Abstract
The first 86 residues of the Rous sarcoma virus (RSV) Gag protein form a membrane-binding (M) domain that directs Gag to the plasma membrane during budding. Unlike other retroviral Gag proteins, RSV Gag is not myristylated; however, the RSV M domain does contain 11 basic residues that could potentially interact with acidic phospholipids in the plasma membrane. To investigate this possibility, we analyzed mutants in which basic residues in the M domain were replaced with asparagines or glutamines. The data show that neutralizing as few as two basic residues in the M domain blocked particle release and prevented Gag from localizing to the plasma membrane. Though not as severe, single neutralizations also diminished budding and, when expressed in the context of proviral clones, reduced the ability of RSV to spread in cell cultures. To further explore the role of basic residues in particle production, we added lysines to new positions in the M domain. Using this approach, we found that the budding efficiency of RSV Gag can be improved by adding pairs of lysines and that the basic residues in the M domain can be repositioned without affecting particle release. These data provide the first gain-of-function evidence for the importance of basic residues in a retroviral M domain and support a model in which RSV Gag binds to the plasma membrane via electrostatic interactions.
Retroviral Gag proteins form particles and bud from cellular membranes in the absence of the other viral components (i.e., env and pol gene products and the mRNA genome). Gag is directed to the site of budding by a membrane-binding (M) domain located at its amino terminus. Rous sarcoma virus (RSV), like all infectious retroviruses, buds from the plasma membrane, and its M domain is located in the first 86 residues of Gag (Fig. 1) (21, 35). Indeed, deletions in this region abolish budding, and particle release is restored by replacing the M domain with the plasma membrane-targeting signals of cellular proteins or of human immunodeficiency virus type 1 (HIV-1) Gag (1, 36, 37). In addition, the first 35 residues of the RSV M domain can be cross-linked to the phospholipids of the viral envelope (26). However, the manner in which the M domain directs Gag to the plasma membrane is unknown.
FIG. 1.
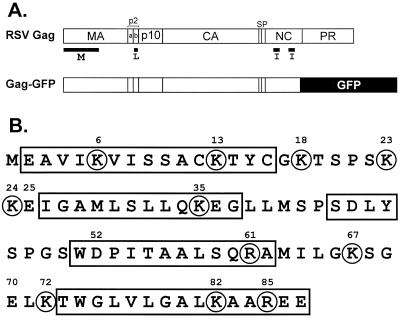
Locations of basic residues in the M domain of RSV Gag. (A) Diagram of Gag and Gag-GFP. The proteolytic products of Gag and locations of the domains required for budding are indicated. The M domain directs Gag to the plasma membrane; the I domains promote interactions between Gag molecules; the L domain is required for separation of the viral particle from the plasma membrane. (B) Primary sequence showing the locations of helices (rectangles) and basic residues (circles) in the M domain. The three acidic residues replaced in the gain-of-function studies are also numbered.
Previous studies have revealed that membrane proteins establish hydrophobic and/or polar interactions with phospholipid bilayers (28). For example, the M domain of HIV-1 Gag uses myristate and a cluster of basic residues to form hydrophobic and electrostatic interactions with the plasma membrane (3, 9, 10, 38, 39). Likewise, fatty acid modifications and basic residues form the plasma membrane targeting signals of many cellular proteins (e.g., Src, K-Ras, and myristoylated alanine-rich protein kinase C substrate) (11, 28, 32, 34).
Although acetylated, the M domain of RSV Gag does not possess any fatty acid modifications that contribute to membrane binding (24, 29). However, inspection of the primary sequence of the M domain reveals the presence of 11 basic amino acids (Fig. 1B). Moreover, the solution structure of the M domain suggests that these basic residues are exposed on the surface of the molecule (18). Thus, we considered whether basic residues in the M domain are important for budding by generating mutants in which the basic residues were neutralized by replacements with asparagines or glutamines. Our data show that these changes caused significant defects in the ability of Gag to localize to the plasma membrane and release particles into the growth media of transfected cells. In addition, single neutralizations caused mild defects in the ability of RSV to spread in culture. Most importantly, we show that the addition of basic residues to new positions in the M domain results in dramatic increases in budding from the plasma membrane. Thus, our data provide the first gain-of-function evidence for the importance of basic residues in a retroviral Gag M domain.
MATERIALS AND METHODS
Expression vectors.
The gag gene used for this study was obtained from the RSV Prague C proviral vector, pATV-8 (13, 30). It was cloned into the polylinker region of M13mp19 to create MGAG, a plasmid used to generate single-stranded DNA for oligonucleotide-directed mutagenesis. To analyze budding in mammalian cells, we used a simian virus 40 (SV40)-based vector, designated pSV.Myr0, in which gag is expressed from the SV40 late promoter (36). Infectivity studies were performed after transferring gag alleles to pRCAS-derived proviral vectors (see below).
Oligonucleotide-directed mutagenesis.
The oligonucleotide 5′-TGCGGGAAGACTAGTCCTTCTAAG-3′ was used to generate an SpeI site between nucleotides (nt) 434 and 439 (underlined) in MGAG, using the method described by Kunkel et al. (15). Clones were identified by digestion with SpeI and designated MGAG-S. The altered alleles were then subcloned into the SV40-based expression vector. As expected, three independent clones of this construct, designated pSV.Myr0-S, bud with an efficiency identical to that of pSV.Myr0, since creation of the SpeI site did not change the amino acid sequence of Gag.
