Abstract
In Côte d'Ivoire, the popularity of ready-to-eat salads has grown substantially. Despite their convenience, these products often face criticism for their microbiological safety. This research was conducted to assess the virulence and antibiotic resistance profiles of Escherichia coli (E. coli), Salmonella spp., and Staphylococcus aureus (S. aureus) isolated from salads available in hypermarkets across Abidjan. The study utilized a combination of microbiological and molecular biology techniques. Results indicated that E. coli isolates harbored virulence genes such as stx2 (50%) and ST (62.50%), though genes stx1 and LT were absent in the samples tested. In S. aureus, virulence genes detected included sea (55.55%), sec (11.110%), and sed (44.44%). The antibiotic resistance assessment revealed high resistance in E. coli to β-lactam antibiotics, with all isolates resistant to cefuroxime (100%) and the majority to ampicillin and cefoxitin (87.5%). Most Salmonella spp. isolates were sensitive to the antibiotics tested, except for cefoxitin and ampicillin, showing resistance rates of 42.85% and 57.15%, respectively. Staphylococcus aureus demonstrated considerable resistance, particularly to cefoxitin (44.44%), benzylpenicillin (100%), and ampicillin (55.55%). In addition, resistance to aminoglycosides (55.55% to both kanamycin and gentamicin) and macrolides (66.66% to erythromycin and 55.55% to clindamycin) was noted. Resistance to various fluoroquinolones ranged between 33.33% and 55.55%. The presence of resistance genes such as blaTEM (10.52%), qnrA (2.26%), qnrB (5.26%), qnrS (5.26%), and mecA (13.15%) in E. coli and S. aureus underscores the challenge of multidrug resistance, exhibiting phenotypes such as ESBL (50%), Meti-R (55.55%), KTG (44.44%), MLSB (44.44%), and FQ-R (25%). These results carry significant epidemiological and public health implications, highlighting the urgent need for improved safety regulations and practices regarding ready-to-eat salads in urban food markets.
1. Introduction
Ready-to-eat fruit and vegetables, also known as 4th-range products, appeared in European supermarkets from 1980 onwards [1]. According to WHO, FAO, and the World Cancer Research Fund, the consumption of 400–600 g of fruit and vegetables a day is recommended [2, 3]. A diet rich in fruits and vegetables is likely to reduce the risk of cardiovascular disease and protect against certain types of cancer [4]. However, several cases of food poisoning associated with the consumption of these products have been observed around the world. For example, in 2012, a foodborne outbreak was reported in a college in China due to the consumption of salad ingredients [5]. In May 2014, an outbreak was observed in military and civilian populations associated with the consumption of ready-to-eat mixed salad in Norway [6]. In April 2019, a cross-border outbreak in Sweden including 37 cases and 20 cases in Denmark following consumption of imported fresh spinach was reported [7]. Based on currently available statistics, bacterial species in this case, Escherichia coli (E. coli), Salmonella spp., and Staphylococcus aureus (S. aureus), are among the important pathogens associated with fourth-range foods [8]. In Africa, the average prevalence of E. coli in ready-to-eat foods is 31.6%. The prevalence of Salmonella spp. is estimated at 21.7% and that of S. aureus at 25.1% [9]. Today, in developing countries such as Côte d'Ivoire, the consumption of ready-to-eat salads has become a cause for concern. Indeed, the desire to eat well is now a general trend [10]. However, high prevalences of E. coli (16%), Salmonella spp. (18%), and S. aureus (24%) have been determined in ready-to-eat salads sold in the city's supermarkets [11]. Thus, this work was carried out to determine the levels of virulence and antibiotic resistance of E. coli, Salmonella spp., and S. aureus isolates isolated from ready-to-eat salads sold in Abidjan supermarkets. This work aims to prevent the dissemination of virulence and resistance genetic material of these pathogens through the consumption of these foods in Côte d'Ivoire.
2. Materials and Methods
2.1. Study Area
This study examines industrially produced ready-to-eat salads available in large supermarkets within Abidjan, Côte d'Ivoire. Abidjan is located in the southern part of the country, along the Gulf of Guinea, and covers an area of 2119 km2, representing 0.6% of the national territory, with a population density of 1475 inhabitants per km2. Sample analyses were conducted in the laboratory at Université Jean Lorougnon Guédé in Daloa, the capital of the Haut-Sassandra region. Positioned centrally in the western part of Côte d'Ivoire, Daloa is situated at a latitude of 6°52′38″ north and a longitude of 6°27′00″ west, approximately 141 km from Yamoussoukro, the political capital and 383 km from Abidjan, the economic hub. Further studies on antibiotic resistance were carried out at the Bacteriology-Virology Department of the Institut Pasteur de Côte d'Ivoire, within the Antibiotics, Natural Substances, and Surveillance of Microorganisms and Antibiotics Unit (ASSURMI), located in Cocody.
2.2. Biological Material
This experimental study analyzed bacterial isolates from 38 samples of ready-to-eat salads collected from hypermarkets in Abidjan, Côte d'Ivoire. These different samples come from 5 supermarkets located in the communes of Cocody (3) and Marcory (2). The research focused on three bacterial species: Escherichia coli (8 isolates), Salmonella spp. (7 isolates), and Staphylococcus aureus (9 isolates). These isolates, previously characterized using conventional microbiological techniques, were sourced from a diverse range of salad types as detailed in Table 1.
Table 1.
Detection of pathogenic bacteria in ready-to-eat salads.
| Salads | Germs | ||
|---|---|---|---|
| E. coli | S. aureus | Salmonella spp. | |
| 100% curly heart (Cf) | − | − | − |
| Baby spinach (Ep) | − | − | − |
| Young shoots (JP) | − | − | + |
| Lamb's lettuce (Ma) | − | − | − |
| Lamb's lettuce + rocket (MR) | − | − | + |
| Rocket (Rq) | − | + | − |
| Aperitif salads (SA) | − | + | + |
| Salads carrots (SCA) | + | + | − |
| Salads cabbage (SCH) | + | − | + |
| Green oak leaf salads (SChV) | + | + | + |
| Mixed salads (SCOM) | + | + | − |
| Pineapple fruit salads (SFA) | − | − | − |
| Mixed fruit salads (SFC) | + | − | − |
| Pineapple + mango fruit salads (SFAM) | − | − | − |
| Papaya + pineapple fruit salads (SFPA) | − | + | − |
| Papaya + lemon fruit salads (SFPC) | − | − | − |
| Grapefruit salads (SFR) | + | + | + |
| Meli melo salads (SME) | + | + | − |
| Nicoise salads (SN) | + | + | + |
+, Presence of pathogens; −, pathogen-free.
