Abstract
Poxviruses encode a broad range of proteins that interfere with host immune functions, such as soluble versions of receptors for the cytokines tumor necrosis factor, interleukin-1β, gamma interferon (IFN-γ), IFN-α/β, and chemokines. These virus-encoded cytokine receptors have a profound effect on virus pathogenesis and enable the study of the role of cytokines in virus infections. The vaccinia virus (VV) Western Reserve gene B18R encodes a secreted protein with 3 immunoglobulin domains that functions as a soluble receptor for IFN-α/β. We have found that after secretion B18R binds to both uninfected and infected cells. The B18R protein present at the cell surface maintains the properties of the soluble receptor, binding IFN-α/β with high affinity and with broad species specificity, and protects cells from the antiviral state induced by IFN-α/β. VV strain Wyeth expressed a truncated B18R protein lacking the C-terminal immunoglobulin domain. This protein binds IFN with lower affinity and retains its ability to bind to cells, indicating that the C-terminal region of B18R contributes to IFN binding. The replication of a VV B18R deletion mutant in tissue culture was restricted in the presence of IFN-α, whereas the wild-type virus replicated normally. Binding of soluble recombinant B18R to cells protected the cultures from IFN and allowed VV replication. This represents a novel strategy of virus immune evasion in which secreted IFN-α/β receptors not only bind the soluble cytokine but also bind to uninfected cells and protect them from the antiviral effects of IFN-α/β, maintaining the cells' susceptibility to virus infections. The adaptation of this soluble receptor to block IFN-α/β activity locally will help VV to replicate in the host and spread in tissues. This emphasizes the importance of local effects of IFN-α/β against virus infections.
Viruses encode several immune evasion strategies to block host antiviral defenses. Vaccinia virus (VV) is the prototypic member of the poxvirus family of cytoplasmic DNA viruses (17). Poxviruses encode a unique set of secreted proteins that function as soluble cytokine receptors, or binding proteins, and modulate virus virulence. These include proteins that bind tumor necrosis factor (TNF), interleukin-1β (IL-1β), alpha/beta interferon (IFN-α/β), gamma interferon (IFN-γ), and chemokines. Poxvirus cytokine receptors are secreted from infected cells, bind cytokines in solution, and inhibit cytokine activity by blocking the interaction of cytokines with specific receptors on the cell surface.
A heterogeneous family of cytokines known as IFNs was defined by their ability to induce resistance to virus infection (12). Type I IFNs are represented by various IFN-α subtypes, IFN-β, IFN-ω, and IFN-τ, and these bind to a common cellular receptor (IFN-α/βR) (21). IFN-γ (type II IFN) binds a different receptor (IFN-γR). Both types of IFN have direct antiviral activity, are proinflammatory, and have immunoregulatory activity. The antiviral effect of IFN-γ is mediated in part by its ability to induce cellular (Th1) immune responses such as the production of cytotoxic T lymphocytes which are important for destruction of infected cells. Type I IFNs mediate their antiviral function mostly by ability to induce a number of proteins that confer an antiviral state on the cell at the site of virus replication (23), and also have some immunoregulatory activity (6). IFNs are important for protection against poxvirus infections. Mice with a targeted disruption of the type I or II IFN system are more susceptible to VV infection (9, 11, 19). Treatment of mousepox virus-infected mice with neutralizing antibodies to either type I or II IFN resulted in defective clearance of virus (13), whereas treatment of mice with IFN prior to infection with VV abrogated the infection (22). The inhibition of nitric oxide synthase in mice, which mediates the antiviral activity of IFN-γ, converts a resolving infection into fulminant mousepox (14).
VV and other poxviruses have evolved strategies to evade the antiviral effects of IFNs (25). First, VV encodes two intracellular proteins that prevent an antiviral state in the cells. VV E3L protein binds double-stranded RNA and prevents the activation of the IFN-induced protein kinase PKR (7). VV K3L protein, which has sequence similarity with eukaryotic translation initiation factor 2α (eIF-2α), binds competitively to PKR and blocks the phosphorylation and inactivation of host eIF-2α (5). Second, poxviruses encode soluble proteins that are secreted from infected cells and function as soluble IFN receptors (IFNRs). A soluble IFN-γR expressed by myxomavirus and orthopoxviruses has sequence similarity to the cellular counterpart and blocks IFN-γ activity. In addition, VV and other orthopoxviruses encode an IFN-α/βR that is encoded by the B18R gene in VV strain Western Reserve (WR) and by the B19R gene in the Copenhagen strain (8, 26). The B18R protein has three immunoglobulin (Ig)-like domains and binds IFN-α/β with high affinity despite showing very limited sequence similarity to cellular IFN-α/βRs, which have fibronectin type III domains. In contrast to the cellular counterparts, both the IFN-γR and IFN-α/βR encoded by VV and cowpox virus bind IFNs from several species (4, 18, 26).