Neutralizations of basic residues in the first half of the M domain were created in the SV40-based expression vector using PCR. Two overlapping oligonucleotides, 5′-GCGGGAA(C/G)ACTAGTCCTTCTAA(C/A)AA(C/A)GAAATAG GGGCCATGTTGTCCCTCTTACAA(C/A)AGGAAGGGTTGCTT-3′ and 5′- TTAGAAGGACTAGT(G/C)TTCCCGCAATAGGTTT(G/T)ACACGCGGA CGAAATCACCT(T/G)TATGACGGCTTCCAT-3′, were used to amplify sequences encoding the first six basic residues of the M domain. These primers were synthesized with a 1:1 mixture of wild-type and mutagenic nucleotides at positions (in parentheses) that resulted in the maintenance or neutralization of basic residues in the M domain. Mutants generated by this method were first identified by the presence of the SpeI site (underlined) and then sequenced to determine the specific mutations they possessed. Additional mutants with neutralizations in the first half of the M domain were then made by exchanging SstI-SpeI or SpeI-XhoI restriction fragments with pSV.Myr0-S.
Neutralizations of basic residues in the second half of the M domain were generated using MGAG-S and combinations of the following oligonucleotides: 5′-CTATCCACGCAGGCTATGATAC-3′ (R61Q), 5′-GATACTTGGGCAATCGGGAG-3′, (K67Q), 5′-GGGAGAGTTACAAACCTGGG-3′ (K72Q), 5′-GGGGCATTGCAGGCGGCTC-3′ (K82Q), and 5′-GGCGGCTCAAGAGGAACAG-3′ (R85Q). Mutant alleles were identified by DNA sequencing and moved to the SV40-based vector for analysis.
Using similar methods, pairs of acidic-to-basic or acidic-to-neutral residue changes were created by mutating MGAG-S sequences. Acidic residue codons (underlined) were mutated using combinations of the following oligonucleotides: 5′-CTAAGAAGCAAATAGGGGCCATG-3′ (E25Q), 5′-CTAAGAAG AAAATAGGGGCCATG-3′ (E25K), 5′-GGTCCTGGCAGCCCATTACCG C-3′ (D52Q), 5′-GGTCCTGGAAGCCCATTACCGC-3′ (D52K), 5′-GAAATCGGGACAGTTAAAAACC-3′ (E70Q), and 5′-GAAATCGGGAAAGTTAAAAACC-3′ (E70K). Mutants were identified by DNA sequencing and subcloned into the SV40 vector. The acidic residues changes were combined with neutralizations of the first and third basic residues in the M domain by subcloning SpeI-XhoI fragments into the 1,3 mutant.
Analysis of particle release.
Mammalian (COS-1) cells were transfected with SV40-based vectors using a DEAE-dextran-chloroquine method previously described (36). Cells were then labeled with l-[35S]methionine (50 μCi, >1,000 Ci/mmol) 48 h after transfection. To determine the initial levels of Gag expression, cells were labeled for only 5 min and lysed in radioimmunoprecipitation assay buffer after removal of the labeling medium. To assay for budding, duplicate cultures were labeled for 2.5 h, and media and cell lysate fractions were collected. After immunoprecipitation of proteins using rabbit antiserum against whole RSV, samples were denatured and separated by polyacrylamide gel electrophoresis (PAGE) using sodium dodecyl sulfate (SDS)–12% polyacrylamide gels. Labeled Gag proteins were detected by autoradiography or by using phosphor screens. Laser scanning densitometry and/or PhosphorImager (Molecular Dynamics) analysis was then used to determine the amount of CA (capsid protein) in the media after the 2.5-h labeling. Budding efficiencies were then calculated by normalizing the amount of released CA to the amount of uncleaved Gag (Pr76) in the cell lysates after the 5-min labeling. Data are expressed as a percentage of wild-type's release efficiency, and error bars indicate standard deviations. Analysis of each mutant was performed at least three times.
Cloning and analysis of Gag-GFP constructs.
The mutagenic oligonucleotide 5′-TCGGGGCCGTGGCCCGGGCCCGAGCCACCTGCCGTCTCG-3′ was used to create an ApaI site (underlined) between nucleotides 2087 and 2092 in the MGAG-S plasmid (near the 3′ end of the NC [nucleocapsid]-coding region). After recombinants were identified by ApaI digestion, the SstI-ApaI fragment containing the truncated gag sequence was inserted into the polylinker of plasmid pEGFP-N2 (Clontech) to create pGag-GFP. In this way, the green fluorescent protein (GFP) variant EGFP (∼27 kDa), replaced the last six residues of NC and the entire PR (protease) domain of Gag (Fig. 1A). Particle production by Gag-GFP was analyzed by transfecting QT6 or COS-1 cells and metabolically labeling as described above. Immunoprecipitations were performed using polyclonal anti-RSV or polyclonal anti-GFP (Clontech product no. 8367).
Sequences encoding neutralizations of basic residues in the M domain were subcloned into pGag-GFP by moving SstI-BspEI fragments from the SV40-based vectors. Biochemical analysis confirmed that these mutants exhibit defects in particle release similar to when the alleles were expressed from the SV40-based vectors.
Subcellular localization of Gag-GFP and M domain mutants was determined using avian (QT6) cells. At 24 to 36 h posttransfection, QT6 cultures were removed from standard incubation conditions (37°C, 5% CO2), washed once, and overlaid with glass coverslips. Cells were immediately observed by confocal microscopy using a Zeiss laser scanning microscope, and Gag-GFP fluorescence was detected by excitation with a helium-argon laser (488-nm peak excitation).