2.3. Searching for Virulence Genes
2.3.1. Preparation of Genetic Material (DNA)
DNA extraction was performed using the CTAB method, as outlined in reference [12]. The process began by centrifuging 1.5 mL of the bacterial preculture in LB medium at 16,000 rpm for 5 minutes to pellet the cells. After discarding the supernatant, the pellet was resuspended in 1.5 mL of CTAB1 extraction buffer (20 g/L CTAB, 1.4 mol/L NaCl, 0.1 mol/L Tris, 0.02 mol/L Na-EDTA, and a pH of 8.0) and 5 μL of RNAse (20 mg/mL). This mixture was then incubated at 60°C for 30 minutes, with intermittent shaking to resuspend the material. Proteinase K (10 μL of 20 mg/mL) was added halfway through the incubation.
Following another round of centrifugation, 900 μL of the supernatant was extracted and mixed with an equal volume of chloroform. After vortexing and subsequent centrifugation at 15, 000g for 15 minutes, 650 μL of the clear supernatant was mixed with 1.3 mL of CTAB2 precipitation buffer and left to stand at room temperature for 60 minutes. Postcentrifugation, the supernatant was discarded, and the DNA pellet was washed with a NaCl solution (700 μL of CTAB3) followed by chloroform. The aqueous phase was then mixed with twice its volume of cold isopropanol and allowed to precipitate at room temperature for 20 minutes.
The DNA was then pelleted by centrifugation, washed with 70% ethanol, dried in an oven at 55°C for 30 minutes, and finally resuspended in 30 μL of TE buffer. The extracted DNA was stored at −20°C for further analysis.
2.3.2. Amplification of Desired Genes
For E. coli, the target genes included those encoding Shiga toxins 1 and 2 (stx1 and stx2), which were amplified using multiplex PCR. The amplification also targeted genes for heat-labile (LT) and heat-stable (ST) toxins. The PCR mix, based on the protocol described in [13], consisted of 20 μL total volume: 10 μL of 2X Phusion Master Mix, 2 μL of primers (both sense and antisense, detailed in Table 2), 2 μL of extracted DNA, and 6 μL of sterilized nuclease-free water. The PCR conditions included an initial denaturation at 94°C for 5 minutes, followed by 30 cycles of amplification (details in Table 3). The PCR products, stained with 1 μL of 6X loading buffer, were analyzed on a 1.2% agarose gel.
Table 2.
Primers used for the detection of virulence genes in E. coli and S. aureus.
| Germs | Genes | Sequences (5′->3′) | Sizes (bp) | References |
|---|---|---|---|---|
| E. coli | stx1 | F: ACACTGGATGATCTCAGTGG R: CGTAATCCCCCTCCATTATG |
614 | [17] |
| stx2 | F: CCATGACAACGGACAGCAGTT R: CCTGTCAACTGAGCAGCACTTTG |
779 | [17] | |
| LT | F: GCGACAAATTATACCGTGCT R: CCGAATTCTGTTATATATGT |
708 | [18] | |
| ST | F: CTGTATTTGTCTTTTTCACCT R: GCACCCGGTACAAGCAGGAT |
182 | [18] | |
|
| ||||
| S. aureus | sea | F: GGTTATCAATGTGCGGGTGG R: CGGCACTTTTTTCTCTTCGG |
102 | [15] |
| seb | F: GTATGGTGGTGTAACTGAGC R: CCAAATAGTGACGAGTTAGG |
164 | [15] | |
| sec | F: AGATGAAGTAGTTGATGTGTATGG R: CACACTTTTAGAATCAACCG |
451 | [15] | |
| sed | F: CCAATAATAGGAGAAAATAAAAG R: ATTGGTATTTTTTTTCGTTC |
278 | [15] | |
| see | F: AGGTTTTTTCACAGGTCATCC R: CTTTTTTTTCTTCGGTCAATC |
209 | [15] | |
stx1, Shiga toxin 1; stx2, Shiga toxin 2; LT, heat-labile toxin; ST, heat-stable toxin; sea, seb, sec, sed, and see: Staphylococcal enterotoxins, a–e.
Table 3.
Virulence gene amplification program.
| Amplification steps | Amplification conditions (temperature and weather) | ||
|---|---|---|---|
| stx1/stx2 | ST/LT | sea/seb/sec/sec/sed/see | |
| Initial denaturation | 94°C/5 min | 94°C/5 min | 94°C/5 min |
| Cyclic denaturation | 94°C/30 s | 94°C/45 s | 94°C/2 min |
| Hybridization | 56°C/30 s | 60°C/1 min | 57°C/2 min |
| Cyclic elongation | 72°C/30 s | 72°C/1 min | 72°C/1 min |
| Final elongation | 72°C/10 min | 72°C/7 min | 72°C/7 min |
| Number of cycles | 30 | 35 | 35 |
For S. aureus, the study focused on characterizing five stereotyped enterotoxins (sea, seb, sec, sed, and see), which are heat-stable proteins linked to food poisoning. The virulence genes were amplified in two multiplex sets: one for sea and seb and another for sec, sed, and see, using primers listed in Table 3. Amplifications were conducted in a mini thermal cycler (miniPCR bioTM), following the protocol in [15]. The resultant PCR products were subjected to electrophoresis on a 2% agarose gel.
2.4. Study of Antibiotic Resistance
2.4.1. Determination of Resistance Profile
This component of the study involved conducting antibiograms using the agar diffusion method (Mueller Hinton, MH) as outlined by the antibiogram committee of the Société Française de Microbiologie [16]. Initially, a 24-hour culture of each isolate was prepared. Subsequently, a bacterial suspension was made in a 2 mL solution of 0.85% NaCl to match the 0.5 McFarland standard, equivalent to approximately 1 to 2 × 108 CFU/mL. Inoculation was performed on the surface of MH agar plates, followed by the application of antibiotic discs, considering the inherent resistance profiles of the bacterial species involved. The specific antibiotics applied are detailed in Table 4. Different antibiotics were used for each bacterial species. Specific antibiotics for S. aureus included FOS, RIF, CHL, CMN, ERY, FTN, KMN, GMN, FOX, AMP, PNG, FAD, NXN, NIR, CIP, MXF, and RIF, while those for Salmonella spp. and E. coli included FOX, CRO, TCC, FIX, FEP, MEC, AMP, TIC, IPM, CXM, NAL, CIP, NXN, NIR, AKN, TGC, TMP, FOS, and NTM.
