Supplemental Digital Content is Available in the Text.
Acetaminophen reduced phase 2 formalin flinching, postformalin neuropathic and paw incision–evoked tactile allodynia, and displayed no evidence of dependence, tolerance, or reward.
Keywords: Acetaminophen, Paracetamol, Tactile allodynia, Formalin, Flinching, Mouse, Tolerance, Dependence, Reward, Conditioned place preference
Abstract
Introduction:
We explored in mice, the analgesic, tolerance, dependency, and rewarding effects of systemic acetaminophen (APAP).
Methods:
Studies employed adult mice (C57Bl6). (1) Intraplantar formalin flinching + post formalin allodynia. Mice were given intraperitoneal APAP in a DMSO (5%)/Tween 80 (5%) or a water-based formulation before formalin flinching on day 1 and tactile thresholds assessed before and after APAP at day 12. (2) Paw incision. At 24 hours and 8 days after hind paw incision in male mice, effects of intraperitoneal APAP on tactile allodynia were assessed. (3) Repeated delivery. Mice received daily (4 days) analgesic doses of APAP or vehicle and tested upon formalin flinching on day 5. (4) Conditioned place preference. For 3 consecutive days, vehicle was given in the morning in either of 2 chambers and in each afternoon, an analgesic dose of morphine or APAP in the other chamber. On days 5 and 10, animals were allowed to select a “preferred” chamber.
Results:
Formalin in male mice resulted in biphasic flinching and an enduring postformalin tactile allodynia. Acetaminophen dose dependently decreased phase 2 flinching, and reversed allodynia was observed postflinching. At a comparable APAP dose, female mice showed similarly reduced phase 2 flinching. Incision allodynia was transiently reversed by APAP. Repeated APAP delivery showed no loss of effect after sequential injections or signs of withdrawal. Morphine, but not APAP or vehicle, resulted in robust place preference.
Conclusions:
APAP decreased flinching and allodynia observed following formalin and paw incision and an absence of tolerance, dependence, or rewarding properties.
1. Introduction
Acetaminophen (APAP) is a modestly efficacious pain therapeutic reducing mild to moderate pain.15,22,39,53,74 It is the most widely used analgesic, with over-the-counter use exceeding 25 × 109 doses/year, worldwide with a market of almost USD 1.6 billion in 2022.82
Preclinical work has demonstrated APAP efficacy in inflammatory and neuropathic pain paradigms.26,33,86 Work has demonstrated the robust effect of APAP upon the facilitated state underlying the biphasic hind paw flinching evoked by intraplantar formalin.8,12,27,46,55 The early phase has been argued to reflect the acute afferent drive initiated by the actions of formalin mediated through the TRPA1 channel.49 Late-phase flinching is considered to reveal the facilitated state initiated by the afferent barrage generated during phase 1.3,64 Although most work has focused on flinching, there is a postflinching, persistent, allodynia (phase 3) that has a neuropathic phenotype.24,25,84
We have undertaken studies focusing on 4 issues related to effects of APAP. (1) Would APAP reverse postintraplantar formalin evoked late-phase allodynia said to have a neuropathic phenotype and in parallel have comparable effects upon allodynia observed early and late after paw incision? (2) Does APAP at analgesic doses display intrinsic rewarding properties? Clinical experience indicates that APAP is without an intrinsic rewarding property, as confirmed by its FDA designation for over-the-counter use worldwide and consistent with preclinical self-administration work.36,56 Accordingly, we sought to determine using the conditioned place preference (CPP) paradigm if repeated delivery of APAP is associated with the development of a rewarding state. (3) As the CPP model required repeated dosing to develop place preference, we queried whether repeated delivery would result in tolerance with repeated exposure, and subsequently, if such repeated dosing led to any sign of dependence/withdrawal upon termination of dosing, a property which, while accepted, is poorly documented. (4) Finally, APAP is poorly soluble in water and is frequently studied with a variety of vehicles (dimethylsulfoxide [DMSO], Tween 80) with varying degree of intrinsic activity. Recent work led to a water-based formulation created by taking advantage of APAP's temperature sensitive solubility and storage in sealed ampoules.
2. Methods
All studies were performed according to protocols that have been approved by the University of California, San Diego (UCSD) Animal Research Committee to ensure compliance with all tenets of the Animal Welfare Act and Public Health service policy.
2.1. Animals
Adult wild type C57Bl/6 male and females 20 to 25 g. were obtained from Envigo. All mice were held in the vivarium for a minimum of 5 days before use.
2.2. Behavioral testing
Behavioral tests were conducted between 9:00 am and 5:00 pm Considering reports of a possible contribution of sex of the experimenter,77 we note that each of these studies were performed by one female investigator, without knowledge as to treatment assignment.
2.3. Drugs
Acetaminophen as a powder (APAPp) (Sigma) was formulated by dissolving in DMSO (5%)/Tween 80 (5%) and brought to a concentration of 10 mg/mL by the addition of sterile water. Acetaminophen was also delivered in a water-based solution provided in sealed ampoules (APAPa) (courtesy of Sintetica Pharma, Switzerland) in a concentration of 30 mg/mL (https://patents.google.com/patent/DK2874602T3/en). Ampoules were opened just before use, and solutions were diluted in sterile water to the desired concentration for injection. In the conditioned place preference studies, morphine sulfate was prepared in saline. Drugs were prepared for delivery by diluting to a final volume of 0.1 mL/10 grams of body weight.
2.4. Drug delivery
2.4.1. Mouse intraplantar injection
Mice were lightly restrained. A 30-G needle was inserted subcutaneously into the plantar surface of the left paw and 20 µL of 2.5%. Formalin was injected over 10 seconds.
2.4.2. Mouse intraperitoneal injection
The mouse was restrained, and its head tilted facing downward with the abdomen exposed. A 30-G needle was inserted through the abdominal skin and musculature on the right side of the animal, and fluid was injected. Aspiration ensured the needle had not punctured a blood vessel, intestines, or bladder.
2.5. Study paradigm
2.5.1. Study 1. Acetaminophen and formalin-evoked phase 1/2 flinching and postformalin allodynia
To assess formalin-evoked flinching, a metal band was placed around the left hind paw of the mouse. Following a 1-hour acclimation, the mouse received an injection of intraplantar (IPLT) formalin (20 µL/2.5%) to induce flinching. The movement of the metal band (mouse flinching) was detected by an automated device.85 Data were collected continuously as flinch counts/minute for a period of 45 minutes following formalin injection. In formalin studies, groups of male mice were randomly assigned to receive intraperitoneal (IP) injections of APAPp (100 or 300 mg/kg) vs vehicle or APAPa (100 or 300 mg/kg) vs saline 30 minutes before the injection of intraplantar formalin and phase 1 and phase 2 formalin was assessed. In female mice, effects of IP APAPp and APAPa at 300 mg/kg vs vehicle on formalin-induced flinching was also examined.