The B18R protein is expressed in VV-infected cells as two molecular species of 52 and 60 to 65 kDa, the latter being secreted into the culture medium (3). Secretion of the B18R protein from cells infected with VV or recombinant baculovirus was in accordance with the presence of a signal peptide at the N terminus and the absence of a hydrophobic transmembrane domain at the C terminus (3, 24). Antiserum raised against VV proteins released from the cell at early times postinfection recognized a protein on the surface of the VV-infected cell (27, 29). For many years this protein was known as the VV early soluble and surface (S) antigen, and subsequently it was shown to be encoded by the VV WR gene B18R (28). Morikawa and Ueda also expressed the B18R protein from recombinant baculovirus and suggested that B18R might be anchored in the plasma membrane through the N-terminal hydrophobic sequence (16). Deletion of the B18R gene attenuates the virus in mice, indicating that the protein plays a role in virus virulence in vivo (26).
Colamonici et al. (8) showed that the soluble VV WR B18R protein attached to the cell surface bound IFN, but the role of the B18R protein present at the cell membrane and the mechanism by which B18R binds to the cell surface were not investigated further in the context of VV infection.
Here we report that VV WR and other orthopoxviruses, including cowpox and camelpox viruses, express IFN-α/βRs at the cell surface. We show that the B18R protein secreted from infected cells can bind to the cell surface and prevent the induction of an antiviral state in the cell. Replication of a VV WR mutant lacking the B18R open reading frame (ORF) in cell monolayers is restricted in the presence of IFN. We propose a mechanism by which the B18R protein mediates its anti-IFN activity at the cell membrane of the uninfected cell, thus preventing the induction of an antiviral state before cells become infected with VV.
MATERIALS AND METHODS
Cells and viruses.
All the cell lines were obtained from the Cell Bank of the Sir William Dunn School of Pathology (University of Oxford). Human TK−143B, D980R, and FS2 cells, monkey Vero, BSC-1, CV1, and Cos7 cells, and rabbit RK13 cells were grown in minimal essential medium (MEM) (GIBCO) with 10% fetal bovine serum (FBS). Human U937, THP-1, HL-60, Molt4, and Raji cells and mouse EL4 cells were grown in RPMI 1640 (GIBCO) and 10% FBS. Spodoptera frugiperda (Sf) insect cells and Autographa californica nuclear polyhedrosis virus (AcNPV) were cultured in TC100 medium (GIBCO) containing 10% FBS. The sources of VV strains and other orthopoxviruses have been described elsewhere (4).
Recombinant viruses.
The VV WR deletion mutant lacking B18R (vΔB18R, vAA6), the recombinant virus containing two copies of the B18R gene (vB18R, vAA4), and the recombinant baculoviruses expressing B15R (AcB15R, AcAA3) or B18R (AcB18R, AcAA4) have been described previously (3).
Reagents.
Human recombinant IFN-α2 (3 × 108 U/mg) was obtained from PeproTech (London, United Kingdom) and was radioiodinated without loss of biological activity by using 125I-labeled Bolton-Hunter reagent to 40 μCi/μg as described. Human natural IFN-α (1.5 × 108 U/mg) was obtained from Wellcome (Beckenham, United Kingdom) and mouse natural IFN-α (4 × 104 U/mg) from Calbiochem. Bovine recombinant IFN-α1 (107 U/mg) was a generous gift from R. A. Collins (Institute of Animal Health, Compton, United Kingdom), and rat and rabbit type I IFN (1.9 × 106 and 3.9 × 105 U/mg, respectively) were from Lee Biomolecular Research Laboratories (San Diego, Calif.).
DNA sequencing.
The B18R gene from VV Wyeth was amplified by PCR using Taq polymerase and virus DNA as the template and by direct sequencing from virus genomic DNA. The PCR product was sequenced using specific oligonucleotides. Sequencing reactions were carried out using the ABI PRISM dye terminator cycle sequencing ready reaction kit, and reaction products were run on an ABI 373 DNA sequencer. The sequence data were assembled and analyzed using the Genetics Computer Group (GCG) computer programs (10).
Metabolic labeling of proteins, immunoprecipitation, and electrophoretic analysis.