Table 4.
List of antibiotic discs tested on bacterial isolates.
| Antibiotic families | Antibiotics | Code | Fillers (μg) |
|---|---|---|---|
| Beta-lactam antibiotics | Benzylpenicillin | PENG | 1 IU |
| Cefoxitin | FOX | 30 | |
| Ceftriaxone | CRO | 30 | |
| Ticarcillin + clavulanic acid | TCC | 85 | |
| Cefixime | FIX | 5 | |
| Cefepime | FEP | 30 | |
| Mecillinam | MEC | 10 | |
| Ticarcillin | TIC | 75 | |
| Cefuroxime | CXM | 30 | |
| Ampicillin | AMP | 10 | |
|
| |||
| Macrolide, lincosamide, and streptogramin | Clindamycin | CMN | 2 |
| Erythromycin | ERY | 15 | |
|
| |||
| Fluoroquinolones | Norfloxacin | NXN | 10 |
| Nitroxoline | NIR | 30 | |
| Ciprofloxacin | CIP | 5 | |
| Moxifloxacin | MXF | 5 | |
| Nalidixic acid | NAL | 30 | |
|
| |||
| Carbapenem | Imipenem | IPM | 10 |
| Meropenem | MEN | 10 | |
|
| |||
| Tetracycline | Tigecycline | TGC | 15 |
|
| |||
| Aminosides | Kanamycin | KMN | 30 |
| Gentamicin | GMN | 10 | |
| Netilmicin | NTM | 10 | |
| Amikacin | AKN | 30 | |
|
| |||
| Others | Fusidic acid | FAD | 10 |
| Rifampicin | RIF | 5 | |
| Fosfomycin | FOS | 200 | |
| Chloramphenicol | CHL | 30 | |
| Nitrofuran | FTN | 300 | |
Plates were incubated within 15 minutes of disc placement, and the zones of inhibition around the antibiotic discs were measured using an automated system (ADAGIO). The diameters of these inhibition zones were used to determine the sensitivity of the isolates, classified as sensitive (S), resistant (R), or intermediate (I), according to the specific criteria for each bacterium [16].
2.4.2. Detection of Genes Encoding Antibiotic Resistance
This analysis included multiplex PCR amplification of beta-lactam resistance genes (blaTEM, blaSHV, and blaCTX-M) and fluoroquinolone resistance genes (qnrA, B, and S) in E. coli and Salmonella species. The method used is that described by the authors in [41]. Indeed, PCR reactions were performed using 2 μL of DNA template (density of 10 ng/μL), 4 μL of Master Mix (5X), 1 μL of each primer (a total of 6 μL per multiplex), and 8 μL of H2O. The reaction mix has a final volume of 20 μL. In addition, the mecA gene, responsible for meticillin resistance, was amplified in staphylococci. The primers for these resistance genes can be found in Table 5. The reaction mixture used for this procedure was identical to that employed in the virulence gene testing. Specific PCR conditions tailored to these resistance genes are detailed in Table 6.
Table 5.
Primers used for the detection of resistance genes in E. coli, Salmonella spp., and S. aureus.
| Germs | Genes | Sequences (5′->3′) | Sizes (bp) | References |
|---|---|---|---|---|
| E. coli and Salmonella spp. | bla TEM | F: ATGAGTATTCAACATTTCCGTG R: TTACCAATGCTTAATCAGTGAG |
840 | [19] |
| bla SHV | F: TTTATGGCGTTACCTTTGACC R: ATTTGTCGCTTCTTTACTCGC |
1051 | [20] | |
| bla CTX –M | F: GGTTAAAAAATCACTGCGTC R: TTGGTGACGATTTTAGCCGC |
863 | [21] | |
| qnrA | F: GATAAAGTTTTTCAGCAAGAGG R: ATCCAGATCGGCAAAGGTTA |
543 | [21] | |
| qnrB | F: GACAGAAACAGGTTCACCGGT R: CAAGACGTTCCAGGAGCAACG |
469 | [21] | |
| qnrS | F: ACGACATTCGTCAACTGCAA R: TAAATTGGCAACCTGTAGGC |
417 | [21] | |
|
| ||||
| S. aureus | mecA | F: TGCTATCCACCCTCAAACAGG R: AACGTTGTAACCACCCCAAGA |
286 | [22] |
blaTEM, blaSHV, and blaCTX-M: beta-lactam resistance genes; qnrA, qnrB, and qnrS: fluoroquinolone resistance marker genes; mecA: meticillin resistance; E. coli, Escherichia coli; S. aureus, Staphylococcus aureus.
Table 6.
Resistance gene amplification conditions.
| Amplification steps | Temperature condition/times | ||
|---|---|---|---|
| blaCTX-M, blaTEM, blaSHV | qnrA, qnrB, qnrS | mecA | |
| Initial denaturation | 94°C/5 min | 95°C/5 min | 94°C/4 min |
| Cyclic denaturation | 94°C/1 min | 95°C/30 s | 94°C/30 s |
| Hybridization | 60°C/1 min | 60°C/30 s | 60°C/1 min |
| Cyclic elongation | 72°C/1 min | 72°C/1 min | 72°C/2 min |
| Final elongation | 72°C/7 min | 72°C/10 min | 72°C/4 min |
| Number of cycles | (30) | (30) | (35) |
3. Results and Discussion
3.1. Results
3.1.1. Prevalences of Virulence Genes in Bacterial Isolates Isolated from 4th-Range Salads
The study identified the presence of key virulence genes within isolates of S. aureus and E. coli isolated from ready-to-eat salads. For S. aureus, the sea gene showed a prevalence of 55.55%, detected in five samples (SA, SCOM, SN, SChV, and SME). The sec gene was less prevalent, found in only one sample (SCOM) at 11.11%. The sed gene was identified in four samples (Rq, SA, SCA, and SCOM), with a prevalence of 44.44%.
In E. coli, the virulence genes detected included ST and stx2, with prevalences of 62.50% (five samples: SN, SCOM, SChV, SFR, and SME) and 50% (four samples: SN, SCOM, SChV, and SME), respectively. Detailed data on these findings are available in Table 7. In addition, electrophoretic profiles illustrating the amplification products of the sea and stx2 genes are presented in Figures 1 and 2, respectively.
Table 7.
