other organs. Transient increases in antibody secreting cells may be beneficial for OUD/SUD vaccines, as recent arguments have suggested that long-lived and/or permanent immunity to drugs of abuse raise ethical issues of bodily autonomy. While long-lasting treatment may be beneficial in some ways for sustained abstinence due to ease of patient burden to receive frequent treatment, some have suggested that permanent blockade of opioids through immunization could lead to ethical considerations relating to patient choice to continue treatment or even the possibility of coerced treatment within vulnerable populations (47). As vaccines for OUD and other SUDs undergo clinical evaluation, these ethical issues will have to be considered carefully. The current results do suggest that further investigation is needed into the mechanisms of IL-4 before considering it to be used as a molecular adjuvant; however, given current results, we hypothesized that IL-4 may hold additional promise as a predictive biomarker of vaccine efficacy.
As OXY-sKLH is currently undergoing clinical evaluation, it is necessary to test putative biomarkers to identify correlates of protection, which may be critical to identifying individuals who would most benefit from OUD vaccines. Previous studies have identified that pre-immunization number of oxycodone-specific naïve B cells correlates positively with vaccine response (48), providing the first putative biomarker for efficacy of the OXY-sKLH vaccine. Due to our findings that IL-4 depletion increases vaccine efficacy, we hypothesized that IL-4 may also have utility as a predictive biomarker, with those who inherently produced lower levels of IL-4 after immunization (Th1 biased immune response) responding better to anti-opioid immunization than those who have an innately Th2 biased immune response. Therefore, we tested whether concentration of pre-immunization IL-4 after non-specific stimulation of either T cells or innate immune cells in vitro would correlate with post-immunization vaccine response in vivo. We found that in genetically diverse outbred mice, higher levels of pre-immunization IL-4 produced by T cells stimulated with PHA negatively correlates with oxycodone-specific IgG titers. These data are consistent with our previous findings that IL-4 depletion affects GC-Tfh cells and the T cell transcription program (16, 36). A similar personalized medicine approach could be implemented as a screening strategy for patients by analyzing the potential of their T cells to produce IL-4 or other cytokines pre-vaccination through in vitro or ex vivo assays to determine which individuals would respond favorably to anti-opioid immunization.
In this final experiment, we did not see a correlation between innate immune cell derived IL-4 through stimulation with LPS and vaccine response. It should be noted that TLR4, the receptor to which LPS binds, has been shown to be necessary for vaccine efficacy after OXY-KLH immunization (14), yet TLR4 agonists have not shown consistent success in increasing the efficacy of vaccines for OUD (14, 15). In the human population, IL-4 and its downstream signaling components (IL-4R, STAT6) are highly polymorphic loci with high minor allele frequencies for individual single nucleotide polymorphisms (49-57), making it an attractive target for a predictive biomarker in clinical studies. In fact, IL-4 has been shown to correlate with efficacy in vaccines against hepatitis B (49, 58), Japanese encephalitis virus (51), measles (52), tetanus and diphtheria (59), and streptococcus pneumoniae (60). The current results support the use of IL-4 as a putative predictive biomarker for patient stratification in future clinical studies for OUD vaccines, including an ongoing Phase I trial for OXY-sKLH, and ultimately for individualized or personalized treatment of patients with OUD.
Supplementary Material
Key Points:
IL-4 depletion increased antigen-specific ASCs after anti-opioid immunization in mice
Recombinant oxycodone-specific IgG1 and IgG2a display equivalent efficacy
T cell derived IL-4 may be a predictive biomarker of anti-oxycodone vaccine efficacy
Acknowledgements:
The authors thank the University of Minnesota Flow Cytometry Resource for technical and research support.
Financial Support:
This work was supported by R01DA041730 (MP), U01DA051658 (MP), T32DA007097 (BC), and F31DA054760 (BC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- OUD
opioid use disorder
- SUD
substance use disorder
- sKLH
subunit keyhole limpet hemocyanin
- TCRα
T cell receptor α
- GC
germinal center
- NKT cells
natural killer T cells
- TLR
toll-like receptor
- APC
antigen presenting cell
- mAb
monoclonal antibody
- Fc
constant fragment
- Th
T helper cell
- PD-L1
programmed death-ligand 1
- ICOSL
inducible T cell co-stimulator ligand
- STAT6
signal transducer and activator of transcription 6
- IRS
insulin receptor substrate
Footnotes
Competing Interests: MP is the inventor of “Cytokine Signaling Immunomodulators and Methods”. MP, CB and DH are inventors on other pending patents related to vaccines or antibodies against opioids. All authors declare that there are no other competing interests.
