Abstract
Omega-3 (n-3) polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA), play important roles in human nutrition and brain health by promoting neuronal functions, maintaining inflammatory homeostasis, and providing structural integrity. As Alzheimer’s disease (AD) pathology progresses, DHA metabolism in the brain becomes dysregulated, the timing and extent of which may be influenced by the APOE4 allele. Here, we discuss how maintaining adequate DHA intake early in life may slow the progression to AD dementia in cognitively normal individuals with APOE4, how recent advances in DHA brain imaging could offer insights leading to more personalized preventive strategies, and how alternative strategies targeting PUFA metabolism pathways may be more effective in mitigating disease progression in patients with existing AD dementia.
Keywords: Alzheimer’s, APOE4, DHA, fatty acids, imaging
DOCOSAHEXANOIC ACID, APOE4 AND ALZHEIMER’S DISEASE
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorders and is characterized by a gradual decline in cognitive function and the emergence of neuropathological hallmarks including abnormal amyloid plaques (see Glossary), neurofibrillary tangle (NFT) deposition, neuroinflammation, and cerebral atrophy. The absence of universally effective treatments and preventative strategies to reverse or delay AD underscores the complexity of its pathophysiology. Understanding this requires a multifaceted and diverse approach owing to the heterogeneity of the disease. AD susceptibility and progression are associated with specific genetic markers and various environmental factors related to lifestyle and nutrition. For example, the apolipoprotein E ε4 (APOE4) allele is considered to be the strongest genetic risk factor for late-onset AD. Human APOE is expressed as one of three isoforms, ε2, ε3, and ε4, and individuals carrying one or more ε4 alleles exhibit a significantly higher risk of developing AD.
Dietary consumption of omega-3 (n-3) polyunsaturated fatty acids (n-3 PUFAs), such as docosahexaenoic acid (DHA), has been extensively investigated in AD treatment and prevention studies due to DHA’s involvement in amyloid production and clearance [1–3], maintenance of synaptic plasticity [4, 5], and other neuroprotective functions [6, 7]. However, randomized clinical trials examining the efficacy of n-3 PUFA supplementation in AD have yielded mostly inconclusive results, possibly due to heterogeneity in patient populations and limitations in trial design [8]. Alternatively, the null hypothesis is correct, and DHA supplementation is not effective in preventing cognitive decline. Emerging evidence suggests that DHA consumed with other antioxidant nutrients in a more complex dietary pattern, such as the Mediterranean or MIND diet, is likely to be more effective [9]. The complex interactions between baseline n-3 PUFA intake, genetics, and environmental factors have gained increasing attention as potentially modifiable risk factors for AD [10, 11]. If successful, this approach could pave the way for the development and implementation of personalized AD prevention strategies by modifying environmental contributions based on an individual’s genetic predisposition to AD. One noteworthy interaction is the relationship between APOE4 and DHA, in part because APOE4 alters DHA transport and metabolism in the brain [12].
This review aims to delineate the effect of APOE4 on DHA brain metabolism and highlights the value of newly developed in vivo DHA imaging techniques. We discuss how APOE4 influences the metabolism of brain DHA across three distinct stages: preclinical AD with normal cognition (Stage I), prodromal AD or mild cognitive impairment (Stage II), and AD dementia (Stage III), as illustrated in Figure 1. We propose that increasing n-3 PUFA intake in APOE4 carriers during the initial two stages may decelerate the progression of AD, while inhibiting the enzymes responsible for catabolizing brain PUFAs may alleviate neuroinflammation and improve outcomes in patients with AD dementia.
Figure 1.
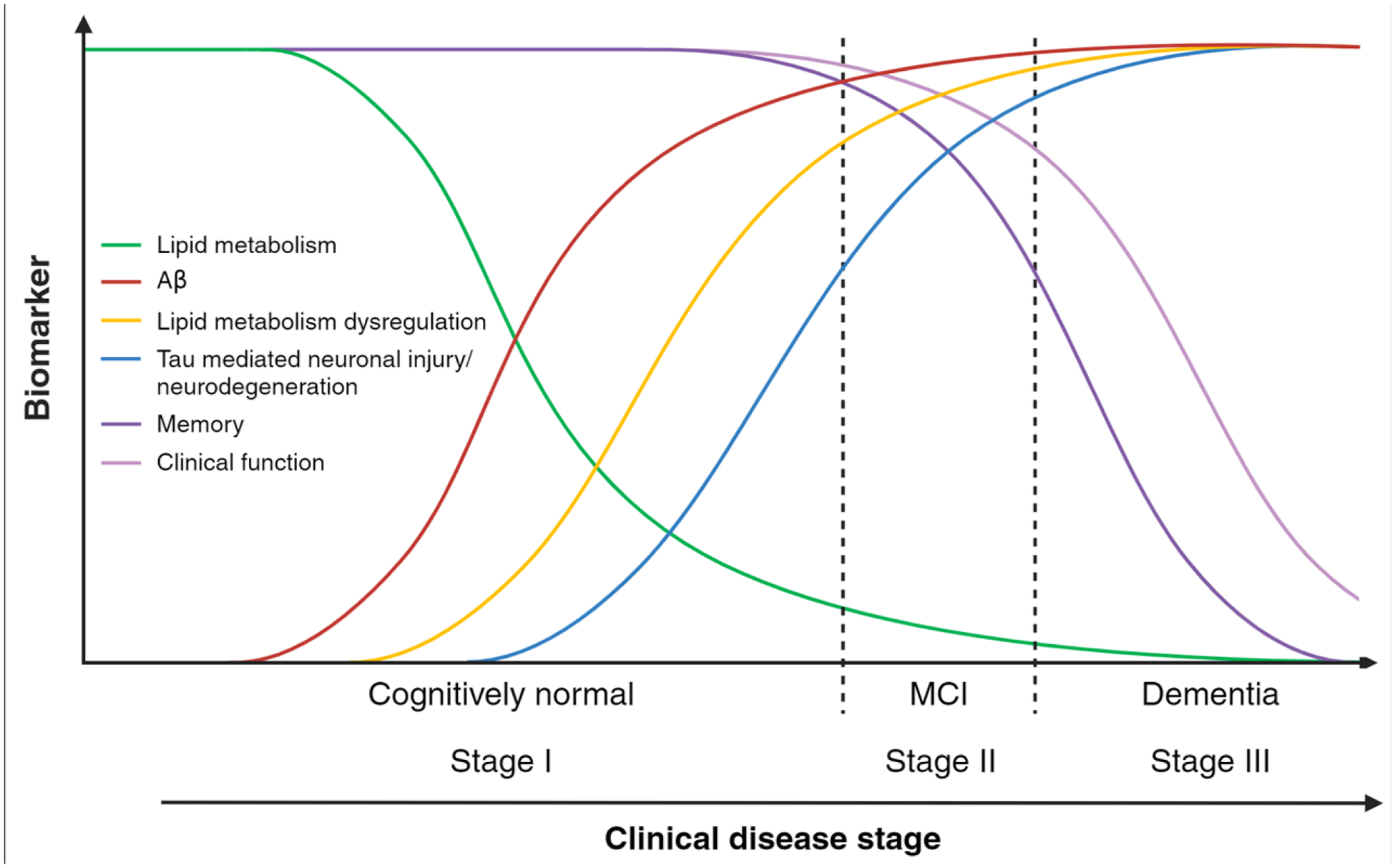
APOE4 and brain lipid metabolism.
In APOE4 carriers, lipid metabolism is differentially altered throughout three AD progression stages. We hypothesized that changes in brain lipids (such as lower DHA and greater cholesterol within lipid rafts of neuronal membranes) appear early and preceding amyloid deposition (Stage I), presenting an opportunity for increasing DHA intake to slow disease progression. As the disease advances to stages II and III, the brain shifts to PUFA oxidation for energy production, which accelerates tau-mediated injury and neurodegeneration. Most n-3 PUFA dietary and supplemental interventions were conducted in disease stages II and III and were not clinically effective. Figure created using BioRender.