Prevalence of virulence genes in E. coli and S. aureus.
| Isolates | Genes sought | Number of samples | Prevalences (%) |
|---|---|---|---|
| E. coli | LT | 8 | 0 (0) |
| ST | 8 | 5 (62.50) | |
| stx1 | 8 | 0 (0) | |
| stx2 | 8 | 4 (50) | |
|
| |||
| S. aureus | sea | 9 | 5 (55.55) |
| seb | 9 | 0 (0) | |
| sec | 9 | 1 (11.11) | |
| sed | 9 | 4 (44.44) | |
| see | 9 | 0 (0) | |
stx1, Shiga toxin 1; stx2, Shiga toxin 2; LT, heat-labile toxin; ST, heat-stable toxin; sea, seb, sec, sed, and see: Staphylococcal enterotoxins, a–e.
Figure 1.
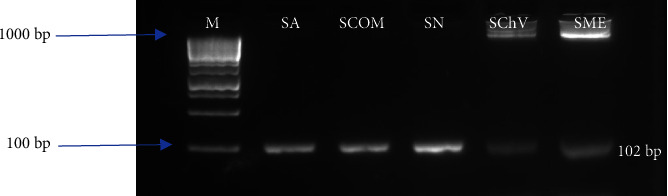
Electrophoretic profile of the amplification product of the S. aureus virulence gene (sea). M: molecular marker (100 bp); isolates from SA: Aperitif lettuces, SChV: green oak leaf salads, SN: Nicoise salads, SCOM: mixed salads, and SME: Meli melo salads.
Figure 2.
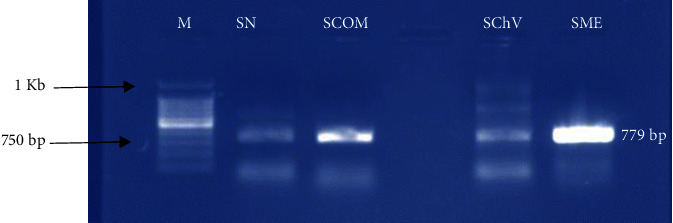
Electrophoretic profile of E. coli virulence gene amplification product (stx2). M: molecular marker (250 bp); isolates from SN: Nicoise salads, SCOM: mixed salads, SME: Meli melo salads, and SChV: green oak leaf salads.
3.1.2. Antibiotic Resistance Profile of Isolated Isolates
In this study, the antibiotic resistance profiles of bacterial isolates isolated from ready-to-eat salads were thoroughly investigated. E. coli exhibited a high level of resistance, with all isolates (100%) resistant to cefuroxime, and a similarly high resistance was observed for cefoxitin and ampicillin at 87.5%. A lower resistance rate was noted for imipenem at 12.5%, while ciprofloxacin displayed a moderate resistance level of 37.5% (Table 8). For Salmonella spp., resistance to beta-lactam antibiotics was significant, with 42.85% of isolates resistant to cefoxitin and 57.15% to ampicillin. S. aureus isolates demonstrated extensive resistance across various antibiotics, with a complete resistance (100%) to benzylpenicillin and substantial resistance rates of 66.66% for both fosfomycin and erythromycin. In addition, resistance was noted at 55.55% for ampicillin, kanamycin, gentamicin, norfloxacin, and rifampicin, while 44.44% of isolates were resistant to cefoxitin and moxifloxacin (Table 8). These findings underscore the critical challenge of combating antibiotic resistance in foodborne pathogens and highlight the need for stringent food safety regulations and proactive antibiotic stewardship.
Table 8.
Resistance profile of bacterial isolates isolated from ready-to-eat salads.
| Antibiotic families | Molecular | E. coli | Salmonella spp. | S. aureus |
|---|---|---|---|---|
| Beta-lactam antibiotics | Benzylpenicillin (PENG).1 IU | 0/8 (0) | 0/7 (0) | 9/(100) |
| Cefoxitin (FOX).30 μg | 7/8 (87.5) | 3/7 (42.85) | 4/9 (44.44) | |
| Ceftriaxone (CRO).30 μg | 0/8 (0) | 0/7 (0) | 0/9 (0) | |
| Ticarcillin + clavulanic acid (TCC).85 μg | 4/8 (50) | 0/7 (0) | 0/9 (0) | |
| Cefixime (FIX).5 μg | 3/8 (37.5) | 0/7 (0) | 0/9 (0) | |
| Cefepime (FEP).30 μg | 0/8 (0) | 0/7 (0) | 0/9 (0) | |
| Mecillinam (MEC).10 μg | 2/8 (25) | 0/7 (0) | 0/9 (0) | |
| Ticarcillin (TIC).75 μg | 4/8 (50) | 0/7 (0) | 0/9 (0) | |
| Cefuroxime (CXM).30 μg | 8/8 (100) | 0/7 (0) | 0/9 (0) | |
| Ampicillin (AMP).10 μg | 7/8 (87.5) | 4/7 (57.14) | 5/9 (55.55) | |
|
| ||||
| Macrolide, lincosamide, and streptogramin | Clindamycin (CMN).2 μg | 0/8 (0) | 0/7 (0) | 5/9 (55.55) |
| Erythromycin (ERY).15 μg | 0/8 (0) | 0/7 (0) | 6/9 (66.66) | |
|
| ||||
| Fluoroquinolones | Norfloxacin (NXN).10 μg | 2/8 (25) | 0/7 (0) | 5/9 (55.55) |
| Nitroxoline (NIR).30 μg | 3/8 (37.5) | 0/7 (0) | 0/9 (0) | |
| Ciprofloxacin (CIP).5 μg | 3/8 (37.5) | 0/7 (0) | 3/9 (33.33) | |
| Moxifloxacin (MXF).5 μg | 0/8 (0) | 0/7 (0) | 4/9 (44.44) | |
| Nalidixic acid (NAL).30 μg | 1/8 (12.5) | 0/7 (0) | 0/9 (0) | |
|
| ||||
| Carbapenem | Imipenem (IPM).10 μg | 1/8 (12.5) | 0/7 (0) | 0/9 (0) |
| Meropenem (MEN).10 μg | 0/8 (0) | 0/7 (0) | 0/9 (0) | |
|
| ||||
| Tetracycline | Tigecycline (TGC).15 μg | 0/8 (0) | 0/7 (0) | 0/9 (0) |
|
| ||||
| Aminosides | Kanamycin (KMN).30 μg | 0/8 (0) | 0/7 (0) | 5/9 (55.55) |
| Gentamicin (GMN).10 μg | 0/8 (0) | 0/7 (0) | 5/9 (55.55) | |
| Netilmicin (NTM).10 μg | 2/8 (25) | 0/7 (0) | 0/9 (0) | |