Mice were assessed for tactile thresholds before the injections of APAPa/APAPp or the respective vehicles and the intraplantar formalin. At 1 hour after cessation of flinching, tactile thresholds were assessed. On day 12 after formalin, allodynia was again assessed, and mice assigned to receive APAPa/APAPp or the respective vehicles. Allodynia thresholds were assessed over the next 4 hours. Following the completion of these studies, animals were euthanized by CO2 inhalation, according to Institutional Animal Care and Use Committee (IACUC)-approved protocols. To assess mechanical thresholds, animals were placed in clear plexiglass chambers (base: 6 cm × 6 cm base 20 cm height), placed on a wire mesh-bottomed cages for 45 minutes before the initiation of testing. Tactile thresholds were measured with a series of von Frey filaments (Seemes Weinstein von Frey Anesthesiometer; Stoelting Co., Wood. Dale, IL) ranging from 2.44 to 4.31 (0.02–2.00 g), using the Dixon up–down method18 to calculate the 50% probability of withdrawal threshold in grams.10
2.5.2. Study 2. Paw incision
Under isoflurane anesthesia, a cutaneous incision was made in male mice on the plantar surface of the left hind paw, and the underlying muscle elevated, but not severed. The cutaneous wound was closed with 3 sutures, and the animal allowed to recover.47 In these studies, APAP or vehicle was delivered after 24 hours, and the effects upon the tactile allodynia assessed, as described in study 1 above. On day 8, given persistent allodynia, a second injection of APAP was given, and effects on tactile threshold are assessed.
2.5.3. Study 3. Conditioned place preference
Using a modification of the previously reported method,57,58 we tested for place preference induced by vehicle, APAP, and morphine in male mice. Each unit consisted of 3 adjoining clear Plexiglas compartments measuring 90 × 90 × 165 mm, with the middle compartment separated from the other 2 by walls with removable entries. The middle compartment was a neutral chamber, whereas the other 2 compartments were distinctly different in terms of wall pattern (diagonal black stripes vs black squares with total light admitted into each chamber being identical) and removable flooring with 1 of 2 textures (granular vs grid). Time the animal spent in each chamber was monitored by the disruption of LED light paths crossing the space of each of the 2 nonneutral chambers. The testing paradigm occurred over 10 days. During the first 2 “adaptation” days (days 1 and 2), mice were placed in the middle chamber and allowed to freely move between the 3 chambers for 30 minutes. The time spent in each chamber was recorded. On the mornings of the following 2 “conditioning” days (days 3 and 4), 10 minutes after vehicle injection, mice were placed in one of 2 outer chambers for 30 minutes. In the afternoons, mice received drug treatments: vehicle (10 mL/kg), morphine (3 mg/kg), or APAP (200 mg/kg), and 10 minutes later, they were restricted to the other outer chamber for 30 minutes. Exposure doses were chosen based on their ability to produce a significant analgesia. On days 5 and 10, mice were placed in the middle chamber to have free access to all 3 compartments for 30 minutes to determine whether they had developed a preference for the drug-paired chamber and whether this drug-chamber pairing was persistent. Time spent in each chamber was recorded. To define drug effect, average time spent in the drug-paired chamber during the 2 adaptation days was subtracted from the time spent in the same chamber on the test days. Assignment of the chambers to drug-paired or vehicle-paired compartments was counterbalanced.
2.5.4. Study 4. Tolerance and withdrawal
Male mice were assigned to receive 4 daily injections of either acetaminophen (IP APAPp, 300 mg/kg) or vehicle (IP, 10 mL/kg) (days 1–4). On day 5, all animals received IP APAPp (300 mg/kg), 30 minutes before intraplantar formalin. Animals from the 2 groups were then systematically noted at 6, 24, and 48 hours for indices of withdrawal (agitation, hyperactivity, and weight loss).35
2.6. General behavioral assessment
During the study, assessment of behavioral function was undertaken by the categorical assessment of the following indices. (1) righting (regaining quadruped posture after inversion), (2) symmetrical ambulation, (3) placing and stepping (dragging of the dorsum of the hind paw over an edge leading to an elevation of the paw and placement), (4) pinnae (twitch of the ear with application of a flexible probe into the auditory canal), or (5) blink (twitch of the eyelid with a light touch of the eye).
2.7. Power analysis–group size calculation
Group sizes for formalin-evoked flinching were estimated based on group mean and SD for phase 2 flinching in groups receiving formalin would nominally be 1100 ± 240 flinches. In this screening work, we wished to show a behaviorally significant reversal (30% = ≈ 330 count change from 1050) with power (1-β) = 80% and α = 0.05. Analysis indicated a minimum group size of 6 to 7 for flinching.
Group sizes for the tactile allodynic comparisons (post formalin, incision model) were based on the estimates of mean ± SD in groups of arthritic allodynic mice as nominally 0.49 ± 0.45 grams (n = 6). Given that normal mouse thresholds are 1.5 to 2 grams and that a 30% reversal of allodynia is judged to be behaviorally significant and assuming an α = P < 0.05 and a power (1-ß) = 80%, we calculated sample sizes in the allodynia studies also in the range of 6 to 8/group.
2.8. Data analysis
For primary statistical analysis, flinching was expressed as area under the curve (AUC) for total flinch counts for phase 1 (0–5 minutes) and for phase 2 (6–45 minutes) for each animal. Comparisons were then made between treatments for phase 1 and separately for phase 2. Repeated measures of per-minute flinch were not performed. For conditioned place preference, drug effect was defined as the time spent in the drug-paired chamber.
2.9. Statistics
Previous work has shown that significant intergroup variations can be demonstrated in formalin flinching, and data may fail to meet assumptions of normality. Accordingly, we conservatively performed comparisons in these AUC data sets using nonparametric statistics (Kruskal–Wallis as required). To compare APAP effect across doses, AUC for phase 1 and phase 2 were normalized by dividing the individual AUC for a given mouse by the median of the respective vehicle treatment at each dose. The plotted data for dosing were compared by Kruskal–Wallis test.
For the tactile allodynia end points, comparisons were undertaken using 2-way ANOVA with repeated measures over time as required. The conditioned place preference was analyzed by a two-way ANOVA over time for the 3 drug treatment groups. In either situation, pairwise, post hoc, multiple comparison analysis was performed using Sidak. All analysis and graphics were performed using GraphPad Prism v. 9.4.0.
3. Results
3.1. Acetaminophen and formalin evoked phase 1/2 flinching
Unilateral hind paw delivery of formalin led to a robust biphasic (phase 1 early phase/phase 2 late phase) flinching of the injected paw in both males and female mice (Fig. 1, and Supplemental Figure S1 and S2, http://links.lww.com/PR9/A235).
Figure 1.