TK−143B or Sf cells were infected with orthopoxviruses or baculoviruses, respectively, at 10 PFU per cell. At the indicated time postinfection, cultures were pulse-labeled with 150 μCi of 35S-labeled Promix (Amersham; 1,200 Ci/mmol)/ml and 150 μCi of [35S]cysteine (DuPont-New England Nuclear; 600 Ci/mmol)/ml in methionine- and cysteine-free medium in the absence of serum. Cells or media were dissociated in radioimmunoprecipitation assay (RIPA) buffer and immunoprecipitated with anti-B18R rabbit Ig and protein A-Sepharose as described previously (3). Specific anti-B18R Ig was purified by affinity chromatography on protein A-Sepharose from a rabbit antiserum raised against recombinant B18R protein expressed in the baculovirus system (3). Immunoprecipitates were dissociated in Laemmli sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% gels and fluorography with Amplify (Amersham).
Sucrose gradient centrifugation.
Samples were layered onto a 5 to 25% sucrose gradient in phosphate-buffered saline (PBS) and centrifuged in a Beckman SW41 Ti rotor (40,000 rpm, 17 h, 4°C). Gradient fractions were mixed with 5 μg of bovine serum albumin, and proteins were precipitated with 10% trichloracetic acid (TCA) (for 10 min at 4°C) and collected by centrifugation (for 15 min in a microcentrifuge). Pellets were dissolved in SDS-PAGE sample buffer, their pH was adjusted by addition of ammonia vapors, and they were analyzed by SDS-PAGE. Prestained molecular size markers were analyzed in parallel, and the sucrose concentration in the fractions was confirmed to be similar by determination of the refractive index.
Binding of B18R to cells.
B18R produced in the baculovirus system and radiolabeled metabolically with [35S]methionine and [35S]cysteine was incubated with various cell lines, in monolayers or in suspension, that had been either left untreated or treated for 2 h at 37°C with 10 ng of phorbol myristate acetate (PMA)/ml. Confluent cell monolayers in 24-well plates (1 × 105 to 3 × 105 cells) were washed twice with cold PBS containing 10% FBS and incubated with 35S-labeled B18R or B15R, in the absence or presence of unlabeled protein, for 3 h at 4°C. Monolayers were washed six times with 10% FBS in PBS, and cells were dissociated in Laemmli buffer. Binding of recombinant radiolabeled protein to cells in suspension (1.5 × 106 cells) was performed in 200 μl for 2 h at 4°C. Cells were washed four times with 10% FBS in PBS and recovered each time by centrifugation before being dissolved in Laemmli buffer. Samples were analyzed by SDS-PAGE and fluorography. Radioactivity incorporated into proteins that bound to cells was determined by precipitation with TCA and filtration through GF/C filters.
Cell surface and soluble IFN binding assays.
Cultures of TK−143B or Sf cells were infected at 10 PFU/cell in serum-free medium. Supernatants from orthopoxvirus- or baculovirus-infected cells were harvested at 1 or 3 days postinfection and prepared as described previously (26). All binding and competition assays were carried out in duplicate using RPMI medium containing 1% FBS and 20 mM HEPES (pH 7.5). Soluble binding assays with human recombinant 125I-labeled IFN-α2 (100 to 250 pM) were performed by precipitation of the ligand-receptor complexes with polyethylene glycol (PEG) and filtration as described previously (3, 26). Nonspecific binding precipitated with binding medium alone or with excess unlabeled IFN-α2 was subtracted. Affinity constants were determined with the LIGAND program (20). In the binding assays with U937 and THP-1 cells, 100 to 250 pM human 125I-labeled IFN-α2 was added and levels of bound 125I-labeled IFN-α2 were determined by phthalate oil centrifugation as described previously (3, 26). For binding assays to TK−143B cells, cell monolayers were grown to confluence in 6-well plates and infected at 10 PFU/cell with various orthopoxviruses in duplicate. Supernatants from the infected cultures were removed at 12 h postinfection, and cell layers were washed twice with cold binding buffer. Cells were then incubated for 3 h on ice with 0.5 ml of binding buffer containing 1 nM 125I-labeled human IFN-α2, washed twice with cold binding buffer to remove unbound 125I-labeled human IFN-α2, and lysed with 1 ml of 1 M NaOH for 5 min. Radioactivity present in cell lysates was then counted in a gamma counter.
Inhibition of IFN-α-mediated signal transduction by cell surface B18R.
HeLa (Flow) cells were detached by incubation in PBS–1 mM EDTA at 37°C, collected by centrifugation, and resuspended at 5 × 107 cells/ml in MEM containing 10% FBS. Cells were either left untreated or incubated with 106 cell equivalents of supernatants from AcNPV-, AcB18R-, or AcB15R-infected cultures for 1 h at 37°C. Cells were then washed twice with medium, resuspended at 5 × 107 cells/ml in MEM containing 10% FBS at 37°C, and either left unstimulated or stimulated with 500 U of human IFN-α (Wellferon)/ml for 15 min. Cells were then collected by centrifugation and resuspended in 1 ml of lysis buffer containing 50 mM Tris-HCl (pH 8.0), 0.5% (vol/vol) NP-40, 10% (vol/vol) glycerol, 0.1 mM sodium orthovanadate, 1 mM dithiothreitol (DTT), 50 mM NaF, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mg of aprotinin/ml. After 5 min at 4°C, cell lysates were clarified by centrifugation, and the supernatants were stored at −70°C.