Brain DHA metabolism and APOE4
Routes of DHA Biosynthesis and Metabolism in the Brain
DHA is primarily obtained directly from dietary sources such as fatty fish [13, 14]. Although the body can indirectly synthesize DHA from its shorter chain precursors α-linolenic acid (ALA) and eicosapentaenoic acid (EPA) via elongation and desaturation, these metabolic processes are relatively inefficient in the brain [15, 16]. In animal models, this has been shown to hold true even when dietary n-3 PUFA intake is limited [17]. For example, in animal models placed on a low ALA diet, plasma DHA levels decline before brain levels are affected [18], suggesting that brain DHA is primarily replenished by the uptake of preformed DHA, as opposed to being metabolically converted from its precursors within the brain.
Peroxisomes play a key role in endogenous DHA synthesis by facilitating the conversion of very long chain fatty acids (VLCFA) to DHA via β-oxidation and are also highly involved in the synthesis of plasmalogens [19], which can contain DHA. Peroxisomes can also metabolize DHA via β-oxidation to generate acetyl-CoA, which can induce reactive oxygen species (ROS) production. Although APOE4 has been associated with dysregulated lipid metabolism, its effects on peroxisomal DHA synthesis and metabolism remain to be elucidated.
In the brain, a large proportion of free DHA is esterified and stored in membrane phospholipids. Acetyl-CoA synthetase 6 (Acsl6) activity is known to be a key contributor to this process by converting free DHA to DHA-CoA. Acsl6−/− mice exhibit depleted DHA enrichment in brain phospholipids, and Acsl6 has been shown to be particularly important for brain DHA retention during aging [20–22]. Free DHA can be liberated from cell membranes when phospholipids are hydrolyzed at the sn-2 position by phospholipase A2 (PLA2) enzymes, of which there are three major types: calcium-dependent cytosolic enzymes (cPLA2), calcium-independent enzymes (iPLA2), and secreted enzymes (sPLA2). Of these, iPLA2 is known to play a critical role in brain DHA metabolism and signaling and was believed to be selective for DHA [18, 23, 24]. However, recent in vitro studies have shown that iPLA2 is far more selective for EPA, whereas sPLA2 is selective for DHA over other PUFAs [25, 26]. The metabolism of n-3 PUFAs is shown in Figure 2. In mouse brains, DHA has been shown to predominantly accumulate in pyramidal neuron-rich gray matter regions, colocalizing with iPLA2 expression [27]. Conversely, cPLA2, which exhibits greater selectivity toward n-6 arachidonic acid (AA), is highly expressed in myelin-rich white matter regions of the brain and colocalizes with high AA levels to enhance eicosanoids but also affects DHA. ApoE4 activates cPLA2 in mice via the MAPK p38 signaling pathway [28] and reflected by pronounced eicosanoid lipids profiled in postmortem human brain tissues [29].
Figure 2.

Cellular PUFA metabolism and APOE4 expression
PUFAs are hydrolyzed and released from cellular membranes via PLA2 enzymes and are subsequently metabolized by COX, LOX, and sEH enzymes to generate pro-inflammatory and/or pro-resolving lipid mediators. cPLA2, which can be activated by Ca2+ influx and Aβ oligomers among other inflammatory stimuli, selectively hydrolyzes AA-containing phospholipids and promotes the production of inflammatory eicosanoids. PUFAs can also be synthesized from peroxisomes via β-oxidation and/or metabolized by FAO in the mitochondria to generate acetyl-CoA for ATP production. APOE4 induces the cPLA2 pathway to promote eicosanoid and PUFA oxidation. Figure created using BioRender.
Once liberated by PLA2 enzymes, DHA can be re-esterified and re-incorporated into membrane phospholipids or metabolized by various mechanisms depending on environmental conditions and needs. For example, DHA can be metabolized via fatty acid oxidation (FAO) to satisfy energy demands, by lipid peroxidation under conditions of oxidative stress, or enzymatically converted to docosanoids to provide immune support and maintain inflammatory homeostasis. The production of DHA-derived docosanoids generally relies on three enzymes: lipoxygenases (LOX), cytochrome P450s (CYP), and soluble epoxide hydrolase (sEH), all of which have been identified as potential drug targets for neurodegenerative diseases [29–31]. To quantify the rate of DHA metabolism, compound-specific isotope analysis (CSIA) can be used to measure the rate of DHA turnover, which is known to differ between various matrices within the body. CSIA studies have revealed a DHA half-life of 46 days in mouse brains compared to 6 days in the plasma and 7 days in the liver [15]. In humans, the half-life of DHA has been estimated using [11C]DHA positron emission tomography (PET) imaging to be much longer, approximately 2.5 years [32]. Furthermore, the APOE genotype affects the rate of DHA turnover or incorporation coefficient. This has been demonstrated in CSIA studies, where higher rates of DHA turnover and altered DHA brain incorporation coefficients have been measured in both human APOE4 carriers and mice expressing human APOE4 [33–37].
Physiological Roles of DHA in the Brain
DHA, a predominant n-3 PUFA in the brain by weight, comprises a substantial portion of the total fatty acids within the grey matter. The esterification of free DHA and its incorporation into phospholipids enhances the biomechanical properties of cell membranes at the structural level by promoting membrane fluidity and protecting neurons against oxidative damage [38, 39]. In addition to its role in maintaining neuronal membranes, DHA enhances synaptic plasticity, promotes the degradation of beta amyloid (Aβ), and facilitates the resolution of inflammation [1, 2, 40–44]. Many of the effects of DHA on inflammation are mediated by its bioactive lipid metabolites or docosanoids, including D-series resolvins, maresins, and neuroprotectins, collectively referred to as specialized pro-resolving mediators (SPMs) [45–51]. These SPMs are capable of mitigating glial cell activation and inflammatory signaling [45, 52]. Notably, neuroprotection D1, among these SPMs, can even stimulate neuronal growth and survival [29, 53–58].
Effect of Increased n-6 PUFA Consumption on Brain n-3 PUFA Metabolism
In humans, higher intake of n-6 PUFAs such as linoleic acid (LA) has been reported to compete with the formation of n-3 PUFAs from ALA [59, 60] and increase the n-6/n-3 PUFA ratio in the brain. However, it is unclear whether this promotes neuroinflammation. More likely, an increased n-6/n-3 PUFA ratio may increase the risk of neuroinflammation in certain individuals with other risk factors, as discussed below [29, 61]. A greater n-6/n-3 PUFA ratio attenuates the effects of DHA derived from ALA, but more research is needed to estimate the ideal balance between n-6 and n-3 PUFA intake for optimal brain function in the general population [62–64]. The omega-3 index has been proposed as a biomarker to assess cardiovascular health and more recently, brain function [65]. It measures the balance between n-6 and n-3 PUFAs by quantifying the percentage of DHA and EPA in erythrocytes in relation to total fatty acids [66]. While a lower omega-3 index associated with accelerated brain aging and cognitive impairment in some elderly populations [67, 68], this association is not consistent. In a study of 832 participants from the Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI-3), no associations between the omega-3 index and cognitive decline were observed [69]. Thus, the omega-3 index may serve as a biomarker for exposure to n-3 PUFA-enriched dietary patterns. However, it may be a more useful tool for predicting AD risk or the response to n-3 supplementation in specific high-risk populations that are more vulnerable to lower n-3 PUFA levels or have dysregulated n-3 PUFA metabolism, such as APOE4 carriers [70].
Effects of Aging and APOE4 on DHA Brain Uptake and Metabolism
Brain uptake of free DHA from systemic circulation can occur through passive diffusion between tight junctions or active transport across the blood-brain barrier (BBB). Active transport is facilitated by fatty acid-binding protein 5 (FABP5) [71] or the major facilitator superfamily domain-containing 2A (MFSD2A) transporter [72]. Esterified DHA, such as DHA-lysophosphatidylcholine (DHA-LPC), are preferentially transported into the brain by the MFSD2A transporter. In a study using dynamic PET imaging to monitor [11C]DHA brain metabolism in young, cognitively healthy individuals, we observed that APOE4 carriers displayed a higher brain DHA affinity, as indicated by incorporation coefficients (K*), than non-APOE4 individuals. This increase suggests compensation for greater brain DHA utilization in younger APOE4 carriers, indicating a potential vulnerability to low-DHA diets [33]. To improve the feasibility of long-term in vivo studies of DHA metabolism and brain uptake, we developed 22-[18F]fluorodocosahexaenoic acid (22-[18F]FDHA) for PET imaging, which offers a longer half-life than radiocarbon-labeled DHA analogs [73]. Proposed mechanisms for the increased DHA brain affinity in younger APOE4 carriers include increased neuronal activity [74] and/or reduced lipidation of ApoE4 lipoproteins compared to ApoE2 or ApoE3 [75]. Given that ApoE lipoprotein particles carry n-3 lipids to neurons [76] and ApoE4 is less efficient in this transport [75], greater extraction of plasma DHA into the APOE4 brain may be required to maintain lipid membrane homeostasis.