Electron microscopy.
QT6 cells were seeded on 60-mm Permanox (Nunc) dishes and then transfected with pGag-GFP or M domain mutants as described above. Approximately 24 h posttransfection, cells were gently washed with ice-cold 0.1 M sodium cacodylate (pH 7.4) and then fixed in 4% paraformaldehyde–0.5% glutaraldehyde for 1 h at 0°C. The cells were subsequently rinsed with 0.1 M sodium cacodylate (pH 7.4) and briefly examined by confocal microscopy to confirm that the subcellular localization patterns were not altered by the fixation procedure. Fixed cells were then processed for analysis by electron microscopy as previously described (5).
Infectivity assays.
To test for infectivity, wild-type and mutant gag alleles were transferred to the proviral vector, pRCAS-EGFP (expresses GFP in place of the nonessential v-Src sequence; kindly provided by Mark Federspiel). Infectivity was then evaluated by determining the expression levels of intracellular Gag since viral protein expression requires the successful completion of the early steps in replication. In the first assay, we examined the abilities of the mutants to spread in transfected cells (20). To do this, duplicate cultures of QT6 cells were transfected with proviral DNAs by the calcium phosphate method. At 18 h posttransfection, the transfection efficiency of each DNA was determined by labeling one culture for 5 min with l-[35S]methionine (50 μCi, >1,000 Ci/mmol). Cells from the duplicate cultures were passaged 1:10 every 3 to 4 days. At 1 and 2 weeks posttransfection, cells (106/35-mm-diameter dish) were seeded from each stock culture and subsequently labeled for 5 min. Gag proteins were immunoprecipitated and quantified as described above. Since the transfected DNA exists only transiently, stable expression of viral proteins (e.g., Gag) is indicative of an infectious phenotype.
To directly measure the ability of mutant viruses to complete the early steps of replication (i.e., entry, reverse transcription, and integration), intracellular Gag expression levels were measured at 24 h postinfection. Specifically, turkey embryo fibroblasts (TEFs) were transfected with proviral DNAs, and the media from these cultures were collected after 48 h and centrifuged for 10 min at 2,000 × g. Half of the supernatant (1 ml) was transferred to 0.4 × 106 uninfected TEFs; the remaining supernatant was centrifuged for 40 min at 126,000 × g to concentrate the extracellular virus. Viral pellets were resuspended in 10 mM Tris-HCl (pH 7.6), and reverse transcriptase assays were performed to determine the amount of virus in each inoculum. After a 24-h incubation with the virus-containing media, the TEFs were labeled for 15 min with l-[35S]methionine. The labeled cells were subsequently lysed with radioimmunoprecipitation assay buffer, and RSV antiserum-reactive proteins were immunoprecipitated. After resolution by SDS-PAGE (12% gel), the Pr76 band from each sample was quantified by PhosphorImager analysis. The volume of each band was then divided by the amount of virus present in the corresponding inoculum. The normalized entry values for each mutant were then compared to wild-type's entry efficiency.
RESULTS
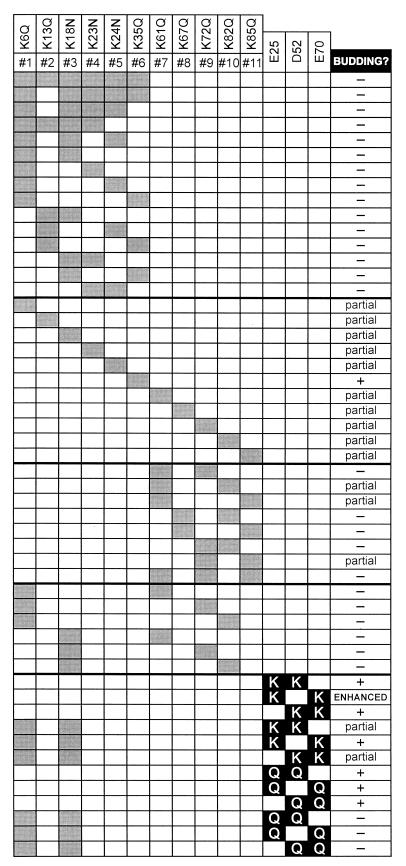
To test whether basic residues are involved in the function of the RSV M domain, we generated mutants in which the basic residues were replaced by the polar, neutral amino acids (asparagine or glutamine). By neutralizing basic residues in a conservative manner, we sought to determine their functional significance without disturbing the overall structure of the M domain. The effects of these changes on the efficiency of particle release are summarized in Fig. 2.
FIG. 2.
Summary of budding phenotypes of M domain mutants. Each row represents a mutant with specific basic residue neutralizations (gray boxes) and/or acidic residue substitutions (black boxes). Budding phenotypes: wild-type level of release (+), less than 20% of wild-type release (−), between 20 and 50% of wild-type release (partial), near-twofold increase in release (enhanced).
Budding defects in mutants with neutralizations of basic residues in the M domain.