Immunoprecipitation and immunoblotting.
Cell lysates were precleared by the addition of 1 μg of rabbit IgG together with 20 μl of protein A-Sepharose CL-4B beads (Sigma) and were then incubated with 1 μg of rabbit anti-JAK1 IgG (Santa Cruz Biotechnology, Inc.) and 20 μl of protein A-Sepharose CL-4B beads overnight at 4°C. Beads were washed three times with lysis buffer and boiled for 3 min in SDS-PAGE sample buffer containing 100 mM DTT. Eluted proteins were analyzed by SDS-PAGE in 7.5% gels and transferred electrophoretically to a nitrocellulose membrane (Hybond-C; Amersham). After blocking overnight at 4°C in PBS containing 5% (wt/vol) low-fat milk and 0.1% (vol/vol) Tween 20, the membrane was probed with a mouse antiphosphotyrosine monoclonal antibody at 2 μg/ml in blocking buffer (4G10; Upstate Biotechnology) for 2 h at room temperature. The membrane was washed three times in PBS containing 0.1% (vol/vol) Tween 20 and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,000 in blocking buffer; Sigma) for 1 h at room temperature. After further washing, bound IgG was detected with an enhanced chemiluminescence kit (ECL; Amersham). To control for equal protein loading, blots were stripped, reprobed with anti-JAK1 antibody, and processed as above.
Nucleotide sequence accession number.
The sequence presented in this paper has been submitted to the EMBL nucleotide sequence database under accession number AJ269556.
RESULTS
Soluble B18R protein secreted from cells binds in a saturable fashion to the cell surface.
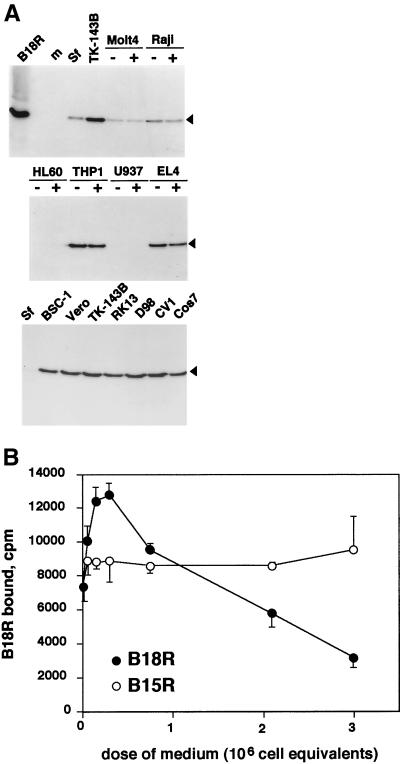
The B18R protein functions as a soluble type I IFN receptor and is secreted from VV-infected cells (8, 26), but evidence for the presence of B18R protein at the cell surface has also been reported (27–29). Morikawa and Ueda (16) suggested that the B18R protein may be anchored at the surfaces of infected cells via the N-terminal signal sequence, thus showing a type 2 membrane protein topology. However, the fact that the B18R protein was secreted in large amounts from VV- and recombinant baculovirus-infected cells suggested the alternative possibility that B18R protein might bind to cells after being secreted into the medium. To test this hypothesis, B18R protein that was secreted from insect cells infected with a recombinant baculovirus (AcB18R) and labeled metabolically with [35S]methionine and [35S]cysteine was used in binding assays with a variety of cells in suspension or monolayer. The SDS-PAGE analysis in Fig. 1A shows that the recombinant B18R protein was the major radiolabeled protein and that the B18R protein bound to most of the cell types tested. Note that it bound very poorly if at all to insect (Sf), U937, and HL-60 cells and that pretreatment of cells with PMA, which induces changes in adhesion properties of the cell surface, did not affect the ability of B18R to bind to cells. Radioactive B18R bound similarly to TK−143B cells that were uninfected or infected with a VV WR mutant lacking the B18R ORF (vΔB18R) (data not shown). In BS-C-1 cell cultures infected at a low multiplicity of infection (MOI), we could detect B18R protein at the cell surface by immunofluorescence with purified anti-B18R Ig (data not shown).
FIG. 1.