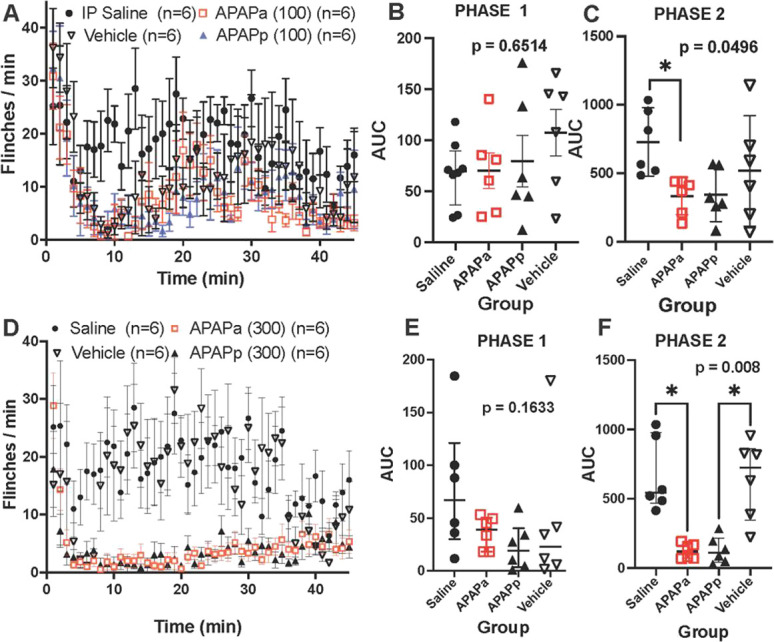
(A, C) Figure presents flinch count (mean ± SEM) for male mice following intraplantar formalin delivered at time 0. Animals received treatment: IP saline, IP vehicle, IP APAPa (ampoule), or IP APAPp (powder) 30 minutes before formalin. (B and E) Scattergram for cumulative flinching count (AUC: median with quartiles) for early phase (phase 1: 0–5 minutes) and (C and F) late phase (phase 2: 6–45 minutes), respectively. (A–C) Data for 100 mg/kg APAP and (D–F) for 300 mg/kg. AUC analysis was performed nonparametrically with Kruskal–Wallis test. Respective P values are presented in the respective scattergrams. Post hoc analysis for significant effects is shown (*P < 0.05). APAP, acetaminophen; AUC, area under the curve; IP, intraperitoneal.
Pretreatment with APAPa and APAPp had no effect upon phase 1 but resulted in a reliable suppression of formalin flinching phase 2 in male mice (see Fig. 1A–F and Supplemental Figure S1A, B, http://links.lww.com/PR9/A235). To assess dose dependency in the male mice of the effects for the 3 doses (100, 200, and 300 mg/kg) of the powdered formulation, the scatter gram of the AUC of the flinching for phase 1 and for phase 2 was normalized by dividing by the median AUC of the respective vehicle effect measured for phase 1 and 2 at each dose. These scattergram plots are presented in Supplemental Figure S3, http://links.lww.com/PR9/A235. For each treatment, the median with 95% CI calculated by PRISM is presented. As shown, calculation of the 95% CI shows no overlap with the ratio = 1 for doses of 200 and 300 mg/kg in phase 2 for male mice, indicating no difference from the respective vehicle control, whereas such overlap was reliably observed for all doses in phase 1. Based on the male data, we chose to assess the effects of a single APAP dose on formalin flinching in the female mice (300 mg/kg) and observed a significant suppression of phase 2, but not phase 1, with both APAPa and APAPp (Supplemental Figure 2, http://links.lww.com/PR9/A235). Plotting the normalized effect of the single 300 mg/kg dose for female mice showed no overlap in phase 2 behavior, like the results with the male mice at the 300 mg/kg dose (Supplemental Figure 3, http://links.lww.com/PR9/A235).
3.2. Acetaminophen and postformalin late-phase allodynia
Tactile thresholds before formalin were around 1.5 grams. Following formalin, there was a significant reduction in the tactile thresholds in control mice (Fig. 2A, B). Treatment before formalin (30 minutes) with APAPa and APAPp at 300 mg/kg (Fig. 2B), but not at 100 mg/kg (Fig. 2A), resulted in a significant reduction in the allodynia (eg, withdrawal thresholds) during the hour after resolution of formalin flinching.
Figure 2.
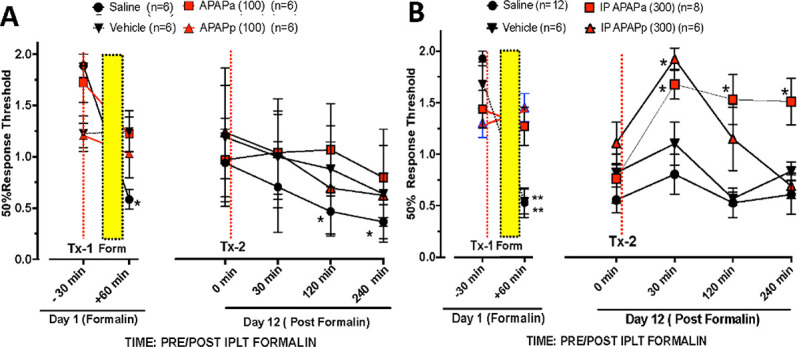
von Frey hair threshold (mean ± SEM) in the male mice receiving intraplantar formalin (Fig. 1) assessed immediately before drug treatment at TX-1(red dashed vertical line: A). IP saline, IP vehicle, IP APAPa (ampoule) (100 mg/kg) or IP APAPp (powder) (100 mg/kg) or (B) IP saline, IP vehicle, IP APAPa (ampoule) (300 mg/kg) or IP APAPp (powder) (300 mg/kg) and formalin on day 1 (formalin) at +30 minutes (indicated by the vertical dotted black line) and after intraplantar formalin (+60 minutes). On day 12, von Frey thresholds were assessed at T = 0 and the animal then administered the same treatment as on day 1 (Tx-2) and then tested at intervals out to 240 minutes. At 1 hour after formalin flinching and then again at 7 and 9 days. Two-way repeated-measures ANOVA were performed with main effects Time and Time × Treatment showing significant (P < 0.01). Post hoc comparisons between treatments were made with Sidak. * (P < 0.05): Day 1 vs Pre Tx1 and Day 12 vs Pre Tx2. APAP, acetaminophen; IP, intraperitoneal.
3.3. Acetaminophen and paw incision
Paw incision resulted in a robust tactile allodynia at 24 hours in the operated paw and a similar allodynia, albeit less robust, in the contralateral paw (Fig. 3). As shown, IP APAP (300 mg/kg), but not vehicle, resulted in a reversal that persisted for 2 hours in the ipsilateral paw (injured). After 8 days, a moderately attenuated allodynia was noted in both treatment groups, and this was similarly reversed by APAP, but not vehicle (Fig. 4A). The contralateral (uninjured) paw in the vehicle treated mice displayed a modest but significant fall in tactile thresholds when examined on day 2 and day 8 (Fig. 4B). By contrast, this fall in threshold in the contralateral paw was not observed in the mice receiving APAP.
Figure 3.
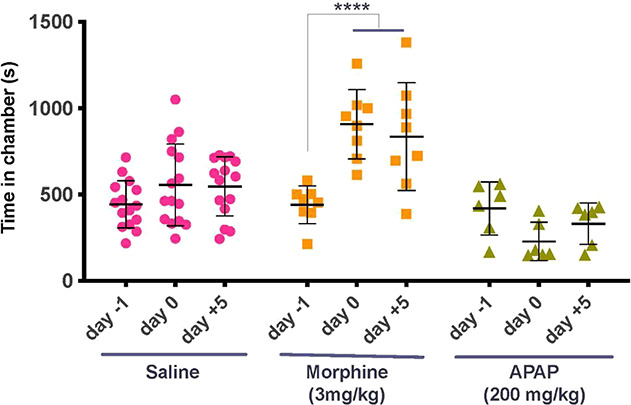
Scattergram showing time in seconds (mean ± SEM) spent in drug-paired chamber for each animal and the drug dosing as assessed before dosing (day 2), on the day after the 4 consecutive days of dosing (day 9) and 5 days later (day 14). Dosing for the 3 groups was saline vs saline, saline vs morphine (3 mg/kg), or saline vs acetaminophen (APAP: 200 mg/kg). Two-way repeated-measures ANOVA was performed with post hoc assessment with Sidak multiple comparison. ****P < 0.001. APAP, acetaminophen.