Previous studies of proteins that use clusters of basic residues to localize to the plasma membrane have shown that substitution of a majority of the basic residues is often necessary to significantly disrupt membrane binding (9, 33, 38). Thus, it seemed likely that several neutralizations would be required to abrogate Gag-mediated particle release if basic residues are involved in the function of the RSV M domain. To make mutants with multiple neutralizations, we initially focused on the six basic residues contained in the first 35 amino acids of the M domain. This decision was motivated by the fact that this portion of the M domain can be cross-linked to the phospholipids of the viral envelope (26). Mutants with multiple neutralizations in the first half of the M domain were generated using two overlapping oligonucleotides. These oligonucleotides were synthesized using a 1:1 mixture of bases at positions that either maintained or mutated each basic residue codon. By simultaneously targeting all six basic residues in this way, we increased the probability of generating mutants with multiple neutralizations in this region.
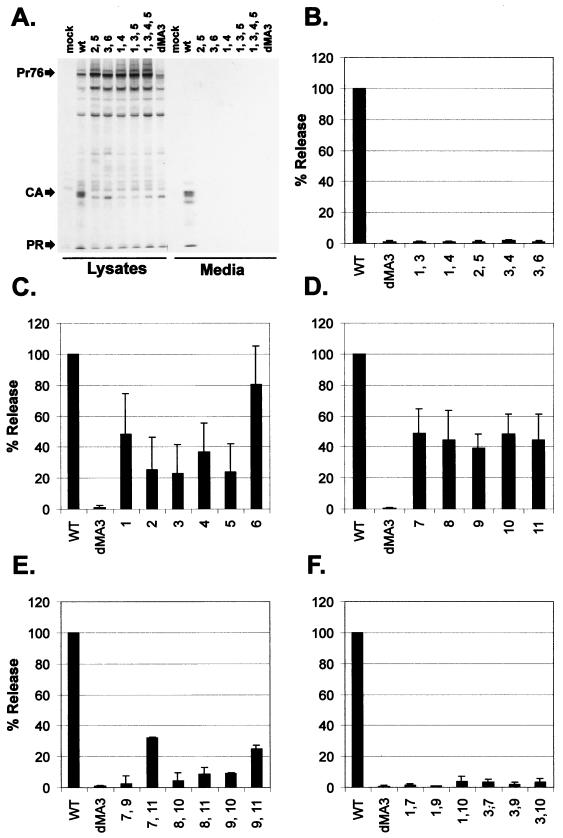
Upon analysis, we found that Gag proteins with neutralizations of two or more basic residues had significant defects in particle release (Fig. 3A and B). Indeed, similar defects were exhibited by every mutant with multiple neutralizations (Fig. 2), suggesting that each basic residue in this region is involved in the function of the M domain.
FIG. 3.
Particle release by mutants with neutralizations of basic residues in the M domain. Mutants with basic residue neutralizations are designated by a simplified nomenclature such that mutant 1,3, for example, has neutralizations of the first and third basic residues in the M domain. To determine the efficiency of release, mammalian (COS-1) cells were metabolically labeled with [35S]methionine 48 h after transfection. Gag proteins were immunoprecipitated from cell lysate and cell growth medium fractions using polyclonal anti-RSV, resolved by SDS-PAGE, and visualized by autoradiography. Wild type (WT) is pSV.Myr0-S. The negative control (dMA3) has residues 16 to 36 deleted from the M domain. (A) Typical autoradiogram showing immunoprecipitated proteins from cell lysate and medium fractions. Positions of full-length Gag (Pr76) and precipitated cleavage products (CA and PR) are indicated. (B) The effects of double neutralizations in the first half of the M domain were quantified by densitometry or PhosphorImager analysis as described in Materials and Methods. Similarly, the effects of single neutralizations in the first half (C) and second half (D) of the M domain were determined. Defects in particle release were also exhibited by mutants with two neutralizations in the second half of the M domain (E) and by mutants containing neutralizations in both halves of the M domain (F).
Since the loss of two basic residues in the first half of the M domain caused significant defects in budding, we considered whether neutralizations of single basic residues would cause similar defects. To test this, we made 11 mutants in which each basic residue in the M domain was neutralized individually. Upon expressing these mutants in transfected cells, we found that single neutralizations reduced budding by 60 to 75% relative to wild-type Gag (Fig. 3C and D). Substitution of the sixth basic residue (K35Q) was one exception; however, this change abrogated budding when combined with a second neutralization in the M domain (e.g., mutant 3, 6 is blocked for budding).
The partial defects caused by single neutralizations in the second half of the M domain suggested that the basic residues in this region are also important for budding. Hence, we considered whether multiple neutralizations in this region would result in more dramatic reductions. Accordingly, we generated mutants using combinations of separate, nonoverlapping oligonucleotides and found that budding was impaired by neutralizing two basic residues in the second half of the M domain (Fig. 3E). However, some mutants (e.g., 7,11 and 9,11) exhibited less severe reductions in particle release.
To further characterize the role of basic residues, we created mutants that contain neutralizations in both the first and second halves of the M domain and found that these changes abrogated particle production (Fig. 3F). Taken together, the budding defects exhibited by mutants with single or multiple neutralizations suggests that each basic residue is involved in the function of the M domain.
Location of M domain mutants in avian cells.