B18R binds specifically to cells. (A) B18R protein produced in the baculovirus system and radiolabeled with [35S]methionine (B18R) was incubated with medium (m) or with the indicated cell lines at 4°C for 2 to 3 h as described in Materials and Methods. Cell lines were preincubated (+) or not preincubated (−) with 10 ng of PMA/ml. The B18R protein bound to cells was analyzed by SDS-PAGE and fluorography. The arrowhead indicates the position of the B18R protein. The results shown are representative of three experiments. (B) B18R protein produced in the baculovirus system and radiolabeled with [35S]methionine was incubated with TK−143B cells for 3 h at 4°C in the presence or absence of increasing doses of medium (equivalent to 3 × 106 cells/ml) from insect cell cultures infected with recombinant baculovirus producing B18R or B15R. Cell monolayers were washed extensively, and the level of B18R protein bound to cells (duplicate samples; mean counts per minute ± standard deviation) was determined by TCA precipitation. The results shown are representative of three experiments.
The binding of 35S-labeled B18R to cells was specific and saturable, because it was inhibited by excess unlabeled recombinant B18R but not B15R, the VV soluble IL-1β receptor with a similar three-Ig-domain structure, both produced in the baculovirus system (Fig. 1B). B18R- and B15R-containing supernatants had similar concentrations of recombinant protein, as determined by Scatchard analysis of binding data with specific ligands (3, 26). The amount of recombinant protein secreted per insect cell is approximately 2 × 106 or 6 × 106 binding sites for B18R and B15R, respectively. The increase in 35S-labeled B18R binding in the presence of low doses of unlabeled protein has been observed in several experiments (data not shown) and could not be explained by induction of B18R aggregation at higher B18R protein concentrations (see below).
In an attempt to identify the component at the cell surface that interacts with B18R, cells prelabeled with [35S]methionine and [35S]cysteine were incubated with unlabeled B18R followed by immunoprecipitation with anti-B18R serum. However, no specific bands were coimmunoprecipitated with B18R (data not shown).
Complex formation between B18R and IFN.
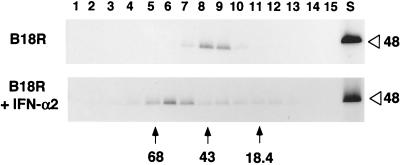
The possible multimerization of B18R protein, before or after binding to IFN, was investigated in two ways. First, the electrophoretic mobility of 35S-labeled B18R produced in the baculovirus system was found to be slightly faster in the absence of reducing agents than in the presence of 2-mercaptoethanol, and no indications of dimeric or oligomeric forms of B18R were found (data not shown). The apparently different molecular size was likely due to the presence of intramolecular disulfide bonds, and this was also observed for the VV B15R protein that also has three Ig domains (data not shown). Second, recombinant 35S-labeled B18R from the baculovirus system was also analyzed by ultracentrifugation in sucrose density gradients. Figure 2 shows that B18R sedimented as a monomer and, after preincubation with an excess of recombinant human IFN-α2, had an increased mobility that was consistent with a 1:1 stoichiometry for the B18R-IFN complex.
FIG. 2.
Complex formation of the B18R protein with IFN-α2. Supernatants from Sf cells infected with AcB18R and labeled metabolically with [35S]methionine and [35S]cysteine were incubated with or without IFN-α2 for 2 h at room temperature and then loaded onto sucrose density gradients (5 to 25% sucrose in PBS) and centrifuged as described in Materials and Methods. The original B18R sample (S) and fractions collected from the bottom of the gradient and precipitated with TCA were analyzed by SDS-PAGE. A fluorograph showing the distribution of radiolabeled B18R incubated or not with IFN-α2 is shown. The positions of the molecular size standards are indicated in kilodaltons. The open arrowhead indicates the position of B18R (48 kDa) in the polyacrylamide gel. The results shown are representative of two experiments.
The possibility that multimers of B18R might be formed at increased protein concentrations, which might account for the increased binding to cells observed at the lowest doses of the competition experiment (Fig. 1B), was also investigated. Preincubation of 35S-labeled B18R with excess unlabeled B18R had no effect on the mobility of the protein in sucrose gradients, suggesting the absence of multimerization under these higher protein concentrations (data not shown).
B18R at the cell surface binds IFN.