| Amikacin (AKN).30 μg | 2/8 (25) | 0/7 (0) | 0/9 (0) | |
|
| ||||
| Others | Fusidic acid (FAD).10 μg | 0/8 (0) | 0/7 (0) | 6/9 (66.66) |
| Rifampicin (RIF).5 μg | 0/8 (0) | 0/7 (0) | 5/9 (55.55) | |
| Fosfomycin (FOS).200 μg | 0/8 (0) | 0/7 (0) | 6/9 (66.66) | |
| Chloramphenicol (CHL).30 μg | 0/8 (0) | 0/7 (0) | 4/9 (44.44) | |
| Nitrofuran (FTN).300 μg | 0/8 (0) | 0/7 (0) | 3/9 (33.33) | |
3.1.3. Prevalence of Antibiotic Resistance Genes and Phenotypes in Isolated Isolates
A comprehensive analysis of resistance genes in E. coli, Salmonella, and S. aureus isolated from ready-to-eat salads was conducted. Thus, the significant resistance of the E. coli species to ticarcillin + clavulanic acid (TCC) and cephalosporin (cefoxitin, cefixime, and cefuroxime) results in the ESBL phenotype (50% of isolates). However, 10.52% of beta-lactam resistant species tested positive for the blaTEM resistance gene. Fluoroquinolone resistance (FQ-R) was identified through the presence of qnrA (2.26%), qnrB (5.26%), and qnrS (5.26%). The presence of resistance genes in E. coli isolates reveals high resistance to fluoroquinolones (25%). No resistance genes were detected in Salmonella isolates. However, most S. aureus isolates (55.55%, or 5 out of 9) harbored the mecA gene, indicative of the Meti-R phenotype, reflecting meticillin resistance. In addition, resistance to aminoglycosides was observed in 55.55% of S. aureus isolates for both gentamicin and kanamycin, contributing to a KTG phenotype affecting 44.44% of these isolates. The MLSB phenotype, linked to resistance to macrolide-lincosamide-streptogramin B antibiotics such as clindamycin (55.55%) and erythromycin (66.66%), was also prevalent in 44.44% of the S. aureus isolates.
These findings on the prevalence of antibiotic resistance genes and corresponding phenotypes are depicted in Figure 3 for genes and Figure 4 for phenotypes.
Figure 3.
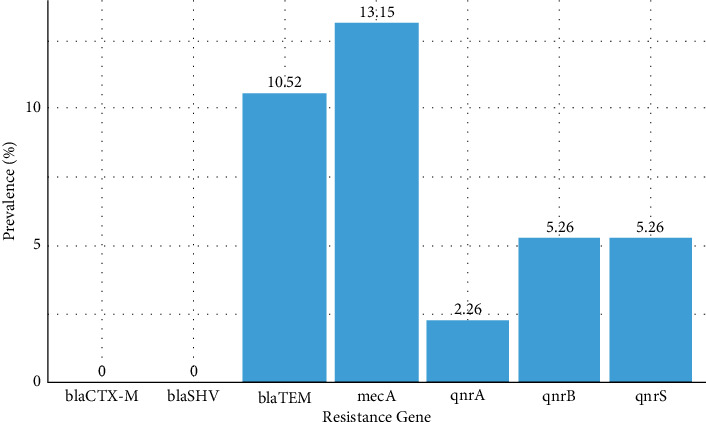
Prevalence of antibiotic resistance genes.
Figure 4.
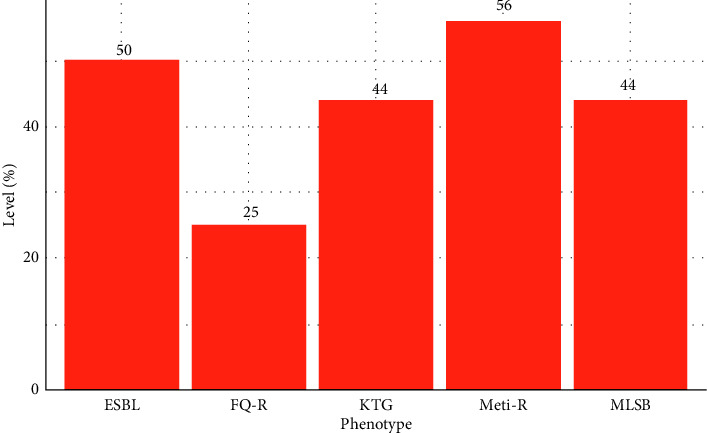
Detected antibiotic resistance phenotypes.
3.2. Discussion
The analysis of virulence and antibiotic resistance in bacterial isolates isolated from ready-to-eat salads sold in supermarkets in Abidjan revealed notable findings. In E. coli isolates, the presence of stx2 genes was detected in four samples, demonstrating a prevalence of 50%. In addition, the ST gene was found in five samples, corresponding to a 62.50% prevalence. Notably, neither stx1 nor LT genes were identified in any of the samples.
Among the four virulence genes (stx1, stx2, ST, and LT) studied, only stx2 was linked to Shiga toxin (STEC) production. A study in the United States found similar trends where the stx1 gene was absent in tested isolates, whereas 93.1% carried the stx2 gene [23]. Moreover, an investigation in Iran revealed a high prevalence of stx2 genes in cattle feces, supporting the potential for fecal contamination in agricultural settings [13].
Regarding the toxins associated with thermolabile (LT) and thermostable (ST) toxins in E. coli, only the ST gene was found, detected in five samples representing a prevalence of 62.50%. Comparable research conducted in Casablanca identified the ST gene in certain food products, suggesting possible fecal contamination through irrigation water used in market gardening [24].
Analysis of S. aureus in this study revealed the presence of the sea, sec, and sed genes with prevalences of 55.55%, 11.11%, and 44.44%, respectively. The results of another study showed the absence of enterotoxin genes in ready-to-eat salads, suggesting significantly better hygiene conditions in the production of 4th-range salads [25] than this. In addition, the literature suggests that the presence of certain preservatives such as lactic acid, produced by bacteria, can inhibit SE formation in foods, potentially via an extracellular protease, leading to a decrease in enterotoxin levels under specific conditions [29]. In this context, the detection of enterotoxin A, C, and D genes in S. aureus in the salads analyzed could indicate deficiencies in personnel hygiene, potentially involving healthy carriers of these genes.