In contrast, there is evidence of a decrease in brain DHA uptake with age. A study on [14C]DHA brain uptake in aged C57BL/6 mice revealed lower DHA brain uptake, attributed to age-related downregulation of DHA transporters at the BBB, such as MFSD2A [77]. Similarly, elderly APOE4 carriers display less efficient n-3 PUFA brain uptake than non-carriers [78]. The decline in DHA brain uptake with age and the presence of APOE4 may also be associated with increased DHA catabolism. In a study of [13C]DHA supplementation in elderly humans without cognitive impairment, it was observed that the whole-body half-life of [13C]DHA was 77% lower in APOE4 carriers compared to non-carriers [34]. This reduction in half-life could be indicative of increased DHA oxidation in the liver and/or brain, potentially explaining the increased DHA oxidation observed in elderly APOE4 carriers.
Individuals with APOE4 are also more susceptible to BBB dysfunction even before the onset of cognitive decline. Much of this is attributed to neuroinflammation, which disrupts the cerebral uptake and metabolism of nutrients [79, 80]. In addition, the APOE4 allele has been linked to a higher Aβ burden [81, 82], reduced glucose brain uptake [83–87], and decreased microvascular blood flow [88] in the brains of cognitively normal adults. These effects have been linked to inflammatory signaling, decreased cerebral vascularization, degradation of tight junctions [89], and overall weakening of the BBB [79]. Other factors, such as exercise, obesity, alcohol intake, smoking, and various genetic polymorphisms can influence systemic DHA levels [90].
The relationship between brain DHA uptake, APOE4, and cognitive decline also appears to be influenced by sex [91, 92]. For example, female mice expressing human APOE4 display lower cortical DHA levels, despite evidence that females have higher rates of DHA biosynthesis from ALA in the human plasma [49, 93, 94]. Similarly, female-specific changes in mitochondrial function due to aging can affect PUFA metabolism. For example, menopausal transition in females is associated with reduced mitochondrial respiration, which has been shown to increase DHA oxidation and PUFA metabolism via myelin degradation [95]. Therefore, trials that account for genetic differences in patient populations and incorporate exercise and dietary modifications in combination with DHA supplementation may prove to be more effective than DHA supplementation alone [96]. In such studies, the use of PET imaging to assess brain DHA requirements can be valuable.
METABOLISM OF DHA IN THE PRECLINICAL AD PHASE (NORMAL COGNITION)
Preclinical AD is characterized by the National Institute on Aging and Alzheimer’s Association (NIA/AA) as the phase preceding noticeable changes in cognitive performance during which abnormal Aβ or NFT deposition can be detected through PET imaging or cerebrospinal fluid (CSF) analysis. There is evidence that amyloid brain accumulation appears in APOE4 carriers in midlife, one to two decades before the onset of clinical disease [97]. Among its pleiotropic effects, APOE4 alters membrane lipid composition [98] which plays a critical role in the formation of amyloid plaques [99]. For example, APOE4 has been shown to increase cellular cholesterol by either promoting cholesterol synthesis or decreasing lysosomal lipophagy [100]. Cholesterol accumulation[101] and lower DHA levels [3], particularly in membrane microdomains or lipid rafts, alter the processing of the amyloid precursor protein (APP) to accelerate Aβ production. The application of DHA PET imaging together with amyloid PET imaging presents an opportunity to capture spatial and temporal data that relate regional brain DHA brain turnover and Aβ deposition in the human brain decades before the onset of AD.
Among APOE4 carriers, the rate of progression from preclinical AD to AD dementia varies significantly and can potentially be influenced by n-3 PUFA or saturated fat intake. For example, in a prospective cohort study of Cardiovascular Risk Factors, Aging and Incidence of Dementia (CAIDE), higher PUFA intake was associated with decreased dementia risk among middle-aged APOE4 carriers (age 44–60) [102]. Additionally, in a randomized clinical trial comparing 1.16 g/day of DHA versus a placebo in cognitively normal individuals (mean age of 33 years), DHA treatment was associated with improved retention times in APOE4 carriers [92]. In contrast, diets enriched in saturated fats and cholesterol accelerated cognitive decline in APOE4 carriers [103].
We hypothesize that a strategy involving greater n-3 PUFA intake during the preclinical AD stage in individuals with the APOE4 allele could effectively slow the rate of cognitive decline and progression to AD dementia. Because cognitive decline may not be readily apparent during this phase, monitoring the efficacy of increased DHA intake in the brain can be guided by DHA PET brain imaging. A reduction in the DHA incorporation coefficient (K*) following DHA consumption would provide a brain DHA “health” biomarker (Figure 3) [12]. The beneficial effects of increased DHA intake on brain health may decrease with age; however, it may still provide clinical advantages. In a recent pilot clinical trial examining the effects of high-dose DHA supplementation in cognitively healthy elderly individuals (mean age 69 years), we found that APOE4 limited the extent of DHA incorporation into triglycerides (TG) in both CSF and plasma. This limitation is consistent with the increased oxidation of DHA carried by TG-rich particles in the liver and brain [104]. However, increases in plasma cholesterol ester (CE)-DHA and phosphatidylcholine (PC)-DHA after supplementation were correlated with greater entorhinal cortex thickness [104].
Figure 3.
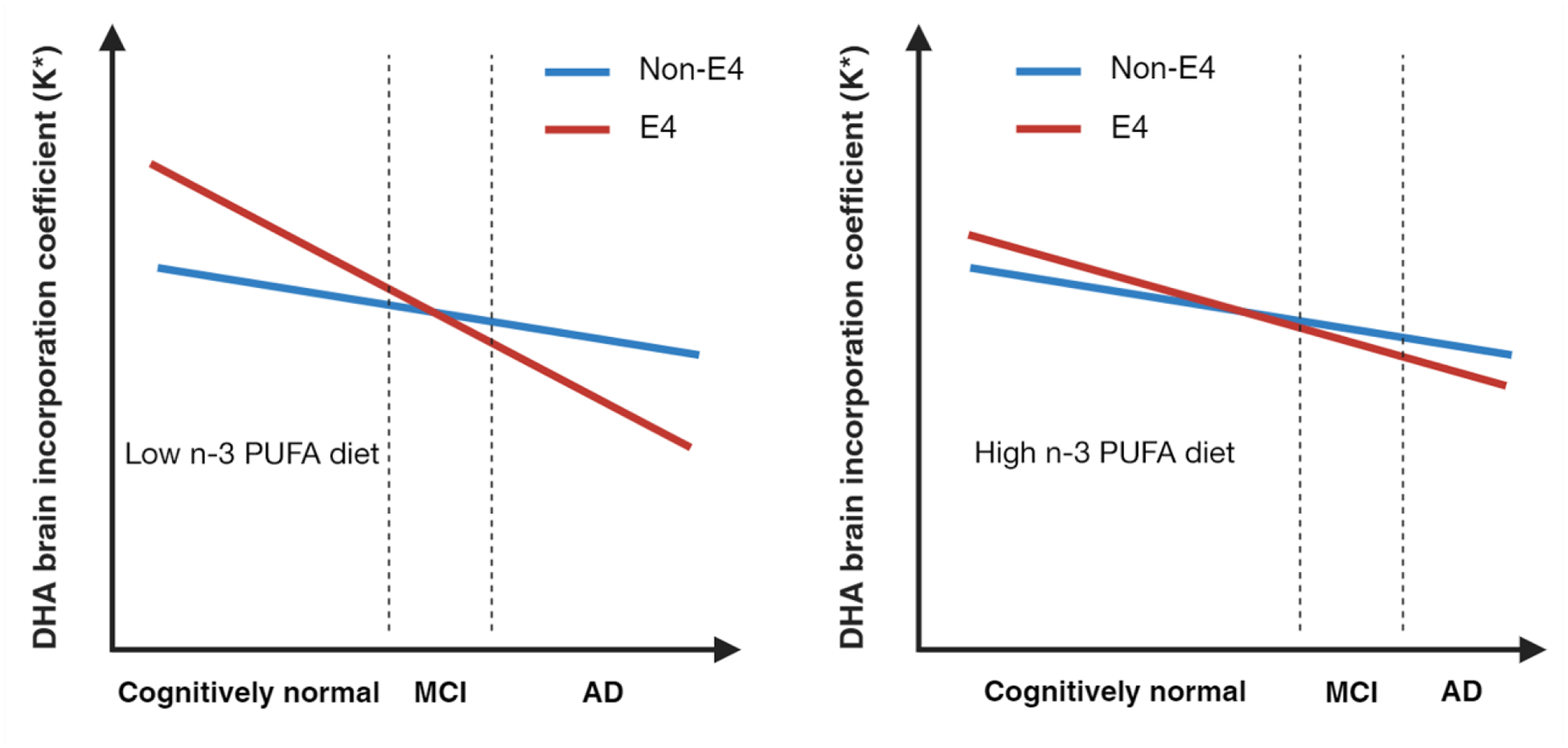
Can DHA supplementation delay APOE4 AD?