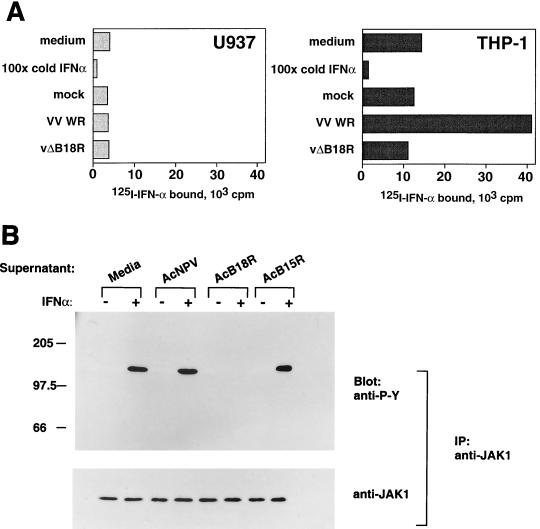
The ability of cell surface B18R to bind type I IFN was tested in binding assays. U937 and THP-1 cells express IFN-α/βRs and accordingly were found to bind 125I-labeled IFN-α2 in a specific manner that was inhibited in the presence of excess IFN-α2 (Fig. 3A). THP-1 cells, but not U937 cells, bind soluble B18R (Fig. 1A). Preincubation of these cells with supernatants from VV WR-infected cultures, but not from uninfected cultures, increased dramatically the ability of THP-1, but not U937, cells to bind IFN-α2 (Fig. 3A). This was due to the presence of B18R, since incubation with supernatants from cultures infected with a VV WR mutant in which the B18R gene has been deleted (vΔB18R) showed no enhancement of the IFN-α2 binding activity in THP-1 cells. These results showed that soluble B18R bound to the cell surface still binds IFN-α2, suggesting that different domains of B18R interacted with the cell and with IFN. Similarly, preincubation of cell cultures with supernatants from VV-infected cells, but not from vΔB18R-infected cells, enhanced sixfold the binding of 125I-labeled IFN-α to human HeLa cells and enabled mouse L929 cells to bind human 125I-labeled IFN-α (data not shown).
FIG. 3.
B18R attached to the cell surface binds IFN-α and prevents activation of IFN-mediated signal transduction. (A) Binding of IFN-α2 to cells coated with B18R. U937 or THP-1 cells (106) were preincubated for 2 h at 4°C with binding medium or supernatants from cultures of TK−143B cells that were mock infected or infected with VV WR or vΔB18R, equivalent to 105 cells. Subsequently, cells were washed twice by centrifugation with binding medium and incubated with 5 nM 125I-labeled IFN-α2 for 2 h at 4°C, and bound IFN levels were determined after centrifugation of cells through phthalate oil. The specificity of the 125I-labeled IFN-α2 binding to cellular receptors was determined in the presence of a 100-fold excess of IFN-α2. (B) Cell surface B18R inhibits signal transduction via the cellular IFN-α receptor. HeLa cells (5 × 107) were incubated with media or supernatants from baculovirus-infected cells, washed to remove soluble proteins, and then incubated with (+) or without (−) 500 U of human IFN-α/ml for 15 min at 37°C. Whole-cell extracts were prepared and immunoprecipitated with anti-JAK1 antiserum. Precipitated proteins were separated by SDS-PAGE (7.5% gel), transferred to nitrocellulose, and probed with the antiphosphotyrosine antibody 4G10 (anti-P-Y) (top panel). As a control for JAK1 expression, blots were stripped and reprobed with anti-JAK1 antibody (bottom panel). The positions of molecular size standards are indicated in kilodaltons.
Signal transduction by IFN is inhibited by B18R at the cell surface.
To determine whether binding of IFN-α2 to the B18R protein on the cell surface was able to block signal transduction mediated by binding of IFN-α to the cellular receptor, the IFN-induced phosphorylation of JAK1 was analyzed (Fig. 3B). HeLa cells were incubated in medium alone or with supernatants from wild-type baculovirus (AcNPV)-, AcB18R-, or AcB15R-infected cells. After washing to remove soluble protein, cells were incubated in medium or stimulated with IFN-α, and the phosphorylation status of JAK1 was assessed by immunoprecipitation of JAK1 followed by SDS-PAGE and immunoblotting with antiphosphotyrosine. Cells incubated without IFN did not demonstrate phosphorylation of JAK1. After 15 min of stimulation with IFN-α, cells incubated with medium, AcNPV, or AcB15R contained phosphorylated JAK1. However, IFN-α2-induced phosphorylation of JAK1 was inhibited completely when cells were preincubated with AcB18R. To confirm that the samples contained equivalent amounts of JAK1, the blots were stripped and reprobed with anti-JAK1 antibody.
Expression of surface B18R by orthopoxviruses.