Figure 4.
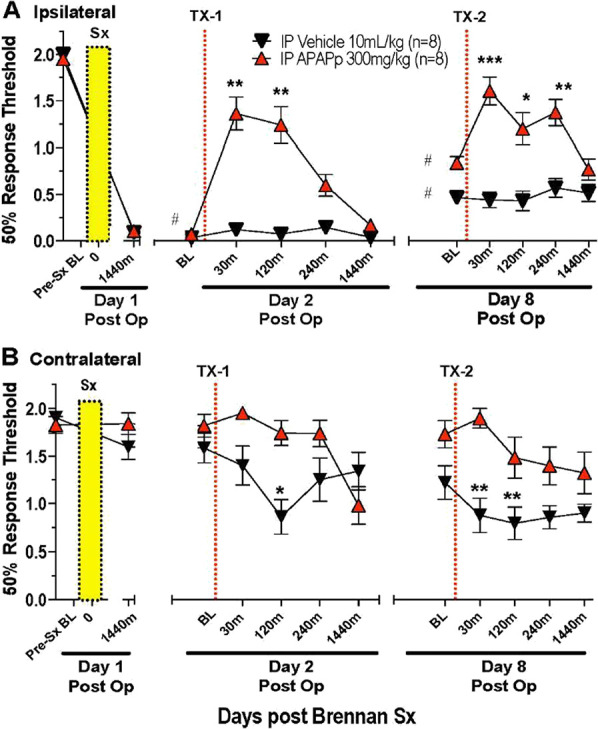
Tactile threshold (mean ± SEM) in male mice measured over time before (baseline: BL) and after unilateral hind paw incision on day 1 (vertical yellow bar) for the (A) ipsilateral and (B) contralateral hind paw in male mice. After 24 hours, on day 2 (Tx-1) again on day 8 (Tx-8), mouse received IP vehicle or acetaminophen (APAP: 300 mg/kg) at vertical dotted line. Two-way repeated-measures ANOVA. Time–treatment main effects were statistically significant (P < 0.0001). Post hoc comparisons vs respective presurgical baseline. P < 0.05. Post hoc comparisons (Sidak) vs respective Pre Tx 1 and Tx2 baselines (BL) *P < 0.05; **P < 0.01; ***P < 0.001. APAP, acetaminophen; IP, intraperitoneal.
3.4. Acetaminophen and Conditioned place preference
Repeated pairing of an analgesic dose of morphine (3 mg/kg) resulted in a significant increase in the time spent in the morphine-paired chamber (Fig. 3) when tested on day 5, and this preference continued to be observed on day 10, although no intermediate drug treatment was administered between day 5 and day 10. These observations suggest a positive reinforcing property of morphine. By contrast, the repeated pairing of an analgesic dose of APAP (200 mg/kg) (Fig. 4), with a given chamber did not lead to a preference for the APAP-paired chamber at any time.
3.5. Acetaminophen and repeated injection
Mice received 4 daily IP injections of acetaminophen (300 mg/kg) or vehicle. On the fifth day, all animals were assessed for formalin flinching. As shown (Fig. 5), APAP on day 5 resulted in similar robust suppressions of phase 2 flinching whether the animal had received prior exposure to vehicle or to the same doses of APAP. Importantly, 5 daily doses of APAPp with 300 mg/kg was well tolerated with no significant change in behavioral assessments or body weight over this 5-day APAP injection protocol (Table 1).
Figure 5.
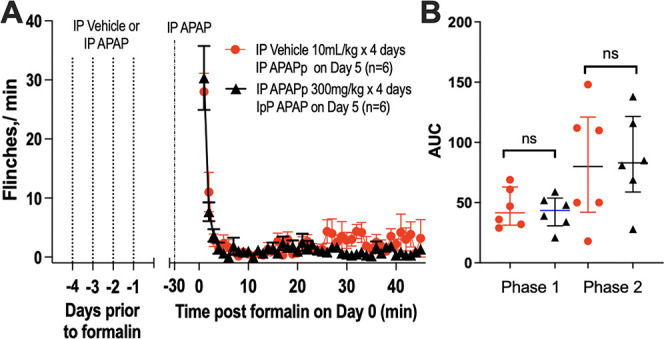
Repeated acetaminophen injections. Male mice were assigned to receive 4 daily injections of either acetaminophen (IP APAP, 300 mg/kg) or vehicle (IP, 10 mL/kg) (days 1–4). On day 5, all animals received IP APAP (300 mg/kg) 30 minutes before intraplantar formalin. (A) Flinch count (mean ± SEM) for mice following intraplantar formalin delivered at time 0. (B) Scattergram for cumulative flinching count (AUC: median/quartiles) for phase 1 (0–10 minutes) and phase 2 (11–45 minutes). AUC analysis for phase 1 and phase 2 flinching between the 2 treatment groups was performed nonparametrically with a rank-sum test; ns: nonsignificant; P > 0.05. APAP, acetaminophen; AUC, area under the curve; IP, intraperitoneal.
Table 1.
Body weight before acetaminophen and after 5 daily injections of acetaminophen (300/mg/kg) or 4 daily injections of vehicle and 1 injection of acetaminophen on day 5 and then 2 days after the last acetaminophen dosing.
| Treatment group | No. of mice | Body weight | ||
|---|---|---|---|---|
| Day 0 Pre APAP |
Day 5 Post 5th APAP dosing |
Day 7 2 d post 5th APAP injection |
||
| Vehicle group | 6 | 24.6 ± 0.3 g | 24.7 ± 0.2 g | 24.1 ± 0.3 g |
| Repeated 55 APAP injection | 6 | 25.2 ± 0.2 g | 25.6 ± 0.2 g | 25.4 ± 0.4 g |
APAP, acetaminophen.
3.6. Acetaminophen effects upon behavior
Assessment of behavior after doses prepared in either formulation showed that at the highest APAP dose (300 mg/kg), mice showed some reduction spontaneous activity otherwise observed in formalin-injected mice. However, categorical examination in the formalin-treated groups revealed no loss of behavioral function as assessed by (1) righting, (2) symmetrical ambulation, (3) placing and stepping, (4) pinnae, or (5) blink. In the mice receiving 5 repeated daily injections of APAP (300 mg/kg), no significant changes in body weight were noted over the study periods. All mice completed the study sequence after the acute and the 5 repeated high doses. Mouse behavior during the period after the repeated delivery of an analgesic doses of APAP was unaccompanied by any behavioral signs of dependence or withdrawal (eg, agitation, urination, defecation, piloerection) or weight loss (Table 1).
4. Discussion
4.1. Study results
Although a therapeutic with a long history, the present preclinical work sought to focus on the common effect profile of APAP.
4.1.1. Acetaminophen dosing
We employed an APAP formulation prepared in DMSO (5%)/Tween 80 (5%) or in a water-based formulation (APAPa). The 300 mg/kg dose was predicated on employing a maximum concentration in which APAPp could be readily formulated to compare with APAPa. We found that for this 7-day time frame, 5 daily injections of 300 mg/kg were well tolerated, showing no adverse behavioral signs and no loss of body weight. Accordingly, we used the 300 mg/kg as the high dose in these studies. The one exception was the use of 200 mg/kg in the CPP study. Although not the highest dose, it meets the criteria for being a robustly effective dose.