Hypothetical models of brain DHA incorporation coefficient as a function of APOE4, age, and diet. Young cognitively healthy APOE4 carriers display higher rates of brain DHA incorporation coefficient (K*) uptake compared to non-carriers, suggesting greater brain reliance on peripheral DHA levels. We hypothesized that maintaining adequate DHA consumption early in life can decrease brain metabolic stress, as reflected by the lowering of the DHA brain incorporation coefficient obtained by PET imaging studies using 22-[18F]DHA. Aging and AD pathology decrease the function of the blood-brain barrier and brain DHA incorporation coefficient, reducing the effectiveness of these interventions later in life. Figure created using BioRender.
To address whether DHA plays a role in preclinical AD, the PreventE4 trial (NCT03613844, 2018–2024) is evaluating the effects of high-dose DHA supplementation on CSF DHA levels, brain imaging, and cognition, when initiated prior to the onset of cognitive decline. In this study, a total of 368 cognitively healthy participants aged between 55 and 80 years (mean age of 65 years) with limited n-3 PUFA intake prior to enrollment were randomized to receive 2 g/day of DHA or a placebo over the course of 24 months [90]. The findings of this trial will provide insights into the potential of n-3 PUFA supplementation to mitigate the risk of preclinical AD and guide the development of early intervention strategies for AD dementia.
METABOLISM OF DHA IN THE PRODROMAL AD PHASE
The prodromal phase, also known as the symptomatic pre-dementia phase or mild cognitive impairment (MCI) due to AD, is the transitional period between the preclinical stage and AD dementia. In the prodromal AD stage, individuals are classified as those displaying abnormalities in at least one AD neuropathology process (AD-P), such as Aβ or NFT deposition, and exhibiting subtle impairment in one or more cognitive domains. A major distinction between prodromal AD and AD dementia lies in the fact that individuals with prodromal AD do not experience significant impairment in their social or occupational functioning or maintain independence in their functional abilities, albeit with greater difficulty compared to cognitively healthy individuals.
In patients with prodromal AD, the accumulation of cerebral Aβ has been shown to correlate with decreased microvascular blood flow within the brain [105], which not only restricts the supply of oxygen and nutrients required for neuronal support but also impairs Aβ clearance. Similarly, lower BBB function, as assessed by the rate of water exchange across the BBB, correlates with lower CSF Aβ levels, indicating that higher cerebral Aβ deposition may impede BBB clearance through the glial lymphatic (glymphatic) pathway [106]. Additionally, the exchange of water, Aβ, and nutrients across the BBB is facilitated by endothelial cells at the blood-brain interface. The brain’s uptake of nutrients, such as DHA, consumes energy and is closely tied to BBB function [79]. In the early prodromal stage, reversible cognitive changes may respond to increased DHA intake. Once tauopathy and neurodegeneration occur, an increase in lipid oxidation pathways alters the metabolic fate of the ingested DHA. Further research is required to identify patients with prodromal AD who would benefit from greater n-3 PUFA intake.
METABOLISM OF DHA IN THE DEMENTIA PHASE
The criteria for diagnosing AD dementia include the requirements for cognitive impairment, as well as the development of significant impairment affecting an individual’s ability to independently carry out work-related or everyday activities, as determined by a clinician. During this stage, individuals with AD dementia experience a rapid decline in both cognitive and motor functions, chronic neuroinflammation, and brain atrophy. Existing evidence indicates that n-3 PUFAs are not beneficial for individuals with AD dementia [8, 10, 12, 90, 96, 107–110].
In the presence of glucose hypometabolism, which is characteristic of individuals with AD dementia, alternative energy sources must be utilized to compensate for the deficiency in adenosine triphosphate (ATP) generated from pyruvate. Considering that cancer cells exhibiting the Warburg effect primarily depend on fatty acids for substantial ATP production [111], it is likely that patients with AD dementia who experience glucose hypometabolism resort to fatty acid metabolism in a similar manner. The conventional pathway for fatty acid metabolism commences at the cellular membrane, where PLA2 enzymes hydrolyze phospholipids containing PUFAs and release them into the cytosol as free fatty acids. Under metabolic stress conditions, these free fatty acids are transported into the mitochondria and converted to acetyl-CoA by fatty acid oxidation (FAO). In an aging mouse brain model, metabolic stress from hypometabolism has been shown to induce white matter degeneration to promote the release of free fatty acids from myelin lipids for FAO [95]. Acetyl-CoA is subsequently incorporated into the tricarboxylic acid (TCA) cycle to generate ATP via oxidative phosphorylation. Overutilization of FAO to meet the brain’s high-energy demands can deplete cellular fatty acid content and reduce the availability of n-3 PUFAs, which are essential for supporting synaptic functions and maintaining membrane fluidity. Furthermore, these processes promote the generation of ROS, which contribute to oxidative stress [112], a well-known hallmark of AD neuropathology [113, 114]. Among these processes, peroxisome dysfunction has been described in AD brains, as reflected by lower plasmalogen levels and VLCFA accumulation [115]. The accumulation of VLCFAs indicates impaired DHA synthesis and has been linked to neurotoxicity and neurodegeneration [116].
In addition to ROS generated by FAO, excessive deposition of Aβ in the brains of individuals with AD dementia triggers an alternative pathway for fatty acid metabolism. In this pathway, ROS, Aβ42 oligomers and ApoE4 can independently induce the overactivation of cPLA2, accentuating the metabolism of arachidonic acid (AA) and inflammatory eicosanoid production (Figure 2) [29, 117, 118]. This is supported by reports of patients with AD dementia exhibiting a greater [11C]AA brain uptake and incorporation [119]. Consequently, the byproducts of cPLA2 activation, along with ROS generated from FAO and Aβ signaling, can further deplete brain n-3 PUFA levels by inducing peroxidation of esterified DHA within membrane-bound phospholipids [120–122]. The factors that trigger cPLA2 activation and their effects on AD pathology are illustrated in Figure 4. The elevated oxidative stress and increased susceptibility to lipid peroxidation in the brains of AD patients raises questions about whether DHA supplementation could potentially be deleterious to brain health and cognitive function at later stages. Increasing the supply of DHA may lead to a higher abundance of lipid peroxidation products, which can exacerbate neuroinflammation, oxidative stress, and structural damage to cell membranes.
Figure 4.