Data availability:
Data are available upon reasonable request by contacting the corresponding author. Oxycodone monoclonal antibody binding sequences are confidential due to intellectual property rights.
References
- 1.Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, Bruneau J, Altice FL, Henderson G, Rahimi-Movaghar A, and Larney S. 2019. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet 394: 1560–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florence C, Luo F, and Rice K. 2021. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend 218: 108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pravetoni M, and Comer SD. 2019. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158: 107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heekin RD, Shorter D, and Kosten TR. 2017. Current status and future prospects for the development of substance abuse vaccines. Expert Rev Vaccines 16: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 5.Hwang CS, and Janda KD. 2017. A Vision for Vaccines: Combating the Opioid Epidemic. Biochemistry 56: 5625–5627. [DOI] [PubMed] [Google Scholar]
- 6.Truong TT, and Kosten TR. 2022. Current status of vaccines for substance use disorders: A brief review of human studies. J Neurol Sci 434: 120098. [DOI] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, and Rennard SI. 2011. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, and Kosten TR. 2009. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 66: 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Pasquale A, Preiss S, Tavares Da Silva F, and Garcon N. 2015. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines (Basel) 3: 320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson C, Baehr C, Schmiel SE, Accetturo C, Mueller DL, and Pravetoni M. 2019. Alum adjuvant is more effective than MF59 at prompting early germinal center formation in response to peptide-protein conjugates and enhancing efficacy of a vaccine against opioid use disorders. Hum Vaccin Immunother 15: 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone AE, Scheuermann SE, Haile CN, Cuny GD, Velasquez ML, Linhuber JP, Duddupudi AL, Vigliaturo JR, Pravetoni M, Kosten TA, Kosten TR, and Norton EB. 2021. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bremer PT, Schlosburg JE, Lively JM, and Janda KD. 2014. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharm 11: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake S, Bremer PT, Zhou B, Petrovsky N, Smith LC, Hwang CS, and Janda KD. 2021. Developing Translational Vaccines against Heroin and Fentanyl through Investigation of Adjuvants and Stability. Mol Pharm 18: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, and Pentel PR. 2014. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 9: e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulima A, Jalah R, Antoline JFG, Torres OB, Imler GH, Deschamps JR, Beck Z, Alving CR, Jacobson AE, Rice KC, and Matyas GR. 2018. A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J Med Chem 61: 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laudenbach M, Baruffaldi F, Robinson C, Carter P, Seelig D, Baehr C, and Pravetoni M. 2018. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci Rep 8: 5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto T. 2018. The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation In Vivo. Front Immunol 9: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junttila IS 2018. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front Immunol 9: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendonça MS, Peraçolli TS, Silva-Vergara ML, Ribeiro SC, Oliveira RF, Mendes RP, and Rodrigues V Jr. 2015. High interleukin-4 expression and interleukin-4 gene polymorphisms are associated with susceptibility to human paracoccidioidomycosis. Mem Inst Oswaldo Cruz 110: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mountford AP, Hogg KG, Coulson PS, and Brombacher F. 2001. Signaling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect Immun 69: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts MT, Stober CB, McKenzie AN, and Blackwell JM. 2005. Interleukin-4 (IL-4) and IL-10 collude in vaccine failure for novel exacerbatory antigens in murine Leishmania major infection. Infect Immun 73: 7620–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe J, Kim HS, Armitage RJ, and Choi YS. 1997. The functional role of B cell antigen receptor stimulation and IL-4 in the generation of human memory B cells from germinal center B cells. J Immunol 159: 3757–3766. [PubMed] [Google Scholar]