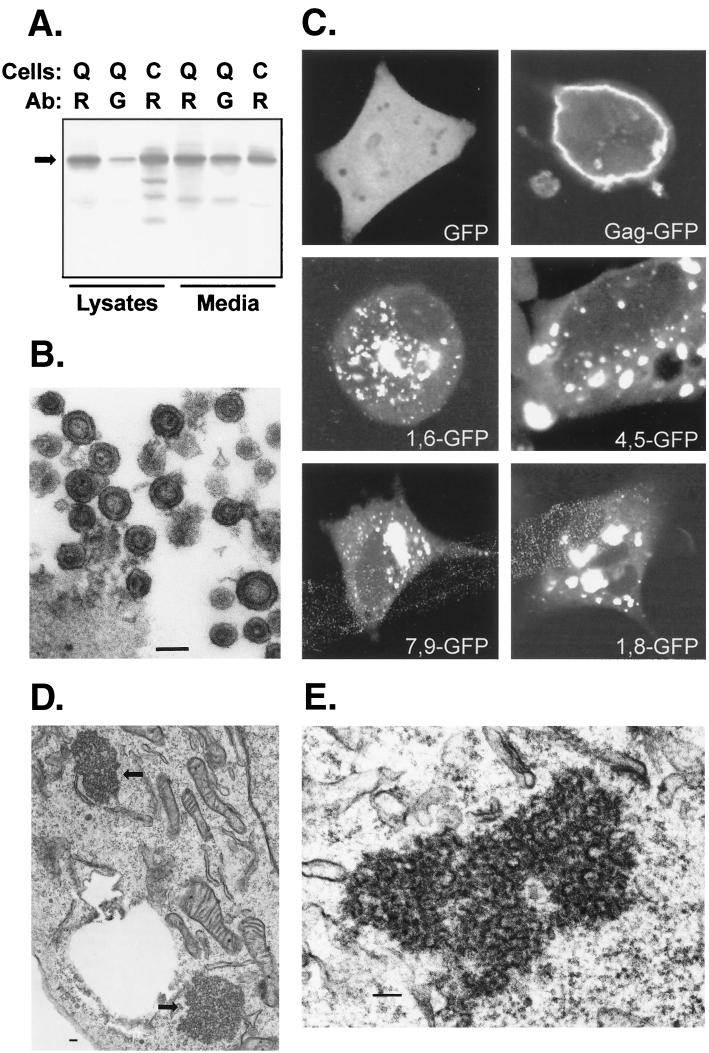
To determine whether neutralizations of basic residues in the M domain prevent Gag targeting to the plasma membrane, we used a construct in which GFP is fused to RSV Gag (Fig. 1A). This approach was chosen over conventional immunofluorescence techniques due to the ease of detection and the ability to observe GFP-tagged proteins in living cells. Gag-GFP was created by replacing the last six residues of NC and the entire PR domain of RSV Gag with GFP. Subsequent analyses confirmed that Gag-GFP is efficiently synthesized, assembled, and released from both mammalian and avian cells (Fig. 4A). In addition, electron microscopy confirmed that particles released from Gag-GFP-transfected cells appear normal and possess a typical immature morphology (due to the lack of PR) (Fig. 4B).
FIG. 4.
Subcellular distribution of M domain mutants. GFP was fused in place of the PR domain of RSV Gag (Fig. 1). (A) Biochemical analysis of Gag-GFP (arrow, 88 kDa) released from QT6 (Q) or COS-1 (C) cells. Immunoprecipitations were performed using polyclonal anti-RSV (R) or polyclonal anti-GFP (G) antibody (Ab) as in Fig. 3. (B) Electron microscopy of Gag-GFP particles released from transfected COS-1 cells. (C) Confocal microscopy of QT6 cells expressing GFP alone, Gag-GFP, or M domain mutants of Gag-GFP. (D) Thin sections of QT6 cells expressing 4,5-GFP, with arrows pointing to the electron-dense aggregates in the cytoplasm. (E) Higher magnification of aggregates formed in QT6 cells expressing 3,4-GFP. In all electron micrographs, bars represent 100 nm.
Confocal microscopy of avian cells transfected with the Gag-GFP expression vector revealed bright rings of fluorescence at the plasma membrane, a pattern reflecting the proper targeting of the fusion protein to the site of RSV Gag assembly and budding (Fig. 4C). In contrast, neutralizations of two basic residues in the M domain of Gag-GFP blocked localization to the plasma membrane. Instead, M domain mutants formed large clusters of fluorescence in the cytoplasm of transfected cells. Subsequent analysis of these cells by electron microscopy revealed aggregates of dense material in the cytoplasm (Fig. 4D and E), and these aggregates were not found in cells expressing budding-competent Gag-GFP (data not shown). While the aggregates contained some structures reminiscent of retroviral particles, they were not associated with any membranes, suggesting that intracellular budding does not occur with these M domain mutants.
Analysis of the infectivity of RSV with single basic residue neutralizations in the M domain of Gag.