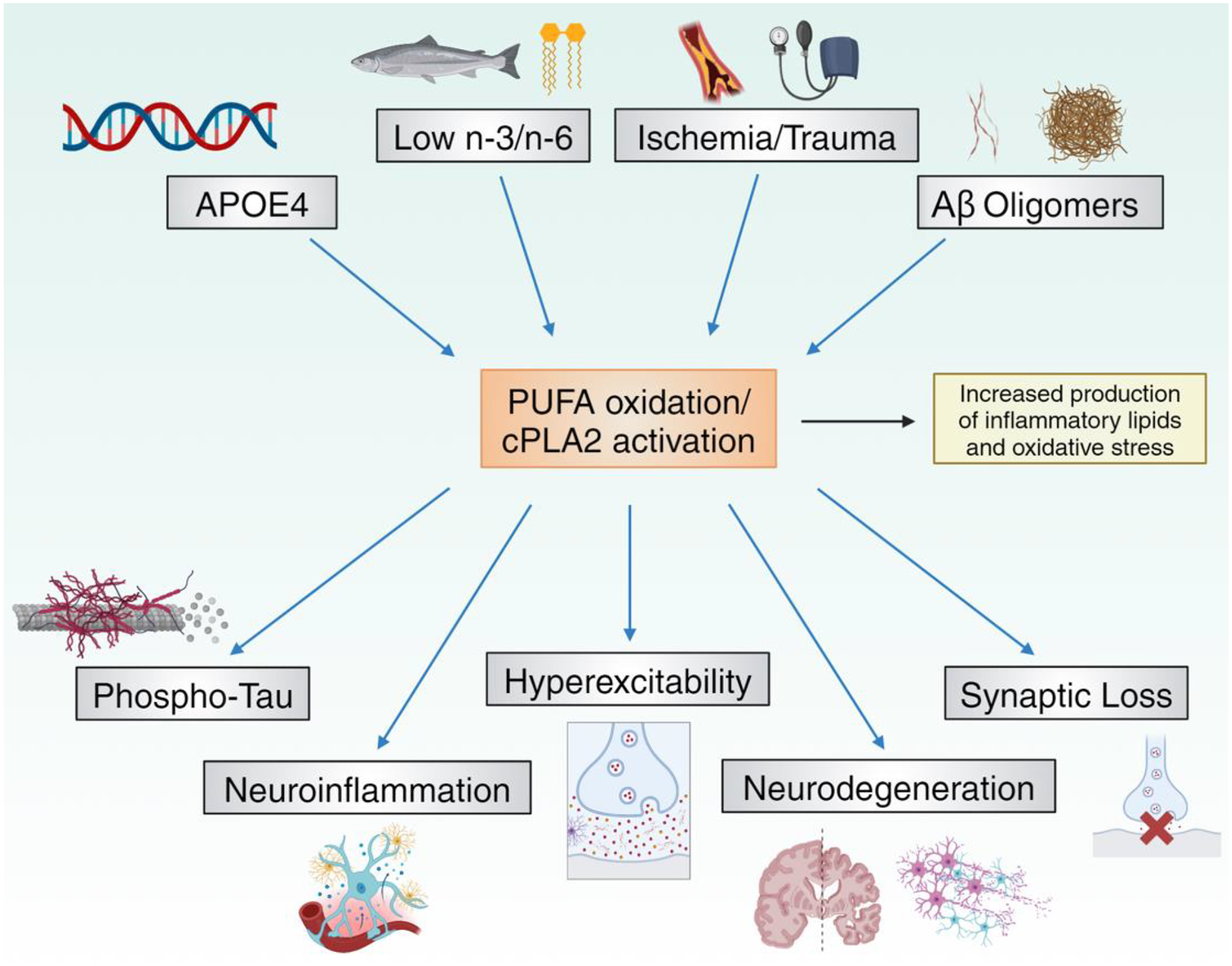
cPLA2 activation in Alzheimer’s disease.
Genetic (APOE4), environmental, and neuropathological factors enhance brain PUFA oxidation and cPLA2 activity, which can in turn accelerate the neuropathological hallmarks of Alzheimer’s disease. Figure created using BioRender.
Therapeutic Potential of Targeting n-3 PUFA Oxidation Pathways in AD Dementia
Several drug targets have been identified that have the potential to mitigate neurodegeneration and cognitive decline in AD dementia by regulating neuroinflammation and oxidative stress. One such target that has garnered focused drug development efforts in recent years is cPLA2, particularly the group IVA isoform (cPLA2α) [123] because of its critical role in selectively releasing arachidonic acid from membrane phospholipids, which initiates the production of inflammatory eicosanoids. The pursuit of safe and effective cPLA2 inhibitors is particularly relevant for individuals carrying the APOE4 allele, as cPLA2 activation has been shown to be significantly higher in postmortem brain tissues of APOE4 carriers with AD dementia than in non-carriers [29, 117]. Selective inhibition of cPLA2 has the potential to reduce neuroinflammation and attenuate glial cell activation by blocking the production of inflammatory eicosanoids, such as prostaglandins, thromboxanes, and leukotrienes, but also offers the potential to alleviate oxidative stress. This is achieved by allowing n-3 PUFA-derived SPMs to promote Aβ clearance and stimulate pro-resolving signaling pathways (Figure 4) [124–131]. Downstream of cPLA2, another calcium-dependent lipid-metabolizing enzyme involved in AD dementia pathogenesis, is arachidonate 5-lipoxygenase (ALOX5). As the primary enzyme responsible for leukotriene biosynthesis, ALOX5 has been implicated as a driver of Aβ and NFT pathologies, as well as neuronal loss in both human and murine AD models [132–138].
The development of novel cPLA2 and ALOX5 inhibitors has the potential to slow the progression of AD dementia, particularly in APOE4 carriers. However, inhibition of cPLA2 and/or ALOX5 would not only suppresses the production of many inflammatory lipid mediators but also hinders lipoxin and epoxyeicosatrienoic acid production from AA, which are SPMs with anti-inflammatory and proresolving capabilities, similar to docosanoids. Furthermore, careful consideration of the specificity of these inhibitors is warranted because iPLA2 and sPLA2 preferentially release n-3 PUFAs from membrane phospholipids, and the three major families of PLA2 enzymes include several subgroups with varying substrate specificities [129, 139]. Finally, single-nucleotide polymorphisms (SNPs) in PLA2VIA, the gene encoding for cPLA2α, revealed interactions between environmental and genetic factors. For example, a particular SNP in cPLA2 was shown to be associated with a lower risk of myocardial infarction, and this interaction was mediated in individuals with greater dietary n-6 PUFA intake [140]. As such, it is possible that SNPs of cPLA2 could modify the risk of AD dementia in a manner that is dependent on dietary PUFA intake and other factors, such as APOE genotype. Further studies on these interactions could improve our understanding of cPLA2 involvement in AD and identify patient populations who could benefit from cPLA2 inhibitors.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The efficiency of DHA metabolism undergoes alterations over the course of AD progression, which are influenced by factors such as age and APOE status. These changes can be closely monitored using DHA PET imaging. The evidence presented in this review supports the hypothesis that the greatest protection against cognitive decline and neurodegeneration in APOE4 carriers can be achieved by increasing n-3 PUFA levels early in life, several years before the onset of AD dementia. PREVENTE4 trial will address whether high dose DHA supplementation could offer benefits to older cognitively normal APOE4 carriers in terms of reducing AD dementia risk. Whether increasing DHA consumption in younger APOE4 carriers modifies brain amyloid accumulation or cognitive performance is not known. In addition, whether DHA taken as a supplement or as part of a complex dietary pattern is more effective remains an area of active investigation. In the later stages of AD, DHA intake appears to be ineffective owing to the oxidation of PUFAs. In such cases, interventions targeting PUFA oxidation pathways (such as cPLA2 inhibitors) may offer a more effective approach to mitigating neuroinflammation and preventing further neurodegeneration in individuals with AD dementia. The development of selective cPLA2 inhibitors and their evaluation aross various AD models will help clarify the role of reducing PUFA oxidation in AD progression and shed light on their utility as therapeutics for AD dementia (see Outstanding Questions). Personalized clinical trials targeting the APOE4 genotype and environmental factors with mechanistic approaches hold significant promise toward preventing and/or treating AD dementia.
OUTSTANDING QUESTIONS.
Is dysregulated PUFA metabolism a cause or effect of neuroinflammation and neurodegeneration in AD dementia?
Does long term supplementation with DHA have beneficial effects on young APOE4 carriers with normal cognition and can this effect be guided using DHA PET imaging?
Should future interventions consider replacing DHA supplements with multi-domain n-3 PUFA dietary patterns that include antioxidant nutrients and exercise?
Can cPLA2 inhibition effectively mitigate neurodegeneration and cognitive decline by suppressing neuroinflammation, and if so, would this type of intervention only offer benefits to APOE4 carriers with AD dementia?
HIGHLIGHTS.