The phenotypic characterization performed on food pathogens isolated from ready-to-eat salads sold in hypermarkets in Abidjan highlights significant concerns, particularly the presence of multiresistant bacteria. These pathogens, which survive without heat treatment in 4th-range foods, pose severe health risks, potentially leading to fatal outcomes for consumers. During this study, antibiotic susceptibility testing revealed a pronounced resistance in E. coli to beta-lactam antibiotics, with 100% resistance to cefuroxime (CXM) and 87.5% resistance to both ampicillin (AMP) and cefoxitin (FOX). Similarly, Salmonella spp. showed resistance rates of 42.85% to cefoxitin (FOX) and 57.14% to ampicillin (AMP). These findings align with those from other regions; for example, a study in northern California found 76% of E. coli isolates resistant to ampicillin and lower resistance to cefoxitin (23%) and cefuroxime (20%) [26]. Contrastingly, a study in southwestern Nigeria reported a 65.7% resistance rate of E. coli to cefuroxime [27], while a different study in Abidjan found no resistance in E. coli to cefuroxime [28], suggesting variability in bacterial resistance based on the source of salad ingredients. Moreover, resistance to fluoroquinolones was also noted, closely mirroring results from other studies on ready-to-eat foods, with 61.29% resistance to ciprofloxacin observed [29]. Similar research found resistance rates to ciprofloxacin at 8.3% and to carbapenems at 5% [30]. However, this study found a low resistance in E. coli to imipenem at 2.9%, akin to findings by another study [31].
For Salmonella spp., the observed resistance profile in this study aligns with the significant resistance to ampicillin reported elsewhere, such as 88% in S. enteritidis [32] and 89.9% in Nigerian samples [33]. Additional work on retail meat revealed a notable resistance in Salmonella serotypes to ampicillin at 29% and to cefoxitin at 30.43%, further supporting the patterns of resistance found in this study [34]. Antibiotic susceptibility testing of S. aureus isolates revealed significant resistance levels to various antibiotic families. High resistance rates were observed with the beta-lactam antibiotics, notably cephalosporins such as cefoxitin (44.44%) and penicillins, with a 100% resistance to benzylpenicillin and 55.55% to ampicillin. For aminoglycosides, resistance levels were 55.55% for both kanamycin (KMN) and gentamicin (GMN). Resistance to macrolides was also substantial, with erythromycin (ERY) at 66.66% and clindamycin (CMN) at 55.55%. Resistance to fluoroquinolones varied, peaking at 55.55% for norfloxacin (NXN). Resistance to beta-lactam antibiotics in S. aureus can be attributed to the production of beta-lactamase, which hydrolyzes the beta-lactam ring of penicillins or intrinsic resistance mechanisms such as modification of penicillin-binding proteins (PBPs) or acquisition of new PBPs. This phenomenon of meticillin resistance, which leads to broad-spectrum resistance to all beta-lactam antibiotics, is described in references [35, 36]. Macrolide resistance generally involves the action of a methylase enzyme that modifies the 23S subunit of ribosomal RNA. Comparatively, these resistance patterns correspond closely to results obtained in Côte d'Ivoire, where one study documented a 50% resistance rate to erythromycin [37].
Fluoroquinolone resistance often involves three primary mechanisms: target modification through mutations in the grlA or grlB genes of topoisomerase IV [38]. Other significant resistance observed included fosfomycin (66.66%), rifampicin (50%), and chloramphenicol (37%). A study conducted in Greece by the authors in [39] explored the prevalence, distribution, and antibiotic susceptibility of S. aureus in ready-to-eat salads and environmental and personnel samples from a salad production facility. They found S. aureus in 27% of samples, with most isolates showing resistance to 2–5 antibiotics, including fosfomycin. This aligns with the findings in the current study, which showed higher resistance rates to fosfomycin (82.6%), rifampicin (55.55%), and chloramphenicol (44.44%), suggesting a more pronounced resistance profile compared to the Greek study.
During this study, the assessment of antibiotic resistance profiles necessitated identifying specific resistance genes in E. coli, Salmonella, and S. aureus isolates from ready-to-eat salads. E. coli resistance to beta-lactam antibiotics in this study was high, with a 50% ESBL (extended-spectrum beta-lactamase) phenotype. However, the blaTEM gene was only identified in 10.52% of isolates. In addition, fluoroquinolone resistance was evident via the qnrA gene (2.26%), In addition, resistance to fluoroquinolones was evident through the qnrA (2.26%), qnrB (5.26%), and qnrS (5.26%) genes, which collectively underpinned fluoroquinolone resistance in 25% of cases. These findings align with those reported in [40]. In S. aureus, the mecA gene was detected with a prevalence of 13.15%, accounting for 55.55% of the isolates and indicating resistance to meticillin, characteristic of the Meti-R phenotype. The study also found significant resistance to aminoglycosides, specifically gentamycin and kanamycin, each at 55.55%, correlating with the KTG phenotype in 44.44% of the samples. In addition, high rates of resistance to clindamycin (55.55%) and erythromycin (66.66%) were observed, contributing to the MLSB phenotype in 44.44% of the isolates. A study cited as [39] noted that in MRSA isolates, resistance rates exceed 90%, mirroring the findings of this study where all identified multiresistant isolates exhibited four resistance phenotypes: Meti-R, KTG, MLSB, and resistance to fluoroquinolones. Such elevated resistance levels could significantly contribute to the rise in nosocomial infections and complicate adherence to antibiotic therapy protocols by both patients and healthcare providers. Ultimately, this situation underscores the critical need for standardized antibiogram practices to guide effective antibiotic treatments, especially in cases where standard protocols may not be adequately followed.
4. Conclusion
The study of virulence and antibiotic resistance in bacterial isolates isolated from ready-to-eat salads has underscored significant health risks to consumers. Particularly, the pathogenic potential of E. coli was demonstrated through the identification of stx2 and ST genes with prevalences of 50% and 62.50%, respectively. Similarly, the detection of sea, sec, and sed genes in S. aureus isolates, having prevalences of 55.55%, 11.11%, and 44.44%, respectively, highlights the virulent nature of these pathogens. The antibiotic resistance profiles further reveal that isolates of S. aureus and E. coli exhibit multiple resistances to antibiotics, complicating treatment options. Notably, E. coli isolates showed substantial multiresistance, evidenced by the presence of blaTEM genes (10.52% prevalence), which contribute to an ESBL phenotype in 50% of the isolates. In addition, the detection of qnrA (2.26%), qnrB (5.26%), and qnrS (5.26%) genes indicates a 25% prevalence of resistance to quinolones. S. aureus isolates displayed resistance to meticillin, as marked by the mecA gene (13.15% prevalence) and a Meti-R rate of 55.55%. High resistance rates were also observed in aminosides and macrolides, characterized by KTG and MLSB phenotypes, both at 44.44%. These findings suggest that the consumption of contaminated ready-to-eat salads could lead to therapeutic challenges in treating human infections due to the high prevalence of antibiotic-resistant bacteria. As these salads become a more common part of the diet, it is imperative to enforce stringent safety measures and monitoring to mitigate the risk of consumer poisoning and limit the spread of multiresistant bacteria. This approach is crucial in preserving the efficacy of antibiotics and ensuring the health and safety of consumers.