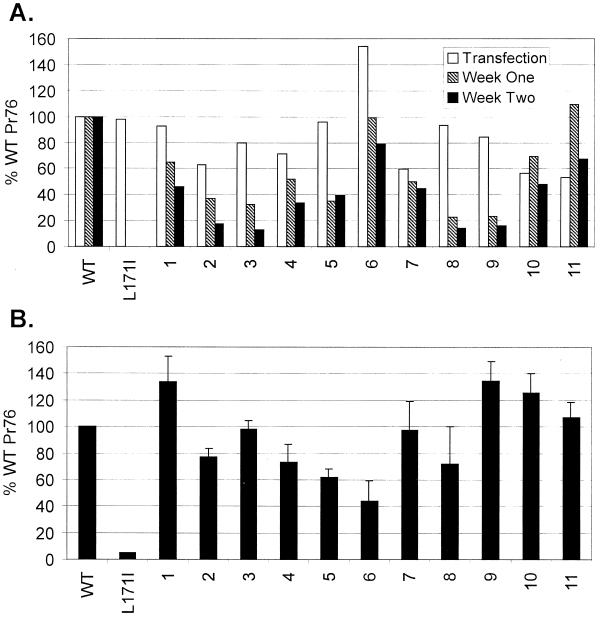
Several authors have argued that MA plays a role in retrovirus entry (8, 14, 21, 25). Therefore, it was of interest to determine whether neutralizations of basic residues in the M domain disrupt infectivity. To test this possibility, we focused on mutants with neutralizations of single basic residues because they produce sufficient numbers of particles for infectivity assays. After moving these gag alleles to proviral vectors, we first tested the mutants' abilities to establish spreading infections in transfected avian cells. To do this, we made use of the fact that the vast majority of transfected DNA is unstable and lost during passage of the cells. Hence, expression of noninfectious mutants is transient, while that of infectious clones persists. Using this approach, we found that single neutralizations in the M domain reduced virus spreading (Fig. 5A). Indeed, the decrease in the relative levels of Pr76 between weeks 1 and 2 suggests that these mutants do not spread with wild-type efficiencies; however, these defects were not as dramatic as that exhibited by the previously described, noninfectious Gag mutant, L171I (6).
FIG. 5.
Infectivity assays. (A) Virus spreading in transfected cultures. Proviral clones of mutants with neutralizations of single basic residues in the M domain were transfected into avian (QT6) cells. Duplicate cultures were either labeled for 5 min with [35S]methionine 18 h after transfection or were passaged every 3 to 4 days and labeled at 1 and 2 weeks posttransfection. Gag proteins from cell lysates were collected by immunoprecipitation, separated by SDS-PAGE, and quantified by PhosphorImager analysis. Infectivity is expressed as a percentage of the total radiolabeled Gag (Pr76) in the lysates of cells transfected with the wild-type (WT) proviral clone. The negative control has a substitution (L171I) in the MHR of CA that renders extracellular particles noninfectious. Values represent the average of two experiments using independent clones for each mutant. (B) Single-cycle infectivity. TEFs were incubated for 48 h after transfection with proviral DNAs. Virus-containing media were then centrifuged to remove cellular debris, and the supernatants were transferred to uninfected TEFs. After 24 h, these cultures were labeled for 15 min with [35S]methionine, and intracellular Gag (Pr76) expression was quantified after immunoprecipitation and SDS-PAGE. Infectivity was then calculated by normalizing Pr76 levels to the amount of virus in the corresponding inocula. The ability of each mutant to enter cells and produce Gag proteins from integrated genomes is expressed as a percentage of wild-type's efficiency (n = 3, error bars indicate standard deviation).
To determine whether the mild deficiencies in spreading were due to defects in the early steps of virus replication (i.e., through integration), each mutant was analyzed in a short-term infectivity assay. In this case, virus-containing media were collected from TEFs transiently transfected with proviral DNAs. Cell-free media were then transferred to uninfected TEFs, and after a 24-h incubation, the amount of intracellular gag expression (Pr76) was determined by metabolic labeling and immunoprecipitation. Pr76 levels were subsequently normalized to the amount of virus present in the inoculum (measured by reverse transcriptase assays), and the ability of each mutant to enter cells was compared to wild-type's efficiency (Fig. 5B). As shown, the ability of basic residue mutants to infect fresh cells was found to be within 50% of wild-type levels. Thus, the deficiencies exhibited by mutant viruses in spreading infections are likely due to the effects of single neutralizations on particle release (i.e., Fig. 3C and D correlate with Fig. 5A, week 2).
Suppression of budding defects.
The failure of double-neutralization mutants to localize to and bud from the plasma membrane is consistent with a role for basic residues in the function of the M domain of RSV Gag. However, loss-of-function data do not rule out the possibility of unintended structural defects (i.e., misfolding). If basic residues are required to form electrostatic interactions with anionic phospholipids, then inserting basic residues at new positions in the M domain should restore membrane binding and budding from the plasma membrane.
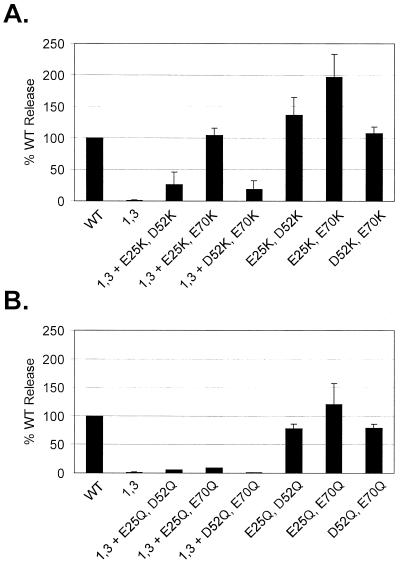
New basic residues were inserted into the M domain by replacing E25, D52, and/or E70 with lysines (Fig. 1A). These acidic residues were chosen so that the lysine side chains would likely orient on the surface of the molecule without perturbing the overall structure of the M domain. When combinations of two acidic-to-basic substitutions were made in M domains containing the 11 original basic residues, we found that budding could actually be enhanced relative to unmodified Gag (Fig. 6A). To determine whether this effect was caused by the loss of two acidic residues rather than the addition of basic residues in the M domain, pairs of acidic residues were neutralized by replacement with glutamine. These acidic-to-neutral substitution mutants were found to bud with an efficiency like that of wild-type Gag (Fig. 6B). Thus, the production of extracellular particles by RSV Gag can be enhanced by increasing the number of basic residues in the M domain.