Inconsistencies between AD clinical trials and prevention studies using n-3 PUFA supplementation can be largely attributed to differences in age, environmental factors, genetic factors, baseline n-3/n-6 intake, and disease stage.
Changes in DHA brain uptake throughout AD progression are influenced by the APOE4 allele and lifestyle factors such as DHA intake or exercise, and can be monitored by DHA PET brain imaging.
APOE4 carriers are more susceptible to blood-brain barrier dysfunction, oxidative stress, neuroinflammation, and fatty acid oxidation with aging compared to non-carriers.
We hypothesize that increasing n-3 PUFA intake provides APOE4 carriers with the highest potential for protection against AD dementia when implemented early in life, many years prior to the onset of cognitive decline.
During the AD dementia phase, alternative strategies targeting neuroinflammation and PUFA metabolism may offer potential benefits.
ACKNOWLEDGEMENTS
HNY holds the Kenneth and Bette Volk Endowed Chair of Neurology. HNY is supported by RF1AG076124, RF1AG078362, R01AG067063, R01AG054434, R01AG055770, R21AG056518, and P30AG066530 from the National Institute on Aging, GC-201711-2014197 from the Alzheimer’s Drug Discovery Foundation (ADDF), and generous donations from the Vranos and Tiny Foundations and from Ms. Lynne Nauss. KC receives partial support from the pilot project funded by P30AG066530.
Glossary
- Amyloid plaque
Extracellular deposits of beta amyloid peptides associated with aging and Alzheimer’s disease
- APOE
A gene that encodes the apolipoprotein E protein, the most abundant lipoprotein in the central nervous system, facilitates lipid transport and metabolism
- Docosahexaenoic acid (DHA)
A n-3 polyunsaturated fatty acid which is highly enriched in the brain and essential for supporting normal neurological development and functions
- Neurofibrillary tangle (NFT)
Intracellular aggregates of hyperphosphorylated tau protein form neurons and are linked to Alzheimer’s disease and cognitive decline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Xiao M, et al. (2022) DHA ameliorates cognitive ability, reduces amyloid deposition, and nerve fiber production in alzheimer’s disease. Front Nutr 9, 852433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hjorth E, et al. (2013) Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J Alzheimers Dis 35, 697–713 [DOI] [PubMed] [Google Scholar]
- 3.Grimm MO, et al. (2011) Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem 286, 14028–14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao D, et al. (2009) Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem 111, 510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talamonti E, et al. (2020) Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J 34, 2024–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joffre C, et al. (2019) N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front Pharmacol 10, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazan NG and Asatryan A (2011) Docosahexaenoic acid (DHA) in stroke, Alzheimer’s disease, and blinding retinal degenerations: coping with neuroinflammation and sustaining cell survival. Oléagineux, Corps gras, Lipides 18, 208–213 [Google Scholar]
- 8.Andriambelo B, et al. (2023) New perspectives on randomized controlled trials with omega-3 fatty acid supplements and cognition: A scoping review. Ageing Res Rev 85, 101835. [DOI] [PubMed] [Google Scholar]
- 9.Morris MC, et al. (2015) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11, 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samieri C, et al. (2022) Personalized nutrition for dementia prevention. Alzheimers Dement 18, 1424–1437 [DOI] [PubMed] [Google Scholar]
- 11.Chouinard-Watkins R, et al. (2015) Interaction between BMI and APOE genotype is associated with changes in the plasma long-chain-PUFA response to a fish-oil supplement in healthy participants. Am J Clin Nutr 102, 505–513 [DOI] [PubMed] [Google Scholar]
- 12.Yassine HN, et al. (2017) Association of docosahexaenoic acid supplementation with alzheimer disease stage in apolipoprotein E epsilon4 carriers: a review. JAMA Neurol 74, 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacombe RJS, et al. (2018) Brain docosahexaenoic acid uptake and metabolism. Mol Aspects Med 64, 109–134 [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, et al. (2016) Fatty acid-binding protein 5 at the blood-brain barrier regulates endogenous brain docosahexaenoic acid levels and cognitive function. J Neurosci 36, 11755–11767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klievik BJ, et al. (2023) Novel 13C enrichment technique reveals early turnover of DHA in peripheral tissues. J Lipid Res 64, 100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi M, et al. (2013) Kinetics of eicosapentaenoic acid in brain, heart and liver of conscious rats fed a high n-3 PUFA containing diet. Prostaglandins Leukot Essent Fatty Acids 89, 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi M, et al. (2007) Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res 48, 1150–1158 [DOI] [PubMed] [Google Scholar]
- 18.Rapoport SI (2013) Translational studies on regulation of brain docosahexaenoic acid (DHA) metabolism in vivo. Prostaglandins Leukot Essent Fatty Acids 88, 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo DS, et al. (2020) Peroxisome quality control and dysregulated lipid metabolism in neurodegenerative diseases. Exp Mol Med 52, 1486–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez RF, et al. (2018) Acyl-CoA synthetase 6 enriches the neuroprotective omega-3 fatty acid DHA in the brain. Proc Natl Acad Sci U S A 115, 12525–12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez RF, et al. (2021) Acyl-CoA synthetase 6 is required for brain docosahexaenoic acid retention and neuroprotection during aging. JCI Insight 6, e144351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouinard-Watkins R and Bazinet RP (2018) ACSL6 is critical for maintaining brain DHA levels. Proc Natl Acad Sci U S A 115, 12343–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basselin M, et al. (2010) Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA(2)beta (VIA)-deficient mice. J Lipid Res 51, 3166–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheon Y, et al. (2012) Disturbed brain phospholipid and docosahexaenoic acid metabolism in calcium-independent phospholipase A(2)-VIA (iPLA(2)beta)-knockout mice. Biochim Biophys Acta 1821, 1278–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi D, et al. (2021) Omega-3 versus Omega-6 fatty acid availability is controlled by hydrophobic site geometries of phospholipase A(2)s. J Lipid Res 62, 100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi D and Dennis EA (2023) Molecular basis of unique specificity and regulation of group VIA calcium-independent phospholipase A(2) (PNPLA9) and its role in neurodegenerative diseases. Pharmacol Ther 245, 108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palavicini JP, et al. (2017) Oligomeric amyloid-beta induces MAPK-mediated activation of brain cytosolic and calcium-independent phospholipase A(2) in a spatial-specific manner. Acta Neuropathol Commun 5, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, et al. (2022) Calcium-dependent cytosolic phospholipase A2 activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Mol Neurodegener 17, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebright B, et al. (2022) Eicosanoid lipidome activation in post-mortem brain tissues of individuals with APOE4 and Alzheimer’s dementia. Alzheimers Res Ther 14, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oguro A, et al. (2021) Contribution of DHA diols (19,20-DHDP) produced by cytochrome P450s and soluble epoxide hydrolase to the beneficial effects of DHA supplementation in the brains of rotenoneinduced rat models of Parkinson’s disease. Biochim Biophys Acta Mol Cell Biol Lipids 1866, 158858. [DOI] [PubMed] [Google Scholar]
- 31.Borkowski K, et al. (2021) Association of plasma and CSF cytochrome P450, soluble epoxide hydrolase, and ethanolamide metabolism with Alzheimer’s disease. Alzheimers Res Ther 13, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umhau JC, et al. (2009) Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res 50, 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassine HN, et al. (2017) DHA brain uptake and APOE4 status: a PET study with [1-11C]-DHA. Alzheimers Res Ther 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chouinard-Watkins R, et al. (2013) Disturbance in uniformly 13C-labelled DHA metabolism in elderly human subjects carrying the apoE epsilon4 allele. Br J Nutr 110, 1751–1759 [DOI] [PubMed] [Google Scholar]
- 35.Hennebelle M, et al. (2014) Ageing and apoE change DHA homeostasis: relevance to age-related cognitive decline. Proc Nutr Soc 73, 80–86 [DOI] [PubMed] [Google Scholar]
- 36.Plourde M (2018) Aging, cognitive decline, apolipoprotein E and docosahexaenoic acid metabolism. Oilseeds & Fats Crops and Lipids 25, D405 [Google Scholar]
- 37.Vandal M, et al. (2014) Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem 129, 516–526 [DOI] [PubMed] [Google Scholar]