4.1.2. Analgesic profile
This work indicates that water or DMSO/Tween vehicle was well tolerated and resulted in comparable vehicle-only effects in these behavioral models. Both APAP formulations resulted in comparable suppression of formalin flinching in male and female mice, and in male mice, the postformalin allodynia and the incision-induced tactile allodynia.8,12,42,72,86 This postformalin allodynia is considered to reflect the appearance of a pain phenotype that shows activation of neuraxial epitopes microglial activation and the pharmacology of a neuropathy.24,25,84 This profile in these models is consistent with previous work in which APAP has been shown in preclinical models to have efficacy in models characterized by facilitated states as revealed by mechanical and thermal hyperalgesic end points in rodent inflammatory models,1,32,54,67 bone cancer,70 and in polyneuropathies (chemotherapy)44 and mononeuropathies.14,16,34 Importantly, the most robust effects are associated with facilitated states, whether those states are driven by inflammation (as in the incision/irritant models) or by models where inflammation is not considered to be the primary driver (as with formalin/nerve injury).
4.1.3. Tolerance, dependence, and withdrawal
An important observation in these studies was that repeated exposure to a high analgesic dose of APAP (300 mg/kg) did not yield any signs of tolerance (tachyphylaxis) or dependence/withdrawal. Furthermore, in contrast to morphine, APAP showed no signs of a developing preference in the conditioned place preference model and again failed to lead to adverse observations during the 5-day period after the last APAP exposure in the CPP paradigm eg, withdrawal. These results are consistent with clinical use, where APAP is not listed as a controlled substance and is available worldwide as an over-the-counter product, listed as an unscheduled over the counter drug that reveals no rewarding properties in humans36,61 or animal models in self-administration paradigms50 or as here in a conditioned place preference (CPP) model, in normal mice56 at a strongly analgesic doses. A single study has reported a degree of APAP preference, although the reason for this difference is not known.2 The preference paradigm as employed in this study was robust and validated by its ability to demonstrate the preference for an opiate, but not APAP.
4.2. Utility of acetaminophen
The clinical utility of APAP is based on 5 properties.
(1). Analgesic efficacy. Meta-analyses report that oral APAP reduces mild-to-moderate pain.15,53,69 Repeated-dose, randomized, double-blind, placebo-controlled trials in orthopedic surgery and abdominal hysterectomy patients showed that APAP significantly decreased pain in prospective, randomized, double-blind, multicenter, clinical trials.9,22,68,74,83 In a Cochrane meta-analysis investigating postoperative pain, APAP (1000 mg) showed numbers needed to treat (NNT) on the order of 3.6,81 comparing favorably with opiates. In human experimental models, IV APAP reduced evoked hyperalgesia.39
(2). Absence of dependence and abuse liability. Decades of clinical use reveal no abuse liability or evidence of an intrinsic rewarding potential.36,50,56,61
(3). Tolerability. Although APAP is widely used and has a record of safety when used at approved doses,23,30,52 overdoses, which can occur when multiple combination medication may be ingested, can lead to severe liver damage and is a common causes of hepatotoxicity.31 Furthermore, APAP use during pregnancy has been associated with an increased risk for neurodevelopmental disorders in prenatally exposed individuals.38,59
(4). Cost-effective. Although not commonly noted, an important metric of the impact of APAP is that with its notable efficacy, it is among the most cost-effective pain medications in the treatment of a variety of clinical pain phenotypes.11,37 In economically disadvantaged countries, APAP plays a major role as a safe, tolerated, nonaddictive, and affordable pain therapeutic.37
4.3. Mechanisms of acetaminophen action
Studies to identify specific APAP/metabolite binding sites have typically revealed little.28,65 Several mechanisms have been hypothesized to reflect an effect of APAP itself, or an action mediated by primary liver/brain metabolites. P-aminophenol can be converted through fatty acid amide hydrolase (FAAH), to N-(4-hydroxyphenyl)-arachidonamide (AM404) and into N-acetyl-p-benzoquinone imine (NAPQI) through cytochrome P450 (CYP) enzymes. Other metabolic pathways less well characterized include glucuronidation and sulfation.17,48 Current thinking has suggested several cellular targets for one or more of these products, including (1) cannabinoid signaling though formation of the AM404 metabolite acting as an inhibitor of anandamide (endogenous CB1 agonist) uptake, (2) activating TRP channels,45,78,87 as with the metabolite NAPQI activating TRYP-A1 and causing a depolarization block of spinal afferents,43 (3) cyclooxygenase inhibition,76 or (4) through serotonin signaling by brainstem activation of bulbospinal serotonergic projections by an undefined mechanism.62,80 Although each mechanism is supported by pharmacologic data, the several mechanisms appear distinct for the profiles of APAP action. Cannabinoid mechanisms would imply evidence of sedation and reward, absent even with high doses of acetaminophen. Similarly, the absence of common COX-mediated effect, such as gastrointestinal, coagulation, cardiovascular, kidney, or particularly an anti-inflammatory actions, is inconsistent with a primary COX-targeted action.5,6,21,41,71,73,76 Regarding a serotonergic mechanism, APAP/metabolites display little or no affinity for 5-HT receptors or actions on neuronal reuptake.65 Although APAP may exert a brainstem activation of bulbospinal serotonergic projections, by an undefined mechanism,62,80 bulbospinal serotonin projections appear to evoke hyperalgesia, likely through an excitatory 5HT receptor such as 5HT3.79 Alternately, the activation of descending pathway may activate a G protein–coupled inhibitory 5HT receptor (eg, 5HT-1 isotypes) or act through an excitatory receptor (5HT2, 3, 7) to activate GABA or enkephalin interneurons.20,51,63 Although inhibition of several excitatory and inhibitory 5HT-r ligands reduce APAP actions, 5-HT3 receptor antisense had no effect.4,13,19,40,60
In summary, although the therapeutic importance of APAP has been disparaged, its wide spread activity has led several groups to seek to recapitulate the APAP profile by creating analogues.7,29,66,75 Although efficacy has been identified with these analogues, there is typically no affirming data that the effects of those analogues are mediated by an APAP-related mechanism. Although current mechanisms point to several linking systems, there is no defining property, which can be compared across drug structures that can point to rational approaches for creating an APAP-linked therapeutic target through which APAP or its metabolites may act to alter nociceptive processing initiated by tissue and/or nerve injury.33 Future work in assessing APAP action requires implementation of agnostic platforms to define pain target engagement of the parent compound and/or its metabolites in animals and humans.
Disclosures
Two authors E.D., A.L. are employees of a company that made the water-based acetaminophen and as stated in the paper. They were not involved in the performance of the experiments or in the analysis of the data but contributed to the manuscript proofing. Other authors report no conflict.
In addition, at the point of revision, each author is required to submit the ICMJE disclosure form, which should be uploaded to Editorial Manager and which can be found on the submission site on the Information for Authors page, or at http://www.icmje.org/downloads/coi_disclosure.pdf. In addition to completing the form, all authors must clearly state all relevant conflicts of interest in the Acknowledgements section of the submitted manuscript.