FIG. 6.
Gain-of-function studies. The efficiency of particle release by M domain mutants with pairs of acidic residue substitutions was determined as in Fig. 3. The acidic substitution mutants contained either no other changes or neutralizations of the first and third basic amino acids in the M domain (1,3 mutants). (A) Effects of acidic-to-basic residue changes; (B) effects of acidic-to-neutral residue changes.
Acidic-to-basic changes also act as second-site suppressors of the budding defect in an M domain mutant containing neutralizations of the first and third basic residues (Fig. 6A). Indeed, replacing E25 and E70 with lysines restored budding to wild-type levels. Moreover, this effect was found to be specific for acidic-to-basic changes because replacing the same acidic residues with glutamine did not restore budding (Fig. 6B). Together, these results show that basic residues are critical determinants of RSV M domain function.
DISCUSSION
In this study, we found the RSV Gag protein to be particularly sensitive to neutralizations of basic residues in the M domain. Indeed, release of Gag can be blocked by neutralizations of just two basic residues. Moreover, single neutralizations in the M domain can also impair budding. In contrast, the M domains of murine leukemia virus and HIV-1 tolerate substitutions of four or more basic residues (9, 33, 38). In addition, an HIV-1 Gag mutant lacking all but one basic residue in MA (matrix) buds 30% as well as the wild type (16). One explanation for the relative insensitivity of the murine leukemia virus and HIV-1 M domains to basic residue changes is the presence of myristate at their N termini. In RSV, however, Gag is not myristylated. Thus, the absence of this second membrane-binding component may explain why the loss of fewer basic residues in the RSV M domain causes dramatic defects in membrane localization. To further examine this possibility, it will be interesting to test whether the addition of myristate to RSV Gag, via an E2G change (36), restores budding in mutants with basic neutralizations in the M domain.
To date, the role of basic residues in binding viral and cellular proteins to biological membranes has been inferred from loss-of-function studies and in vitro membrane-binding data (7, 9, 19, 27, 31, 38, 39). In contrast, this report provides gain-of-function evidence for the importance of basic residues by demonstrating that particle release can be enhanced through the addition of lysines in the M domain. Furthermore, acidic-to-basic residue changes act as second-site suppressors of the budding defect in an M domain mutant. Interestingly, nonconservative substitutions in HIV-1 MA can also act as second-site suppressors of membrane-binding defects (22, 23). Unlike in RSV, however, the restoration effects in HIV-1 Gag have not been attributed to the addition of basic residues. Moreover, these second-site suppressors do not enhance HIV-1 particle production when expressed in the absence of the original substitution.
Rescue of a budding-defective mutant using pairs of acidic-to-basic substitutions suggests that the basic residues involved in membrane binding can be repositioned. Interestingly, the 1,3 mutant with additional substitutions at E25 and E70 was rescued to wild-type levels of release, while only partial restorations were observed when one of the substitutions was at D52. One explanation for these differences is that, in contrast to new lysines at residues 25 and 70, a lysine at residue 52 may be located in a region of the M domain that does not interface with the membrane. This would also explain why Gag proteins containing all of the original basic residues are most dramatically enhanced for budding when lysines are introduced at E25 and E70. Thus, the basic residues involved in membrane binding can be repositioned but their locations may be constrained to the regions of the M domain that interact with the plasma membrane.
Insights into how basic residues might direct Gag to the plasma membrane are provided by studies of membrane-binding proteins containing pleckstrin homology domains and FYVE domains (e.g., dynamin, phospholipase C-δ1, and EEA1). In both domains, basic residues exhibit remarkable specificities for particular phosphoinositides present in the target membrane(s) (2, 4, 12, 17). Likewise, basic residues in the M domain of RSV Gag might determine membrane specificity by binding to the anionic phospholipids found predominantly in the plasma membrane. However, the substrate specificity of the M domain might not be as fine-tuned as that observed in the pleckstrin homology or FYVE domains because repositioning the basic residues did not prevent Gag from localizing to the plasma membrane. Instead, the simple enrichment of the inner leaflet of the plasma membrane with acidic phospholipids may determine membrane specificity. Examination of this possibility will likely require an in vitro system in which the ability of RSV Gag to bind membranes of different phospholipid compositions can be measured.
ACKNOWLEDGMENTS
We thank Carol Wilson for technical assistance with the construction of proviral clones. We also thank Roland Meyers for performing thin sectioning and electron microscopy. pRCAS-EGFP was kindly provided by Mark Federspiel.
This research was sponsored by a National Institutes of Health grant CA47482 to J.W.W.