- 23.Defrance T, Vanbervliet B, Pène J, and Banchereau J. 1988. Human recombinant IL-4 induces activated B lymphocytes to produce IgG and IgM. J Immunol 141: 2000–2005. [PubMed] [Google Scholar]
- 24.Duan L, Liu D, Chen H, Mintz MA, Chou MY, Kotov DI, Xu Y, An J, Laidlaw BJ, and Cyster JG. 2021. Follicular dendritic cells restrict interleukin-4 availability in germinal centers and foster memory B cell generation. Immunity 54: 2256–2272.e2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Warren Navia A, Aid M, Tsui C, Maldonado P, Nair U, Ghneim K, Fallon PG, Sekaly RP, Barouch DH, Shalek AK, Bruckbauer A, Strid J, and Batista FD. 2018. Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells. Cell 172: 517–533.e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez DG, Cote CM, Patel JR, Smith CB, Zhang Y, Nickerson KM, Zhang T, Kerfoot SM, and Haberman AM. 2018. Nonredundant Roles of IL-21 and IL-4 in the Phased Initiation of Germinal Center B Cells and Subsequent Self-Renewal Transitions. J Immunol 201: 3569–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon HB, Severinson E, Heusser C, Johansson SG, Moller G, and Persson U. 1989. Regulation of IgG1 and IgE synthesis by interleukin 4 in mouse B cells. Scand J Immunol 30: 355–361. [DOI] [PubMed] [Google Scholar]
- 28.Turqueti-Neves A, Otte M, Prazeres da Costa O, Hopken UE, Lipp M, Buch T, and Voehringer D. 2014. B-cell-intrinsic STAT6 signaling controls germinal center formation. Eur J Immunol 44: 2130–2138. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, and Craft J. 2016. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 17: 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, and Crotty S. 2010. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol 185: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinhardt RL, Liang HE, and Locksley RM. 2009. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 10: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelman FD, Katona IM, Urban JF Jr., Holmes J, Ohara J, Tung AS, Sample JV, and Paul WE. 1988. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol 141: 2335–2341. [PubMed] [Google Scholar]
- 33.Andoh A, Masuda A, Yamakawa M, Kumazawa Y, and Kasajima T. 2000. Absence of interleukin-4 enhances germinal center reaction in secondary immune response. Immunol Lett 73: 35–41. [DOI] [PubMed] [Google Scholar]
- 34.Jackson RJ, Worley M, Trivedi S, and Ranasinghe C. 2014. Novel HIV IL-4R antagonist vaccine strategy can induce both high avidity CD8 T and B cell immunity with greater protective efficacy. Vaccine 32: 5703–5714. [DOI] [PubMed] [Google Scholar]
- 35.Tang YW, and Graham BS. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest 94: 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crouse B, Robinson C, Huseby Kelcher A, Laudenbach M, Abrahante JE, and Pravetoni M. 2020. Mechanisms of interleukin 4 mediated increase in efficacy of vaccines against opioid use disorders. NPJ Vaccines 5: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baruffaldi F, Kelcher AH, Laudenbach M, Gradinati V, Limkar A, Roslawski M, Birnbaum A, Lees A, Hassler C, Runyon S, and Pravetoni M. 2018. Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Mol Pharm 15: 4947–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, and Pentel PR. 2012. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 341: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baehr C, Kelcher AH, Khaimraj A, Reed DE, Pandit SG, AuCoin D, Averick S, and Pravetoni M. 2020. Monoclonal Antibodies Counteract Opioid-Induced Behavioral and Toxic Effects in Mice and Rats. J Pharmacol Exp Ther 375: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho IY, Bunker JJ, Erickson SA, Neu KE, Huang M, Cortese M, Pulendran B, and Wilson PC. 2016. Refined protocol for generating monoclonal antibodies from single human and murine B cells. J Immunol Methods 438: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo M, Kim HS, Tong RK, Bainbridge TW, Vernes JM, Zhang Y, Lin YL, Chung S, Dennis MS, Zuchero YJ, Watts RJ, Couch JA, Meng YG, Atwal JK, Brezski RJ, Spiess C, and Ernst JA. 2017. Effector-attenuating Substitutions That Maintain Antibody Stability and Reduce Toxicity in Mice. J Biol Chem 292: 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broekman MM, Roelofs HM, Hoentjen F, Wiegertjes R, Stoel N, Joosten LA, de Jong DJ, and Wanten GJ. 2015. LPS-Stimulated Whole Blood Cytokine Production Is Not Related to Disease Behavior in Patients with Quiescent Crohn's Disease. PLoS One 10: e0133932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson C, Gradinati V, Hamid F, Baehr C, Crouse B, Averick S, Kovaliov M, Harris D, Runyon S, Baruffaldi F, LeSage M, Comer S, and Pravetoni M. 2020. Therapeutic and Prophylactic Vaccines to Counteract Fentanyl Use Disorders and Toxicity. J Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huseby Kelcher AM, Baehr CA, Hamid FA, Hart GT, and Pravetoni M. 2021. Contribution of Antibody-Mediated Effector Functions to the Mechanism of Efficacy of Vaccines for Opioid Use Disorders. J Immunol 207: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lischke A, Moriggl R, Brändlein S, Berchtold S, Kammer W, Sebald W, Groner B, Liu X, Hennighausen L, and Friedrich K. 1998. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J Biol Chem 273: 31222–31229. [DOI] [PubMed] [Google Scholar]