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
This work involved the use of bacterial isolates from plant product samples but did not involve participants or human biological material. Consequently, this study is not subject to the Ethical Conduct for Research Involving Humans (TCPS 2).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
Angaman D.M., Gbonon V.C., and N'zi N.P. were responsible for the conception. Angaman D.M., Gbonon V.C., N'zi N.P., and Guédé K.B. were responsible for the design. N'zi N.P., Gbonon V.C., Guédé K.B., Afran S.A., and Angaman D.M. were responsible for the analysis and interpretation of data. N'zi N.P. was responsible for the drafting of the article. Angaman D.M., N'zi N.P., Gbonon V.C, Guédé K.B., and Afran S.A. were responsible for the critical revision for important intellectual content. Angaman D.M., N'zi N.P., Gbonon V.C., Guédé K.B., and Afran S.A. were responsible for the final approval of the version to be published.
References
- 1.Djioua T. Avignon, France: Université d’Avignon et des Pays de Vaucluse; 2010. Amélioration de la conservation des mangues 4ème gamme par application de traitements thermiques et utilisation d’une conservation sous atmosphère modifiée. Thèse de Doctorat en Sciences Agronomiques. [Google Scholar]
- 2.Pollard C., Miller M., Woodman R. J., Meng R., Binns C. Changes in knowledge, beliefs, and behaviors related to fruit and vegetable consumption among western Australian adults from 1995 to 2004. American Journal of Public Health . 2009;99(2):355–336. doi: 10.2105/AJPH.2007.131367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adjrah Y., Karou D. S., Djéri B., et al. Hygienic quality of commonly consumed vegetables, and perception about disinfecting agents in Lomé. International Food Research Journal . 2011;18(4):1499–1503. [Google Scholar]
- 4.FAO. Promotion of Fruit and Vegetables for Health. Human Nutrition Meetings Oceania Nutrition Education Fruit Vegetables Public Health . Rome, Italy: FAO; 2015. [Google Scholar]
- 5.Zhang Q., Qing S., Tang G. P., Zou Z. T., Yao G. H., Zeng G. A foodborne outbreak of Aeromonas hydrophila in a college, Xingyi City, Guizhou, China. Western Pacific surveillance and response journal: Western Pacific Surveillance and Response journal . 2012;3:39–43. doi: 10.5365/WPSAR.2012.3.4.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald E., Einöder-Moreno M., Borgen K., et al. National out break of Yersinia enterocolitica infections in military and civilian populations associated with consumption of mixed salad, Norway, 2014. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin . 2016;21(34):30312–30321. doi: 10.2807/1560-7917.ES.2016.21.34.30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espenhain L., Riess M., Müller L., et al. Cross-border outbreak of Yersinia enterocolitica O3 associated with imported fresh spinach, Sweden and Denmark. Euro Surveillance . 2019;24(24):1–5. doi: 10.2807/1560-7917.ES.2019.24.24.1900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mritunjay S. K., Kumar V. A study on prevalence of microbial contamination on the surface of raw salad vegetables. Biotechonology . 2017;7(1):1–13. doi: 10.1007/s13205-016-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paudyal N., Anihouvi V., Hounhouigan J., et al. Prevalence of foodborne pathogens in food from selected African countries - a meta-analysis. International Journal of Food Microbiology . 2017;249:35–43. doi: 10.1016/j.ijfoodmicro.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Dony A. Bordeaux, France: Institut des Sciences et Techniques des Aliments de Bordeau, Université Bordeaux; 2009. Essais de mise au point de produits de 4eme gamme à base de banane et de noix de coco, Mémoire d’ingénieur. [Google Scholar]
- 11.N’Zi N. P., Angaman D. M., Gbonon V. C., Tiekoura K. B. Etude de la qualité microbiologique des salades de 4ème gamme vendues dans des supermarchés de la ville d’Abidjan (Côte d’Ivoire) durant la période de conservation domestique après ouverture des emballages. International Journal of Innovation and Applied Studies . 2022;36(2):577–587. [Google Scholar]
- 12.Gérard A. Liège, Belgium: Université de Liège (Gembloux, Belgique); 2016. Conception et évaluation de méthodes de détection d’insectes dans les matrices alimentaires. Master en Bioingénieur, Chimie et Bio-Industries. [Google Scholar]
- 13.Tahamtan Y., Hayati M., Namavari M. Prevalence and distribution of the stx1, stx2 genes in Shiga toxin producing E. coli (STEC) isolates from cattle. Iranian Journal of Microbiology . 2010;2(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Hennekinne J. A., Gautier M. V. F. Detection and quantification of staphylococcal enterotoxin A in foods with specific and sensitive polyclonal antibodies. Food Control . 2013;32:55–261. [Google Scholar]
- 15.Mehrotra M., Wang G., Johnson W. M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exf ‘liative toxins, toxic shock syndrome toxin 1, and Methicillin Resistance. Journal of Clinical Microbiology . 2000;38(3):1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CASFM/EUCAST, Recommandations, version1.0. Rapport Technique, No. 610, 128, 2021.