REFERENCES
- 1.Bennett R P, Nelle T D, Wills J W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1993;67:6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottomley M J, Salim K, Panayotou G. Phospholipid-binding proteins. Biochim Biophys Acta. 1998;1436:165–183. doi: 10.1016/s0005-2760(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 3.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burd C G, Emr S D. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;3:805–811. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 5.Craven R C, Leure-duPree A E, Erdie C R, Wilson C B, Wills J W. Necessity of the spacer peptide between CA and NC in the Rous sarcoma virus Gag protein. J Virol. 1993;67:6246–6252. doi: 10.1128/jvi.67.10.6246-6252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craven R C, Leure-duPree A E, Weldon R A, Wills J W. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J Virol. 1995;69:4213–4227. doi: 10.1128/jvi.69.7.4213-4227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich L S, Fong S, Scarlata S, Zybarth G, Carter C. Partitioning of HIV-1 Gag and Gag-related proteins to membranes. Biochemistry. 1996;35:3933–3943. doi: 10.1021/bi952337x. [DOI] [PubMed] [Google Scholar]
- 8.Freed E O. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 9.Freed E O, Englund G, Martin M A. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock J F, Paterson H, Marshall C J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 12.Harlan J E, Hajduk P J, Yoon H S, Fesik S W. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 13.Katz R A, Omer J H, Weis J H, Mitsialis S A, Fara A J, Guntaka R V. Restriction endonuclease and nucleotide sequence analysis of molecularly cloned unintegrated avian tumor virus DNA: structure of large terminal repeats in circle junctions. J Virol. 1982;42:346–351. doi: 10.1128/jvi.42.1.346-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiernan R E, Ono A, Freed E O. Reversion of a human immunodeficiency virus type 1 matrix mutation affecting Gag membrane binding, endogenous reverse transcriptase activity, and virus infectivity. J Virol. 1999;73:4728–4737. doi: 10.1128/jvi.73.6.4728-4737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkel T A, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 16.Lee P P, Linial M. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J Virol. 1994;68:6644–6654. doi: 10.1128/jvi.68.10.6644-6654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemmon M A, Ferguson K M, O'Brien R, Sigler P B, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonnell J M, Fushman D, Cahill S M, Zhou W, Wolven A, Wilson C B, Nelle T D, Resh M D, Wills J W, Cowburn D. Solution structure and dynamics of the bioactive retroviral M domain from Rous sarcoma virus. J Mol Biol. 1997;279:921–928. doi: 10.1006/jmbi.1998.1788. [DOI] [PubMed] [Google Scholar]
- 19.Mosior M, McLaughlin S. Binding of basic peptides to acidic lipids in membranes: effects of inserting alanine(s) between the basic residues. Biochemistry. 1992;31:1767–1773. doi: 10.1021/bi00121a026. [DOI] [PubMed] [Google Scholar]
- 20.Nelle T D, Verderame M F, Leis J, Wills J W. The major site of phosphorylation within the Rous sarcoma virus matrix protein is not required for replication. J Virol. 1998;72:1103–1107. doi: 10.1128/jvi.72.2.1103-1107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelle T D, Wills J W. A large region within the Rous sarcoma virus matrix protein is dispensable for budding and infectivity. J Virol. 1996;70:2269–2276. doi: 10.1128/jvi.70.4.2269-2276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono A, Freed E O. Binding of human immunodeficiency virus type I Gag to membrane: role of the matrix amino terminus. J Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono A, Huang M, Freed E O. Characterization of human immunodeficiency virus type 1 matrix revertants: effects on virus assembly, Gag processing, and Env incorporation into virions. J Virol. 1997;71:4409–4418. doi: 10.1128/jvi.71.6.4409-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmiter R D, Gagnon J, Vogt V M, Ripley S, Eisenman R N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag) Virology. 1978;91:423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- 25.Parent L J, Wilson C B, Resh M D, Wills J W. Evidence for a second function of the MA sequence in the Rous sarcoma virus Gag protein. J Virol. 1996;70:1016–1026. doi: 10.1128/jvi.70.2.1016-1026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pepinsky R B, Vogt V M. Fine-structure analyses of lipid-protein and protein-protein interactions of Gag protein p19 of the avian sarcoma and leukemia viruses by cyanogen bromide mapping. J Virol. 1984;52:145–153. doi: 10.1128/jvi.52.1.145-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebecchi M, Peterson A, McLaughlin S. Phosphoinositide-specific phospholipase C-δ1 binds with high affinity to phospholipid vesicles containing phosphatidylinositol 4,5-biphosphate. Biochemistry. 1992;31:12742–12747. doi: 10.1021/bi00166a005. [DOI] [PubMed] [Google Scholar]
- 28.Resh M D. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 29.Schultz A M, Henderson L E, Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz D E, Tizard R, Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983;32:853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- 31.Sigal C T, Zhou W-J, Buser C A, McLaughlin S, Resh M D. The amino-terminal basic residues of Src mediate membrane binding through electrostatic interactions with acidic phospholipids. Proc Natl Acad Sci USA. 1994;91:12253–12257. doi: 10.1073/pnas.91.25.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman L, Resh M D. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristylated pp60v-src. J Biol Chem. 1992;119:415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soneoka Y, Kingsman S M, Kingsman A J. Mutagenesis analysis of the murine leukemia virus matrix protein: identification of regions important for membrane localization and intracellular transport. J Virol. 1997;71:5549–5559. doi: 10.1128/jvi.71.7.5549-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi H, Maneti S. Interaction of myristoylated alanine-rich protein kinase C substrate (MARCKS) with membrane phospholipids. J Biol Chem. 1993;268:9960–9963. [PubMed] [Google Scholar]
- 35.Verderame M F, Nelle T D, Wills J W. The membrane-binding domain of the Rous sarcoma virus Gag protein. J Virol. 1996;70:2664–2668. doi: 10.1128/jvi.70.4.2664-2668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wills J W, Craven R C, Achacoso J A. Creation and expression of myristylated forms of Rous sarcoma virus Gag protein in mammalian cells. J Virol. 1989;63:4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wills J W, Craven R C, Weldon R A, Jr, Nelle T D, Erdie C R. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J Virol. 1991;65:3804–3812. doi: 10.1128/jvi.65.7.3804-3812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan X, Yu X, Lee T-H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]