Supplementary Material
Acknowledgments
The water-based acetaminophen formulation employed in these studies was kindly provided by Sintetica Pharma, Switzerland. Authors state no other conflicts of interest. E.D. and E.D., A.L. are employees of Sintetica SA and provided the acetaminophen in water formulation. They were not involved in the performance of the experiments or in the analysis of the data.
Role of author in performance of this study: Study design: M.Y., T.Y. Equipment construction: S.R., T.Y. Performance of study: M.Y., N.R., Y.A., K.E. Preparation of formulations: M.Y., Y.A., E.D., A.L. Data analysis: M.Y., N.R., K.E. Graphic and data analysis: M.Y., K.E., Y.A., T.Y. Preparation of Manuscript; M.Y., K.E., Y.A., E.D., A.L. T.Y.
Ethics statements: Generated Statement: The animal study was reviewed and approved by The University of California, San Diego is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (NIH Assurance Number; A3033-1, USDA Animal Research Facility Registration Number: 93-R-0437, AAALAC Institutional Number: 00050) and holds an approved NIH Assurance and USDA License.
Studies involving human subjects: Generated Statement: No human studies are presented in this manuscript.
Inclusion of identifiable human data: Generated Statement: No potentially identifiable human images or data is presented in this study.
Data availability statement: Generated Statement: The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A235.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Mijung Yun, Email: mijung.yun@nmc.or.kr.
Naemi Ditlevsen Regen, Email: naemiregen@hotmail.com.
Yuvicza Anchondo, Email: yanchondo@health.ucsd.edu.
Kelly Eddinger, Email: keddinger@health.ucsd.edu.
Shelle Malkmus, Email: smalkmus@health.ucsd.edu.
Steven W. Roberts, Email: s3roberts@ucsd.edu.
Elisabetta Donati, Email: edonati@sintetica.com.
Antonio Leonardi, Email: aleonardi@sintetica.com.
References
- [1].Abbadie C, Besson JM. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. PAIN 1994;57:45–54. [DOI] [PubMed] [Google Scholar]
- [2].Abbott FV, Hellemans KG. Phenacetin, acetaminophen and dipyrone: analgesic and rewarding effects. Behav Brain Res 2000;112:177–86. [DOI] [PubMed] [Google Scholar]
- [3].Abram SE, Dean C, O'Connor TC. Peroneal afferent nerve discharges underlying the behavioral response to the formalin test. Reg Anesth 1996;21:226–33. [PubMed] [Google Scholar]
- [4].Alloui A, Chassaing C, Schmidt J, Ardid D, Dubray C, Cloarec A, Eschalier A. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur J Pharmacol 2002;443:71–7. [DOI] [PubMed] [Google Scholar]
- [5].Ayoub SS, Colville-Nash PR, Willoughby DA, Botting RM. The involvement of a cyclooxygenase 1 gene-derived protein in the antinociceptive action of paracetamol in mice. Eur J Pharmacol 2006;538:57–65. [DOI] [PubMed] [Google Scholar]
- [6].Ayoub SS, Flower RJ. Loss of hypothermic and anti-pyretic action of paracetamol in cyclooxygenase-1 knockout mice is indicative of inhibition of cyclooxygenase-1 variant enzymes. Eur J Pharmacol 2019;861:172609. [DOI] [PubMed] [Google Scholar]
- [7].Bazan HA, Bhattacharjee S, Burgos C, Recio J, Abet V, Pahng AR, Jun B, Heap J, Ledet AJ, Gordon WC, Edwards S, Paul D, Alvarez-Builla J, Bazan NG. A novel pipeline of 2-(benzenesulfonamide)-N-(4-hydroxyphenyl) acetamide analgesics that lack hepatotoxicity and retain antipyresis. Eur J Med Chem 2020;202:112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bonnefont J, Alloui A, Chapuy E, Clottes E, Eschalier A. Orally administered paracetamol does not act locally in the rat formalin test: evidence for a supraspinal, serotonin-dependent antinociceptive mechanism. Anesthesiology 2003;99:976–81. [DOI] [PubMed] [Google Scholar]
- [9].Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA 2017;318:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [11].Chidambaran V, Subramanyam R, Ding L, Sadhasivam S, Geisler K, Stubbeman B, Sturm P, Jain V, Eckman MH. Cost-effectiveness of intravenous acetaminophen and ketorolac in adolescents undergoing idiopathic scoliosis surgery. Paediatr Anaesth 2018;28:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Choi SS, Lee JK, Suh HW. Antinociceptive profiles of aspirin and acetaminophen in formalin, substance P and glutamate pain models. Brain Res 2001;921:233–9. [DOI] [PubMed] [Google Scholar]
- [13].Courade JP, Chassaing C, Bardin L, Alloui A, Eschalier A. 5-HT receptor subtypes involved in the spinal antinociceptive effect of acetaminophen in rats. Eur J Pharmacol 2001;432:1–7. [DOI] [PubMed] [Google Scholar]
- [14].Cui JG, Zhang X, Zhao YH, Chen C, Bazan N. Allodynia and hyperalgesia suppression by a novel analgesic in experimental neuropathic pain. Biochem Biophys Res Commun 2006;350:358–63. [DOI] [PubMed] [Google Scholar]
- [15].Dalton JD, Schweinle JE. Randomized controlled noninferiority trial to compare extended release acetaminophen and ibuprofen for the treatment of ankle sprains. Ann Emerg Med 2006;48:615–23. [DOI] [PubMed] [Google Scholar]
- [16].Dani M, Guindon J, Lambert C, Beaulieu P. The local antinociceptive effects of paracetamol in neuropathic pain are mediated by cannabinoid receptors. Eur J Pharmacol 2007;573:214–5. [DOI] [PubMed] [Google Scholar]
- [17].Dargue R, Zia R, Lau C, Nicholls AW, Dare TO, Lee K, Jalan R, Coen M, Wilson ID. Metabolism and effects on endogenous metabolism of paracetamol (acetaminophen) in a porcine model of liver failure. Toxicol Sci 2020;175:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980;20:441–62. [DOI] [PubMed] [Google Scholar]
- [19].Dogrul A, Seyrek M, Akgul EO, Cayci T, Kahraman S, Bolay H. Systemic paracetamol-induced analgesic and antihyperalgesic effects through activation of descending serotonergic pathways involving spinal 5-HT₇ receptors. Eur J Pharmacol 2012;677:93–101. [DOI] [PubMed] [Google Scholar]
- [20].Dupuis A, Wattiez AS, Pinguet J, Richard D, Libert F, Chalus M, Aissouni Y, Sion B, Ardid D, Marin P, Eschalier A, Courteix C. Increasing spinal 5-HT2A receptor responsiveness mediates anti-allodynic effect and potentiates fluoxetine efficacy in neuropathic rats. Evidence for GABA release. Pharmacol Res 2017;118:93–103. [DOI] [PubMed] [Google Scholar]
- [21].Engström Ruud L, Wilhelms DB, Eskilsson A, Vasilache AM, Elander L, Engblom D, Blomqvist A. Acetaminophen reduces lipopolysaccharide-induced fever by inhibiting cyclooxygenase-2. Neuropharmacology 2013;71:124–9. [DOI] [PubMed] [Google Scholar]