- 17.Yaron S., Kolling G. L., Simon L., Matthews K. R. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Applied and Environmental Microbiology . 2000;66(10):4414–4420. doi: 10.1128/aem.66.10.4414-4420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazquez-Marrufo G., Rosales-Castillo J. A., Robinson-Fuentes V. A., Tafolla-Munoz I., Carreras-Villase N., Vazquez-Garcidue M. S. Multi-typing of enterobacteria harboring LT and ST enterotoxin genes isolated from Mexican children. Japanese Journal of Infectious Diseases . 2000;66(10):4414–4420. doi: 10.7883/yoken.JJID.2015.454. [DOI] [PubMed] [Google Scholar]
- 19.Essack S. Y. Laboratory detection of extended-spectrum β-lactamases (ESBLs)—the need for a reliable, reproducible method. Diagnostic Microbiology and Infectious Disease . 2000;37(4):293–295. doi: 10.1016/s0732-8893(00)00152-8. [DOI] [PubMed] [Google Scholar]
- 20.Birkett C. I., Ludlam H. A., Woodford N., et al. Real-time TaqMan PCR for rapid detection and typing of genes encoding CTX-M extendedspectrum blactamases. Journal of Medical Microbiology . 2007;56(1):52–55. doi: 10.1099/jmm.0.46909-0. [DOI] [PubMed] [Google Scholar]
- 21.Meradi L., Djahoudi A., Abdi A., Bouchakour M., Perrier Gros Claude J.-D., Timinoun M. Résistance aux quinolones de types qnr, aac (6′)-Ib-cr chez les entérobactéries isolées à Annaba en AlgérieQnr and aac (6′)-Ib-cr types quinolone resistance among Enterobacteriaceae isolated in Annaba, Algeria. Pathologie Biologie . 2011;59(4):73–78. doi: 10.1016/j.patbio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Yoko K., Teruyo I., Xiao Xue M., et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec TypeAssignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrobial Agents and Chemotherapy . 2007;51(1):264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karmali M. A., Mascarenhas M., Shen S., et al. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. Journal of Clinical Microbiology . 2003;41(11):4930–4940. doi: 10.1128/JCM.41.11.4930-4940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badri S., Filliol I., Carle I., Hassar M., Fassouane A., Cohen N. Prévalence of virulence genes in Escherichia coli isolated from food in Casablanca (Morocco) Food Control . 2009;20:560–564. [Google Scholar]
- 25.Chau M., Aung K., Hapuarachchi C., et al. Microbial survey of ready-to-eat salad ingredients sold at retail reveals the occurrence and the persistence of Listeria monocytogenes sequence types 2 and 87 in pre-packed smoked salmon. BMC Microbiology . 2017;17(1):33–46. doi: 10.1186/s12866-017-0956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao N., Borges C. A., Rubin J., et al. Prevalence of β-lactam drug-resistance genes in Escherichia coli contaminating ready-to-eat lettuce. Foodborne Pathogens and Disease . 2020;17(12):739–742. doi: 10.1089/fpd.2020.2792. [DOI] [PubMed] [Google Scholar]
- 27.Oje O., David O., Adeosun O., Adebayo A., Famurewa O. Multiple antibiotic-resistant Escherichia coli in ready-to-eat foods from food outlets in ekiti state university and its environ. British Microbiology Research Journal . 2016;13(1):1–11. [Google Scholar]
- 28.Toe E., Dadié A. T., Dako E., Loukou G., Djé K. M., Blé Y. C. Prevalence and potential virulence of Escherichia coli in ready-to-eat raw mixed vegetable salads in collective catering in Abidjan, Côte d’Ivoire. British Food Journal . 2018;20(1):32–44. [Google Scholar]
- 29.Abass A., Adzitey F., Huda N. Escherichia coli of Ready-to-Eat (RTE) Meats origin showed resistance to antibiotics used by farmers. Antibiotics . 2020;9(12):861–869. doi: 10.3390/antibiotics9120869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos J., Mourão J., Pestana N., Peixe L., Novais C., Antunes P. Microbiological quality of ready-to-eat salads: an underestimated vehicle of bacteria and clinically relevant antibiotic resistance genes. International Journal of Food Microbiology . 2013;166(3):464–470. doi: 10.1016/j.ijfoodmicro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Goepfert J. M. Microbial Ecology of Foods . Vol. 2. New York, NY, USA: Academic Press; 1980. Vegetables, fruits, nuts and their products; pp. 606–642. [Google Scholar]
- 32.Al-Zenki S., Al-Nasser A., Al-Safar A., et al. Prevalence and antibiotic resistance of Salmonella isolated from a poultry farm and processing plant environment in the State of Kuwait. Foodborne Pathogens and Disease . 2007;4(3):367–373. doi: 10.1089/fpd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 33.Kemajou T. S., Awemu G. A., Digban K. A., Oshoman C. E., Ekundayo O. I., Ajugwo A. O. Microbiological studies of vegetable leaves sold in elele market, rivers-state, Niger. Journal of Transmitted Diseases and Immunity . 2017;1(1):1–5. [Google Scholar]
- 34.Chen S., Zhao S., White D. G., et al. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Applied and Environmental Microbiology . 2004;70(1):1–7. doi: 10.1128/AEM.70.1.1-7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quincampoix J. C., Mainardi J. L. Mécanismes de résistance des cocci à Gram positif. Reanimation . 2001;10(3):267–275. [Google Scholar]
- 36.Ryffel C., Kayser F. H., Berger-Bächi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrobial Agents and Chemotherapy . 1992;36(1):25–31. doi: 10.1128/aac.36.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ago P. D., Boko C. K., Adoligbe C. M., Farougou S., Dougnon V. Profil de résistance et gènes de virulence des souches de Salmonella spp. isolées dans les élevages de poules pondeuses au Sud du Bénin . Departement de Production et Santé Animales, Université d’Abomey-Calavi, Cotonou, Benin; 2021. p. p. 63. [Google Scholar]
- 38.Bunnueang N., Kongpheng S., Singkhamanan K., et al. Methicillin-resistant staphylococcus aureus from ready-to-eat foods in a hospital canteen, southern Thailand: virulence characterization and genetic relationship. The Southeast Asian Journal of Tropical m Médicine and Public Health . 2015;46(1):86–96. [PubMed] [Google Scholar]
- 39.Mainardi J. L., Goldstein F. W., Gutmann L. Mécanismes de résistance bactérienne aux antibiotiques, Encyclopédie médico-chirurgicale. Maladies Infectieuses . 1996;1:1–8. [Google Scholar]
- 40.Vounba P. Prevalence of antimicrobial resistance and potential pathogenicity, and possible spread of third generation cephalosporin resistance, in Escherichia coli isolated from healthy chicken farms in the region of Dakar, Senegal. PLoS One . 2019;14(3):1–23. doi: 10.1371/journal.pone.0214304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad H. P., Khalil M. K. Prevalence of blaTEM, blaSHV, and blaCTX-M genes among ESBL-producing Klebsiella pneumoniae and Escherichia coli isolated from thalassemia patients in erbil, Iraq. Mediterranean Journal of Hematology and Infectious Diseases . 2019;11(1):1–7. doi: 10.4084/MJHID.2019.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