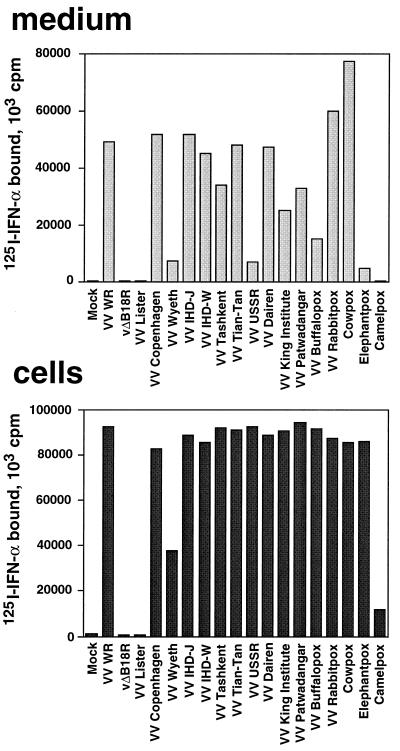
B18R protein was identified at the surface of VV-infected cells by immunofluorescence with specific antibodies (27, 29). Type I IFN binding activity was also reported to be encoded by B18R and to be secreted from cells infected with a number of orthopoxviruses (26). To determine whether orthopoxvirus-infected cells expressed IFN binding activity at the cell surface, a binding assay with 125I-labeled IFN-α2 was performed. Figure 4 shows a dramatic increase in IFN-α2 binding activity at the surfaces of cells infected with 14 VV strains (including rabbitpox and buffalopox), two strains of cowpox virus (Brighton red and elephantpox), and camelpox virus. No enhanced binding activity was detected in cells infected with a VV strain known not to express B18R (Lister) or with a VV WR lacking the B18R gene (vΔB18R). Binding activity for 125I-labeled IFN-α2 was secreted from cultures infected with all viruses tested, except with VV Lister and vΔB18R, as described previously (26). The low soluble binding activity expressed by camelpox virus in this experiment has been reported previously (26). Note that whereas the level of IFN binding activity in the supernatants varied among viruses, the IFN binding activity at the cell surface was consistently high for most of them. This suggested that the B18R protein secreted from cells is targeted primarily to the cell surface, and that soluble B18R may represent excess B18R protein secreted from these cultures once the majority of cell surface binding sites are occupied.
FIG. 4.
Expression of IFN binding activity at the surfaces of cells infected with orthopoxviruses. TK−143B cells were mock infected or infected at 1 PFU/cell with the indicated orthopoxviruses. At 12 h postinfection, cell monolayers were washed twice in binding medium and incubated for 2 h at 4°C with 1 nM 125I-labeled IFN-α2. Cells were washed twice with binding medium and lysed with 0.1% SDS, and the radioactivity bound to cells was determined (cells). The production of viral IFN-α/βRs secreted from the infected cultures was determined in a soluble binding assay with 125I-labeled IFN-α2 by precipitating ligand-receptor complexes with PEG and filtration through GF/C filters (medium).
IFN binding properties of B18R expressed at the cell surface.
To characterize further the IFN binding properties of the B18R protein at the cell surface, binding assays with human 125I-labeled IFN-α2 were performed on mouse L929 cells infected with different VVs. The cellular IFN receptors are highly species specific, and in these experiments human IFN will bind to the B18R protein but not to the mouse IFN receptor expressed by L929 cells.
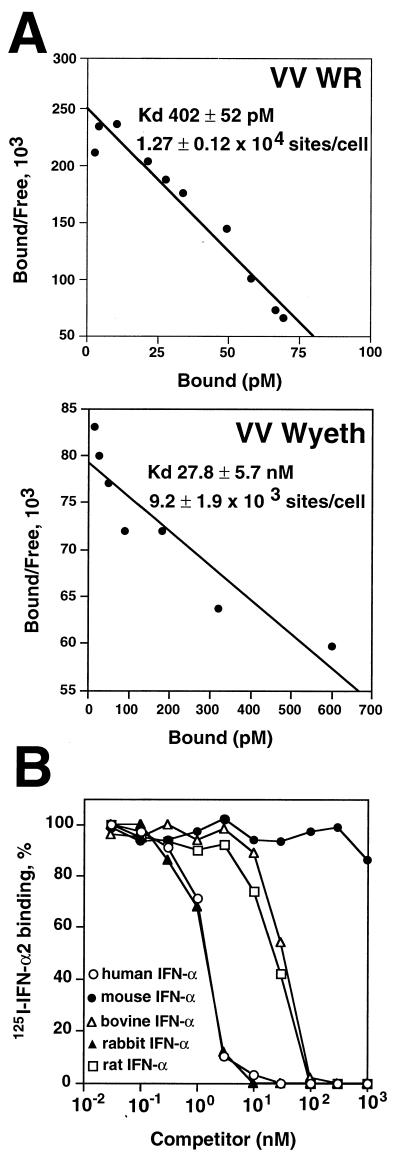
Scatchard analysis of saturation binding assays with increased doses of 125I-labeled IFN-α2 (Fig. 5A) indicated that VV WR-infected cells express large numbers of IFN binding sites at the cell surface (1.27 × 104 ± 0.12 × 104 sites per cell), similar to the number of IFN binding sites secreted from VV WR-infected cells (1.13 × 104 ± 0.05 × 104 sites per cell) (26). The affinity of membrane-bound B18R for IFN-α2 was high (Kd, 402 ± 52 pM) but slightly lower than that reported for the binding of human IFN-α2 to soluble B18R (Kd, 174 ± 15 pM) (26). The soluble B18R protein secreted from cells infected with VV Wyeth (1.5 × 104 ± 0.08 × 104 sites/cell) was reported to have a 85-fold lower affinity for human IFN-α2 (Kd, 13.5 ± 0.83 nM) (26). Nonetheless, we found that the VV Wyeth B18R protein is still able to bind to cells (9.2 × 103 ± 1.9 × 103 sites/cell), where it displays an affinity for IFN-α2 similar to that of the secreted form of this protein (Kd, 27.8 ± 5.7 nM) (Fig. 5A).