- 38.Wang YW, et al. (2023) A comparative study about the neuroprotective effects of DHA-enriched phosphatidylserine and EPA-enriched phosphatidylserine against oxidative damage in primary hippocampal neurons. Mar Drugs 21, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs ML, et al. (2021) EPA and DHA differentially modulate membrane elasticity in the presence of cholesterol. Biophys J 120, 2317–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kousparou C, et al. (2023) DHA/EPA (omega-3) and LA/GLA (omega-6) as bioactive molecules in neurodegenerative diseases. Int J Mol Sci 24, 10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong JR, et al. (2023) Decreased DHA-containing phospholipids in the neocortex of dementia with Lewy bodies are associated with soluble Abeta(42), phosphorylated alpha-synuclein, and synaptopathology. Brain Pathol, e13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evbuomwan SA, et al. (2022) Roles and mechanisms of docosahexaenoic acid (DHA) in neurodevelopment, neuronal functions, learning and memory. World News of Natural Sciences 40, 104–119 [Google Scholar]
- 43.Cutuli D (2017) Functional and structural benefits induced by omega-3 polyunsaturated fatty acids during aging. Curr Neuropharmacol 15, 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, et al. (2015) Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 11, 40–50 e41–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwab JM, et al. (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinohara M, et al. (2012) Functional metabolomics reveals novel active products in the DHA metabolome. Front Immunol 3, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spite M, et al. (2014) Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 19, 21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calder PC (2017) Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 45, 1105–1115 [DOI] [PubMed] [Google Scholar]
- 49.Calder PC (2020) Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 178, 105–123 [DOI] [PubMed] [Google Scholar]
- 50.Ferreira I, et al. (2022) Resolvins, protectins, and maresins: DHA-derived specialized pro-resolving mediators, biosynthetic pathways, synthetic approaches, and their role in inflammation. Molecules 27, 1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alba MM, et al. (2023) Eicosanoids and other oxylipins in liver injury, inflammation and liver cancer development. Front Physiol 14, 1098467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong S, et al. (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 278, 14677–14687 [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, et al. (2022) Inflammation and infection in pain and the role of GPR37. Int J Mol Sci 23, 14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HY, et al. (2022) Molecular and signaling mechanisms for docosahexaenoic acid-derived neurodevelopment and neuroprotection. Int J Mol Sci 23, 4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asatryan A and Bazan NG (2017) Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J Biol Chem 292, 12390–12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stark DT and Bazan NG (2011) Neuroprotectin D1 induces neuronal survival and downregulation of amyloidogenic processing in Alzheimer’s disease cellular models. Mol Neurobiol 43, 131–138 [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, et al. (2011) Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS One 6, e15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu M, et al. (2016) Pro-resolving lipid mediators improve neuronal survival and increase Abeta42 phagocytosis. Mol Neurobiol 53, 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lands B, et al. (2018) Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins Leukot Essent Fatty Acids 136, 15–21 [DOI] [PubMed] [Google Scholar]
- 60.Bibus D and Lands B (2015) Balancing proportions of competing omega-3 and omega-6 highly unsaturated fatty acids (HUFA) in tissue lipids. Prostaglandins Leukot Essent Fatty Acids 99, 19–23 [DOI] [PubMed] [Google Scholar]
- 61.Cisbani G, et al. (2020) Peripheral cytokine and fatty acid associations with neuroinflammation in AD and aMCI patients: An exploratory study. Brain Behav Immun 87, 679–688 [DOI] [PubMed] [Google Scholar]
- 62.Vellas B, et al. (2014) Mapt study: a multidomain approach for preventing alzheimer’s disease: design and baseline data. J Prev Alzheimers Dis 1, 13–22 [PMC free article] [PubMed] [Google Scholar]
- 63.Hooper C, et al. (2017) Cognitive changes with omega-3 polyunsaturated fatty acids in non-demented older adults with low omega-3 index. J Nutr Health Aging 21, 988–993 [DOI] [PubMed] [Google Scholar]
- 64.Macaron T, et al. (2021) Associations of Omega-3 fatty acids with brain morphology and volume in cognitively healthy older adults: A narrative review. Ageing Res Rev 67, 101300. [DOI] [PubMed] [Google Scholar]
- 65.von Schacky C (2020) Omega-3 index in 2018/19. Proc Nutr Soc, 1–7 [DOI] [PubMed] [Google Scholar]
- 66.Harris WS and Von Schacky C (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 39, 212–220 [DOI] [PubMed] [Google Scholar]
- 67.Tan ZS, et al. (2012) Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology 78, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lukaschek K, et al. (2016) Cognitive impairment is associated with a low omega-3 index in the elderly: results from the KORA-age study. Dement Geriatr Cogn Disord 42, 236–245 [DOI] [PubMed] [Google Scholar]
- 69.Rouch L, et al. (2022) Associations of erythrocyte omega-3 fatty acids with cognition, brain imaging and biomarkers in the Alzheimer’s disease neuroimaging initiative: cross-sectional and longitudinal retrospective analyses. Am J Clin Nutr 116, 1492–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yassine HN (2022) The omega-3 index in Alzheimer’s disease: Ready for prime time? Am J Clin Nutr 116, 1474–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan Y, et al. (2015) Fatty acid-binding protein 5 facilitates the blood-brain barrier transport of docosahexaenoic acid. Mol Pharm 12, 4375–4385 [DOI] [PubMed] [Google Scholar]
- 72.Nguyen LN, et al. (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 [DOI] [PubMed] [Google Scholar]
- 73.Duro MVV, et al. (2023) Synthesis and preclinical evaluation of 22-[18F]fluorodocosahexaenoic acid as a positron emission tomography probe for monitoring brain docosahexaenoic acid uptake kinetics. ACS Chem Neurosci 14, 4409–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sorg C, et al. (2007) Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 104, 18760–18765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rawat V, et al. (2019) ApoE4 alters ABCA1 membrane trafficking in astrocytes. J Neurosci 39, 9611–9622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakato M, et al. (2015) Neurite outgrowth stimulation by n-3 and n-6 PUFAs of phospholipids in apoE-containing lipoproteins secreted from glial cells. J Lipid Res 56, 1880–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwao T, et al. (2023) Aging decreases docosahexaenoic acid transport across the blood-brain barrier in C57BL/6J mice. PLoS One 18, e0281946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arellanes IC, et al. (2020) Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 59, 102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yassine HN, et al. (2022) Nutritional metabolism and cerebral bioenergetics in Alzheimer’s disease and related dementias. Alzheimers Dement 19, 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montagne A, et al. (2020) APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong YJ, et al. (2022) Correlations between APOE4 allele and regional amyloid and tau burdens in cognitively normal older individuals. Sci Rep 12, 14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uchida Y, et al. (2022) APOE e4 dose associates with increased brain iron and beta-amyloid via blood-brain barrier dysfunction. J Neurol Neurosurg Psychiatry 93, 772. [DOI] [PubMed] [Google Scholar]
- 83.Farmer BC, et al. (2021) APOEpsilon4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis. Mol Neurodegener 16, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams HC, et al. (2020) APOE alters glucose flux through central carbon pathways in astrocytes. Neurobiol Dis 136, 104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yassine HN and Finch CE (2020) APOE alleles and diet in brain aging and alzheimer’s disease. Front Aging Neurosci 12, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiman EM, et al. (2004) Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A 101, 284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mosconi L, et al. (2008) Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci 1147, 180–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madsen LS, et al. (2023) Capillary dysfunction in healthy elderly APOE epsilon4 carriers with raised brain Abeta deposition. Alzheimers Dement 20, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patrick RP (2019) Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J 33, 1554–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yassine H, et al. (2023) Baseline findings of PreventE4: a double-blind placebo controlled clinical trial testing high dose DHA in APOE4 carriers before the onset of dementia. J Prev Alzheimers Dis 10, 810. [DOI] [PubMed] [Google Scholar]
- 91.Pontifex M, et al. (2018) The effect of APOE genotype on Alzheimer’s disease risk is influenced by sex and docosahexaenoic acid status. Neurobiol Aging 69, 209–220 [DOI] [PubMed] [Google Scholar]
