Abstract
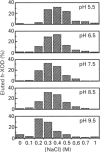
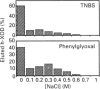
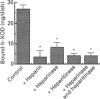
Much evidence has suggested that the superoxide generated by xanthine oxidase (XOD) within the endothelial cell triggers characteristic free-radical-mediated tissue injuries. Although it has been reported that XOD exists not only in the cytoplasm, but also on the outside surface of the endothelial cell membrane, it is not clear how XOD localizes on the outside of the plasma membrane. Purified human xanthine oxidase (h-XOD) had an affinity for heparin-Sepharose. The binding was largely independent of the pH over the physiological range, whereas it tended to increase at lower pH and to decrease at higher pH. Exposure of h-XOD to the lysine-specific reagent trinitrobenzenesulphonic acid or the arginine-specific reagent phenylglyoxal caused it to lose its affinity for heparin-Sepharose. The binding of h-XOD to heparin is apparently of electrostatic nature, and both lysine and arginine residues are involved in the binding. h-XOD was found to bind to cultured porcine aortic endothelial cells, and this binding was inhibited by the addition of heparin or pretreatment of the cells with heparinase and/or heparitinase. Intravenous injection of heparin into two healthy persons led to a prompt increase in plasma h-XOD concentration. These results suggest that XOD localizes on the outside surface of endothelial cells by association with polysaccharide chains of heparin-like proteoglycans on the endothelial-cell membranes. Superoxide extracellularly generated by XOD may injure the source-endothelial-cell membrane and also attract and activate closely appositional neutrophils, which themselves actually cause progressive oxidative damage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Kodera T., Ohta H., Hayashi K., Hirano K. The heparin binding site of human extracellular-superoxide dismutase. Arch Biochem Biophys. 1992 Aug 15;297(1):155–161. doi: 10.1016/0003-9861(92)90654-f. [DOI] [PubMed] [Google Scholar]
- Adachi T., Marklund S. L. Interactions between human extracellular superoxide dismutase C and sulfated polysaccharides. J Biol Chem. 1989 May 25;264(15):8537–8541. [PubMed] [Google Scholar]
- Adachi T., Usami Y., Kishi T., Hirano K., Hayashi K. An enzyme immunoassay for cuprozinc superoxide dismutase using monoclonal antibodies. Application for pharmacokinetic study. J Immunol Methods. 1988 Apr 22;109(1):93–101. doi: 10.1016/0022-1759(88)90446-2. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Treby D. A., Halliwell B., Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1981 Oct;61(4):483–486. doi: 10.1042/cs0610483. [DOI] [PubMed] [Google Scholar]
- Chambers D. E., Parks D. A., Patterson G., Roy R., McCord J. M., Yoshida S., Parmley L. F., Downey J. M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985 Feb;17(2):145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Ikebuchi Y., Masumoto N., Tasaka K., Koike K., Kasahara K., Miyake A., Tanizawa O. Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. J Biol Chem. 1991 Jul 15;266(20):13233–13237. [PubMed] [Google Scholar]
- Inoue M., Watanabe N., Matsuno K., Sasaki J., Tanaka Y., Hatanaka H., Amachi T. Expression of a hybrid Cu/Zn-type superoxide dismutase which has high affinity for heparin-like proteoglycans on vascular endothelial cells. J Biol Chem. 1991 Sep 5;266(25):16409–16414. [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Lindahl U., Marklund S. L. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem J. 1988 Nov 15;256(1):29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Spector T., Hall W. W. Xanthine oxidase from human liver: purification and characterization. Arch Biochem Biophys. 1986 May 15;247(1):108–119. doi: 10.1016/0003-9861(86)90539-4. [DOI] [PubMed] [Google Scholar]
- Liu C. S., Chang J. Y. The heparin binding site of human antithrombin III. Selective chemical modification at Lys114, Lys125, and Lys287 impairs its heparin cofactor activity. J Biol Chem. 1987 Dec 25;262(36):17356–17361. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Williams T. K., Beckman J. S. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988 May;254(5 Pt 1):G768–G774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratych R. E., Chuknyiska R. S., Bulkley G. B. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987 Aug;102(2):122–131. [PubMed] [Google Scholar]
- Saxena U., Klein M. G., Goldberg I. J. Metabolism of endothelial cell-bound lipoprotein lipase. Evidence for heparan sulfate proteoglycan-mediated internalization and recycling. J Biol Chem. 1990 Aug 5;265(22):12880–12886. [PubMed] [Google Scholar]
- Sullivan C. H., Mather I. H., Greenwalt D. E., Madara P. J. Purification of xanthine oxidase from the fat-globule membrane of bovine milk by electrofocusing. Mol Cell Biochem. 1982 Apr 16;44(1):13–22. doi: 10.1007/BF00573841. [DOI] [PubMed] [Google Scholar]
- Thomson C. D. Selenium-dependent and non-selenium-dependent glutathione peroxidase in human tissues of New Zealand residents. Biochem Int. 1985 Apr;10(4):673–679. [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]