- 46.Lin JX, and Leonard WJ. 2000. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene 19: 2566–2576. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe D, and Saucier R. 2021. Biotechnologies and the future of opioid addiction treatments. Int J Drug Policy 88: 103041. [DOI] [PubMed] [Google Scholar]
- 48.Laudenbach M, Baruffaldi F, Vervacke JS, Distefano MD, Titcombe PJ, Mueller DL, Tubo NJ, Griffith TS, and Pravetoni M. 2015. The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J Immunol 194: 5926–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roh EY, Song EY, Yoon JH, Oh S, Chang JY, Park H, Seo SH, and Shin S. 2017. Effects of interleukin-4 and interleukin-12B gene polymorphisms on hepatitis B virus vaccination. Ann Hepatol 16: 63–70. [DOI] [PubMed] [Google Scholar]
- 50.Movahedi M, Amirzargar AA, Nasiri R, Hirbod-Mobarakeh A, Farhadi E, Tavakol M, Behniafard N, Ansaripour B, Moradi B, Zare A, and Rezaei N. 2013. Gene polymorphisms of Interleukin-4 in allergic rhinitis and its association with clinical phenotypes. Am J Otolaryngol 34: 676–681. [DOI] [PubMed] [Google Scholar]
- 51.Yao Y, Xu X, Li Y, Wang X, Yang H, Chen J, Liu S, Deng Y, Zhao Z, Yin Q, Sun M, and Shi L. 2020. Study of the association of seventeen single nucleotide polymorphisms and their haplotypes in the TNF-α, IL-2, IL-4 and IL-10 genes with the antibody response to inactivated Japanese encephalitis vaccine. Hum Vaccin Immunother 16: 2449–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clifford HD, Hayden CM, Khoo SK, Naniche D, Mandomando IM, Zhang G, Richmond P, and Le Souëf PN. 2017. Genetic Variants in the IL-4/IL-13 Pathway Influence Measles Vaccine Responses and Vaccine Failure in Children from Mozambique. Viral Immunol 30: 472–478. [DOI] [PubMed] [Google Scholar]
- 53.Rogoveanu OC, Calina D, Cucu MG, Burada F, Docea AO, Sosoi S, Stefan E, Ioana M, and Burada E. 2018. Association of cytokine gene polymorphisms with osteoarthritis susceptibility. Exp Ther Med 16: 2659–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu N, Gong Y, Chen XD, Zhang J, Long F, He J, Xia JW, and Dong L. 2013. Association between the polymorphisms of interleukin-4, the interleukin-4 receptor gene and asthma. Chin Med J (Engl) 126: 2943–2951. [PubMed] [Google Scholar]
- 55.Useche YM, Restrepo BN, Salgado DM, Narváez CF, Campo O, and Bedoya G. 2019. Association of IL4R-rs1805016 and IL6R-rs8192284 polymorphisms with clinical dengue in children from Colombian populations. J Infect Public Health 12: 43–48. [DOI] [PubMed] [Google Scholar]
- 56.Slager RE, Otulana BA, Hawkins GA, Yen YP, Peters SP, Wenzel SE, Meyers DA, and Bleecker ER. 2012. IL-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor α antagonist. J Allergy Clin Immunol 130: 516–522.e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Ginkel CD, Pettersson ME, Dubois AEJ, and Koppelman GH. 2018. Association of STAT6 gene variants with food allergy diagnosed by double-blind placebo-controlled food challenges. Allergy 73: 1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui W, Sun CM, Deng BC, and Liu P. 2013. Association of polymorphisms in the interleukin-4 gene with response to hepatitis B vaccine and susceptibility to hepatitis B virus infection: a meta-analysis. Gene 525: 35–40. [DOI] [PubMed] [Google Scholar]
- 59.Baynam G, Zhang G, Khoo SK, Sly P, Holt P, Goldblatt J, and Le Souef PN. 2008. Gender-specific effects of cytokine gene polymorphisms on childhood vaccine responses. Vaccine 26: 3574–3579. [DOI] [PubMed] [Google Scholar]
- 60.Wiertsema SP, Baynam G, Khoo SK, Veenhoven RH, van Heerbeek N, Zhang G, Laing IA, Rijkers GT, Goldblatt J, Sanders EA, and Le Souef PN. 2007. Impact of genetic variants in IL-4, IL-4 RA and IL-13 on the anti-pneumococcal antibody response. Vaccine 25: 306–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by contacting the corresponding author. Oxycodone monoclonal antibody binding sequences are confidential due to intellectual property rights.