- [22].Faiz HR, Rahimzadeh P, Visnjevac O, Behzadi B, Ghodraty MR, Nader ND. Intravenous acetaminophen is superior to ketamine for postoperative pain after abdominal hysterectomy: results of a prospective, randomized, double-blind, multicenter clinical trial. J Pain Res 2014;7:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fisher ES, Curry SC. Evaluation and treatment of acetaminophen toxicity. Adv Pharmacol 2019;85:263–72. [DOI] [PubMed] [Google Scholar]
- [24].Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience 2000;101:1127–35. [DOI] [PubMed] [Google Scholar]
- [25].Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain 2001;2:2–11. [DOI] [PubMed] [Google Scholar]
- [26].Füredi R, Bölcskei K, Szolcsányi J, Petho G. Effects of analgesics on the plantar incision-induced drop of the noxious heat threshold measured with an increasing-temperature water bath in the rat. Eur J Pharmacol 2009;605:63–7. [DOI] [PubMed] [Google Scholar]
- [27].Garrido-Suárez BB, Garrido G, Bellma Menéndez A, Merino N, Valdés O, Delgado-Hernández R, Granados-Soto V. Synergistic interaction between amitriptyline and paracetamol in persistent and neuropathic pain models: an isobolografic analysis. Neurochem Int 2021;150:105160. [DOI] [PubMed] [Google Scholar]
- [28].Godfrey L, Morselli A, Bennion P, Clarke GD, Hourani SM, Kitchen I. An investigation of binding sites for paracetamol in the mouse brain and spinal cord. Eur J Pharmacol 2005;508:99–106. [DOI] [PubMed] [Google Scholar]
- [29].González-Trujano ME, Uribe-Figueroa G, Hidalgo-Figueroa S, Martínez AL, Déciga-Campos M, Navarrete-Vazquez G. Synthesis and antinociceptive evaluation of bioisosteres and hybrids of naproxen, ibuprofen and paracetamol. Biomed Pharmacother 2018;101:553–62. [DOI] [PubMed] [Google Scholar]
- [30].Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013;21:201–32. [DOI] [PubMed] [Google Scholar]
- [31].Holubek WJ, Kalman S, Hoffman RS. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2006;43:880–2. author reply 882. [DOI] [PubMed] [Google Scholar]
- [32].Honoré P, Buritova J, Besson JM. Aspirin and acetaminophen reduced both Fos expression in rat lumbar spinal cord and inflammatory signs produced by carrageenin inflammation. PAIN 1995;63:365–75. [DOI] [PubMed] [Google Scholar]
- [33].Hoshijima H, Hunt M, Nagasaka H, Yaksh T. Systematic review of systemic and neuraxial effects of acetaminophen in preclinical models of nociceptive processing. J Pain Res 2021;14:3521–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Im KS, Jung HJ, Kim JB, Lee JM, Park HJ, Joo CH, Moon DE. The antinociceptive effect of acetaminophen in a rat model of neuropathic pain. Kaohsiung J Med Sci 2012;28:251–8. [DOI] [PubMed] [Google Scholar]
- [35].Jhamandas KH, Marsala M, Ibuki T, Yaksh TL. Spinal amino acid release and precipitated withdrawal in rats chronically infused with spinal morphine. J Neurosci 1996;16:2758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jouanjus E, Guernec G, Lapeyre-Mestre M, French Addictovigilance Network. Medical prescriptions falsified by the patients: a 12-year national monitoring to assess prescription drug diversion. Fundam Clin Pharmacol 2018;32:306–22. [DOI] [PubMed] [Google Scholar]
- [37].Kamath CC, Kremers HM, Vanness DJ, O'Fallon WM, Cabanela RL, Gabriel SE. The cost-effectiveness of acetaminophen, NSAIDs, and selective COX-2 inhibitors in the treatment of symptomatic knee osteoarthritis. Value Health 2003;6:144–57. [DOI] [PubMed] [Google Scholar]
- [38].Khan FY, Kabiraj G, Ahmed MA, Adam M, Mannuru SP, Ramesh V, Shahzad A, Chaduvula P, Khan S. A systematic review of the link between autism spectrum disorder and acetaminophen: a mystery to resolve. Cureus 2022;14:e26995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Koppert W, Wehrfritz A, Korber N, Sittl R, Albrecht S, Schuttler J, Schmelz M. The cyclooxygenase isozyme inhibitors parecoxib and paracetamol reduce central hyperalgesia in humans. PAIN 2004;108:148–53. [DOI] [PubMed] [Google Scholar]
- [40].Libert F, Bonnefont J, Bourinet E, Doucet E, Alloui A, Hamon M, Nargeot J, Eschalier A. Acetaminophen: a central analgesic drug that involves a spinal tropisetron-sensitive, non-5-HT(3) receptor-mediated effect. Mol Pharmacol 2004;66:728–34. [DOI] [PubMed] [Google Scholar]
- [41].Lucas R, Warner TD, Vojnovic I, Mitchell JA. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. FASEB J 2005;19:635–7. [DOI] [PubMed] [Google Scholar]
- [42].Luccarini P, Childeric A, Gaydier AM, Voisin D, Dallel R. The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception. J Pain 2006;7:908–14. [DOI] [PubMed] [Google Scholar]
- [43].Lutz CT, Galles ME, Kemp JD, Goeken JA, Dick FR. Kappa immunoglobulin light chain gene rearrangement in a T-lineage chronic lymphocytic leukemia. Am J Clin Pathol 1990;93:702–5. [DOI] [PubMed] [Google Scholar]
- [44].Lynch JJ, III, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. PAIN 2004;110:56–63. [DOI] [PubMed] [Google Scholar]
- [45].Mallet C, Barriere DA, Ermund A, Jonsson BA, Eschalier A, Zygmunt PM, Hogestatt ED. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS One 2010;5:e12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 1992;263:136–46. [PubMed] [Google Scholar]
- [47].Mamet J, Klukinov M, Yaksh TL, Malkmus SA, Williams S, Harris S, Manning DC, Taylor BK, Donahue RR, Porreca F, Xie JY, Oyarzo J, Brennan TJ, Subieta A, Schmidt WK, Yeomans DC. Single intrathecal administration of the transcription factor decoy AYX1 prevents acute and chronic pain after incisional, inflammatory, or neuropathic injury. PAIN 2014;155:322–33. [DOI] [PubMed] [Google Scholar]
- [48].Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics 2015;25:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A 2007;104:13525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mickley GA, Hoxha Z, Biada JM, Kenmuir CL, Bacik SE. Acetaminophen self-administered in the drinking water increases the pain threshold of rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 2006;45:48–54. [PubMed] [Google Scholar]
- [51].Miller KE, Salvatierra AT. Apposition of enkephalin- and neurotensin-immunoreactive neurons by serotonin-immunoreactive varicosities in the rat spinal cord. Neuroscience 1998;85:837–46. [DOI] [PubMed] [Google Scholar]
- [52].Mitchell RA, Rathi S, Dahiya M, Zhu J, Hussaini T, Yoshida EM. Public awareness of acetaminophen and risks of drug induced liver injury: results of a large outpatient clinic survey. PLoS One 2020;15:e0229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Moore A, Collins S, Carroll D, McQuay H, Edwards J. Single dose paracetamol (acetaminophen), with and without codeine, for postoperative pain. Cochrane Database Syst Rev 2000:CD001547. 10.1002/14651858.CD001547 [DOI] [PubMed] [Google Scholar]
- [54].Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther 2003;306:490–7. [DOI] [PubMed] [Google Scholar]