- 92.Stonehouse W, et al. (2013) DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr 97, 1134–1143 [DOI] [PubMed] [Google Scholar]
- 93.Martinsen A, et al. (2019) Altered SPMs and age-associated decrease in brain DHA in APOE4 female mice. FASEB J 33, 10315–10326 [DOI] [PubMed] [Google Scholar]
- 94.Eriksdotter M, et al. (2015) Plasma fatty acid profiles in relation to cognition and gender in alzheimer’s disease patients during oral omega-3 fatty acid supplementation: the OmegAD study. J Alzheimers Dis 48, 805–812 [DOI] [PubMed] [Google Scholar]
- 95.Klosinski LP, et al. (2015) White matter lipids as a ketogenic fuel supply in aging female brain: implications for alzheimer’s disease. EBioMedicine 2, 1888–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrieu S, et al. (2017) Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 16, 377–389 [DOI] [PubMed] [Google Scholar]
- 97.Jansen WJ, et al. (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313, 1924–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yassine HN and Finch CE (2020) APOE alleles and diet in brain aging and alzheimer’s disease. Front Aging Neurosci 12, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reiman EM, et al. (2020) Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun 11, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tcw J, et al. (2022) Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233.e2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simons K and Ehehalt R (2002) Cholesterol, lipid rafts, and disease. J Clin Invest 110, 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laitinen M, et al. (2006) Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord 22, 99–107 [DOI] [PubMed] [Google Scholar]
- 103.Fote GM, et al. (2021) A scoping review of dietary factors conferring risk or protection for cognitive decline in APOE ε4 carriers. J Nutr Health Aging 25, 1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bantugan MA, et al. (2023) Associations of ApoE4 status and DHA supplementation on plasma and CSF lipid profiles and entorhinal cortex thickness. J Lipid Res 64, 100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Madsen LS, et al. (2023) Capillary dysfunction correlates with cortical amyloid load in early Alzheimer’s disease. Neurobiol Aging 123, 1–9 [DOI] [PubMed] [Google Scholar]
- 106.Gold BT, et al. (2021) Water exchange rate across the blood-brain barrier is associated with CSF amyloid-beta 42 in healthy older adults. Alzheimers Dement 17, 2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kosti RI, et al. (2022) Fish intake, n-3 fatty acid body status, and risk of cognitive decline: a systematic review and a dose-response meta-analysis of observational and experimental studies. Nutr Rev 80, 1445–1458 [DOI] [PubMed] [Google Scholar]
- 108.Tseng PT, et al. (2023) Efficacy and acceptability of anti-inflammatory eicosapentaenoic acid for cognitive function in Alzheimer’s dementia: A network meta-analysis of randomized, placebo-controlled trials with omega-3 fatty acids and FDA-approved pharmacotherapy. Brain Behav Immun 111, 352–364 [DOI] [PubMed] [Google Scholar]
- 109.Wu S, et al. (2015) Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci Biobehav Rev 48, 1–9 [DOI] [PubMed] [Google Scholar]
- 110.Brainard JS, et al. (2020) Omega-3, omega-6, and polyunsaturated fat for cognition: systematic review and meta-analysis of randomized trials. J Am Med Dir Assoc 21, 1439–1450 e1421 [DOI] [PubMed] [Google Scholar]
- 111.Lee JS, et al. (2020) ATP production relies on fatty acid oxidation rather than glycolysis in pancreatic ductal adenocarcinoma. Cancers (Basel) 12, 2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dewanjee S, et al. (2022) Altered glucose metabolism in Alzheimer’s disease: Role of mitochondrial dysfunction and oxidative stress. Free Radic Biol Med 193, 134–157 [DOI] [PubMed] [Google Scholar]
- 113.Bai R, et al. (2022) Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev 77, 101619. [DOI] [PubMed] [Google Scholar]
- 114.Rummel NG and Butterfield DA (2022) Altered metabolism in alzheimer disease brain: role of oxidative stress. Antioxid Redox Signal 36, 1289–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kou J, et al. (2011) Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol 122, 271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarrouk A, et al. (2012) Induction of mitochondrial changes associated with oxidative stress on very long chain fatty acids (C22:0, C24:0, or C26:0)-treated human neuronal cells (SK-NB-E). Oxid Med Cell Longev 2012, 623257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang S, et al. (2022) Calcium-dependent cytosolic phospholipase A(2) activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Mol Neurodegener 17, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duro MV, et al. (2022) Lipids and brain inflammation in APOE4-associated dementia. Curr Opin Lipidol 33, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Esposito G, et al. (2008) Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J Nucl Med 49, 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shichiri M, et al. (2023) Application of regulation of reactive oxygen species and lipid peroxidation to disease treatment. J Clin Biochem Nutr 72, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maiti AK (2023) The genetics of phospholipase A2 induced redox signaling in neuroinflammation and neuronal diseases. In Phospholipases in Physiology and Pathology, pp. 307–327, Elsevier [Google Scholar]
- 122.Panov A (2023) Mitochondrial production of perhydroxyl radical (HO2.) as inducer of aging and age-related pathologies. J Biochem Bioph 4, 307–237 [Google Scholar]
- 123.Soubhye J, et al. (2018) Targeting cytosolic phospholipase A2alpha for novel anti-inflammatory agents. Curr Med Chem 25, 2418–2447 [DOI] [PubMed] [Google Scholar]
- 124.Asante I, et al. (2022) Uncovering mechanisms of brain inflammation in Alzheimer’s disease with APOE4: Application of single cell-type lipidomics. Ann N Y Acad Sci 1518, 84–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gynther M, et al. (2022) Increased expression and activity of brain cortical cPLA2 due to chronic lipopolysaccharide administration in mouse model of familial alzheimer’s disease. Pharmaceutics 14, 2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin F (2023) Lipid metabolism and Alzheimer’s disease: clinical evidence, mechanistic link and therapeutic promise. FEBS J 290, 1420–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Premrajan P, et al. (2023) PLA2: Implications in neurological disorders as a prospective therapeutic target. In Phospholipases in Physiology and Pathology, pp. 139–158, Elsevier [Google Scholar]
- 128.Hegde KU, et al. (2023) Cytosolic phospholipase A2 (cPLA2)-mediated oxidative and inflammatory responses in neurodegenerative diseases. In Phospholipases in Physiology and Pathology, pp. 79–90, Elsevier [Google Scholar]
- 129.Khan SA and Ilies MA (2023) The phospholipase A2 superfamily: structure, isozymes, catalysis, physiologic and pathologic roles. Int J Mol Sci 24, 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teng T, et al. (2019) Cytosolic phospholipase A(2) facilitates oligomeric amyloid-beta peptide association with microglia via regulation of membrane-cytoskeleton connectivity. Mol Neurobiol 56, 3222–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Czapski GA, et al. (2016) The lipoxygenases: their regulation and implication in alzheimer’s disease. Neurochem Res 41, 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guan YH, et al. (2022) The role of microglia in Alzheimer’s disease and progress of treatment. Ibrain 8, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahnashi MH, et al. (2022) In-vitro, in-vivo, molecular docking and ADMET studies of 2-substituted 3,7-dihydroxy-4H-chromen-4-one for oxidative stress, inflammation and alzheimer’s disease. Metabolites 12, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Somin S, et al. (2023) Alleviating the unwanted effects of oxidative stress on Abeta clearance: a review of related concepts and strategies for the development of computational modelling. Transl Neurodegener 12, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bitto A, et al. (2017) Effects of COX1–2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm Res 66, 389–398 [DOI] [PubMed] [Google Scholar]
- 136.Chu J, et al. (2013) Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS One 8, e70991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Giannopoulos PF, et al. (2019) Learning impairments, memory deficits, and neuropathology in aged tau transgenic mice are dependent on leukotrienes biosynthesis: role of the cdk5 kinase pathway. Mol Neurobiol 56, 1211–1220 [DOI] [PubMed] [Google Scholar]
- 138.Vagnozzi AN, et al. (2018) Brain 5-lipoxygenase over-expression worsens memory, synaptic integrity, and tau pathology in the P301S mice. Aging Cell 17, e12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen S and Subbaiah PV (2013) Regioisomers of phosphatidylcholine containing DHA and their potential to deliver DHA to the brain: role of phospholipase specificities. Lipids 48, 675–686 [DOI] [PubMed] [Google Scholar]
- 140.Hartiala J, et al. (2012) Association of PLA2G4A with myocardial infarction is modulated by dietary PUFAs. Am J Clin Nutr 95, 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]