- [55].Nakamura S, Nonaka T, Komatsu S, Yamada T, Yamamoto T. Oral acetaminophen-induced spinal 5-hydroxytriyptamine release produces analgesic effects in the rat formalin test. Biomed Pharmacother 2022;146:112578. [DOI] [PubMed] [Google Scholar]
- [56].Nazarian A, Are D, Tenayuca JM. Acetaminophen modulation of hydrocodone reward in rats. Pharmacol Biochem Behav 2011;99:307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Park HJ, Sandor K, McQueen J, Woller SA, Svensson CI, Corr M, Yaksh TL. The effect of gabapentin and ketorolac on allodynia and conditioned place preference in antibody-induced inflammation. Eur J Pain 2016;20:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg 2013;116:224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Parker W, Anderson LG, Jones JP, Anderson R, Williamson L, Bono-Lunn D, Konsoula Z. The dangers of acetaminophen for neurodevelopment outweigh scant evidence for long-term benefits. Children (Basel) 2023;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pelissier T, Alloui A, Caussade F, Dubray C, Cloarec A, Lavarenne J, Eschalier A. Paracetamol exerts a spinal antinociceptive effect involving an indirect interaction with 5-hydroxytryptamine3 receptors: in vivo and in vitro evidence. J Pharmacol Exp Ther 1996;278:8–14. [PubMed] [Google Scholar]
- [61].Pickworth WB, Klein SA, George FR, Henningfield JE. Acetaminophen fails to inhibit ethanol-induced subjective effects in human volunteers. Pharmacol Biochem Behav 1992;41:189–94. [DOI] [PubMed] [Google Scholar]
- [62].Pini LA, Sandrini M, Vitale G. The antinociceptive action of paracetamol is associated with changes in the serotonergic system in the rat brain. Eur J Pharmacol 1996;308:31–40. [DOI] [PubMed] [Google Scholar]
- [63].Pini LA, Vitale G, Ottani A, Sandrini M. Naloxone-reversible antinociception by paracetamol in the rat. J Pharmacol Exp Ther 1997;280:934–40. [PubMed] [Google Scholar]
- [64].Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. PAIN 1996;64:345–55. [DOI] [PubMed] [Google Scholar]
- [65].Raffa RB, Codd EE. Lack of binding of acetaminophen to 5-HT receptor or uptake sites (or eleven other binding/uptake assays). Life Sci 1996;59:PL37–40. [DOI] [PubMed] [Google Scholar]
- [66].Reddoch-Cardenas KM, Cheppudira BP, Garza T, Hopkins CD, Bunker KD, Slee DH, Cap AP, Bynum JA, Christy RJ. Evaluation of KP-1199: a novel acetaminophen analog for hemostatic function and antinociceptive effects. Transfusion 2021;61(suppl 1):S234–42. [DOI] [PubMed] [Google Scholar]
- [67].Rezende RM, França DS, Menezes GB, dos Reis WG, Bakhle YS, Francischi JN. Different mechanisms underlie the analgesic actions of paracetamol and dipyrone in a rat model of inflammatory pain. Br J Pharmacol 2008;153:760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ridderikhof ML, Lirk P, Goddijn H, Vandewalle E, Schinkel E, Van Dieren S, Kemper EM, Hollmann MW, Goslings JC. Acetaminophen or nonsteroidal anti-inflammatory drugs in acute musculoskeletal trauma: a multicenter, double-blind, randomized, clinical trial. Ann Emerg Med 2018;71:357–68.e8. [DOI] [PubMed] [Google Scholar]
- [69].Sachs CJ. Oral analgesics for acute nonspecific pain. Am Fam Physician 2005;71:913–8. [PubMed] [Google Scholar]
- [70].Saito O, Aoe T, Yamamoto T. Analgesic effects of nonsteroidal antiinflammatory drugs, acetaminophen, and morphine in a mouse model of bone cancer pain. J Anesth 2005;19:218–24. [DOI] [PubMed] [Google Scholar]
- [71].Saliba SW, Marcotegui AR, Fortwangler E, Ditrich J, Perazzo JC, Munoz E, de Oliveira ACP, Fiebich BL. AM404, paracetamol metabolite, prevents prostaglandin synthesis in activated microglia by inhibiting COX activity. J Neuroinflammation 2017;14:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sandrini M, Romualdi P, Capobianco A, Vitale G, Morelli G, Pini LA, Candeletti S. The effect of paracetamol on nociception and dynorphin A levels in the rat brain. Neuropeptides 2001;35:110–6. [DOI] [PubMed] [Google Scholar]
- [73].Schwartz JI, Musser BJ, Tanaka WK, Taggart WV, Mehta A, Gottesdiener KM, Greenberg HE. Inhibition of prostacyclin and thromboxane biosynthesis in healthy volunteers by single and multiple doses of acetaminophen and indomethacin. Clin Pharmacol Drug Develop 2015;4:337–45. [DOI] [PubMed] [Google Scholar]
- [74].Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology 2005;102:822–31. [DOI] [PubMed] [Google Scholar]
- [75].Sinning C, Watzer B, Coste O, Nüsing RM, Ott I, Ligresti A, Di Marzo V, Imming P. New analgesics synthetically derived from the paracetamol metabolite N-(4-hydroxyphenyl)-(5Z,8Z,11Z,14Z)-icosatetra-5,8,11,14-enamide. J Med Chem 2008;51:7800–5. [DOI] [PubMed] [Google Scholar]
- [76].Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician 2009;12:269–80. [PubMed] [Google Scholar]
- [77].Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011;31:15450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Stueber T, Meyer S, Jangra A, Hage A, Eberhardt M, Leffler A. Activation of the capsaicin-receptor TRPV1 by the acetaminophen metabolite N-arachidonoylaminophenol results in cytotoxicity. Life Sci 2018;194:67–74. [DOI] [PubMed] [Google Scholar]
- [79].Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res 2004;1019:68–76. [DOI] [PubMed] [Google Scholar]
- [80].Tjølsen A, Lund A, Hole K. Antinociceptive effect of paracetamol in rats is partly dependent on spinal serotonergic systems. Eur J Pharmacol 1991;193:193–201. [DOI] [PubMed] [Google Scholar]
- [81].Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev 2008;2008:CD004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Watch M. Acetaminophen (paracetamol) market share, size 2022-industry trends, progress insight, developing technologies, competitive, regional, and global industry forecast to 2029, New York, NY: Dow Jones & Company. 2022. [Google Scholar]
- [83].Westrich GH, Birch GA, Muskat AR, Padgett DE, Goytizolo EA, Bostrom MP, Mayman DJ, Lin Y, YaDeau JT. Intravenous vs oral acetaminophen as a component of multimodal analgesia after total hip arthroplasty: a randomized, blinded trial. J Arthroplasty 2019;34:S215–20. [DOI] [PubMed] [Google Scholar]
- [84].Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun 2016;56:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol (1985) 2001;90:2386–402. [DOI] [PubMed] [Google Scholar]
- [86].Zhu Q, Sun Y, Mao L, Liu C, Jiang B, Zhang W, Li JX. Antinociceptive effects of sinomenine in a rat model of postoperative pain. Br J Pharmacol 2016;173:1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol 2000;396:39–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