FIG. 5.
IFN binding properties of the B18R protein at the cell surface. (A) Scatchard analysis of binding of human 125I-labeled IFN-α2 to cell surface B18R. Mouse L929 cells (107) were infected with either VV strain WR or VV strain Wyeth at 10 PFU/cell for 12 h and then were incubated in 0.2 ml with different concentrations of 125I-labeled human IFN-α2. Specific binding was determined after centrifugation of cells through phthalate oil. Data were converted to the Scatchard coordinate system. (B) Species specificity of cell surface B18R binding; cross-competition of human 125I-labeled IFN-α2 binding to L929 cells infected with VV WR. 125I-labeled IFN-α2 (10 nM) was incubated with L929 cells (2 × 106) infected with VV strain WR at 10 PFU/cell for 12 h together with increasing concentrations of cold human recombinant IFN-α2 (open circles), mouse natural IFN-α (solid circles), bovine recombinant IFN-α1 (open triangles), rabbit natural type I IFN (solid triangles), and rat natural type I IFN (open squares) for 3 h at 4°C. 125I-labeled IFN-α2 that had bound to the cell surface was separated from free ligand by centrifugation through phthalate oil. The level of binding in the absence of competitor was 17,936 ± 186 cpm.
The B18R protein secreted from infected cells binds type I IFN from different species (26). The relative binding affinities of cell surface B18R for type I IFN from different species were compared by competition binding of unlabeled IFNs with human 125I-labeled IFN-α2. Figure 5B shows that the B18R expressed at the cell surface retained the broad species specificity described for the secreted protein, binding IFN from the rat, rabbit, cow, and, at much lower affinity, mouse.
Expression of B18R by VV strains WR and Wyeth and by cowpox virus.
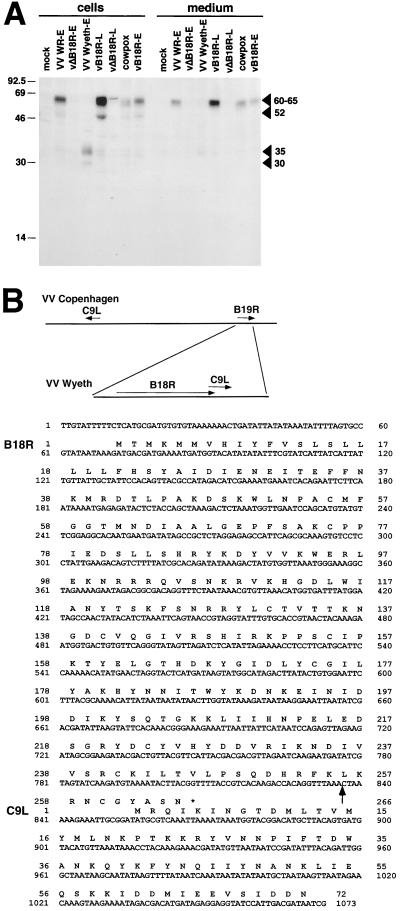
The expression of the B18R protein by VV strains WR and Wyeth and by cowpox virus was investigated by immunoprecipitation with B18R-specific antibodies of infected cells radiolabeled metabolically at early times of infection. Figure 6A shows that the B18R protein was detected in supernatants (60 to 65 kDa) and cell extracts (52 kDa and 60 to 65 kDa) from cultures infected with VV WR and Lister. As controls, a VV WR mutant containing a second copy of the B18R gene under the control of the late VV 4b promoter (vB18R) produced larger amounts of B18R at late times of infection, whereas a VV WR mutant lacking the B18R gene (vΔB18R) did not produce the B18R protein (3). The cowpox virus B18R protein had a similar molecular size, but, notably, the B18R protein from VV Wyeth showed a smaller molecular size (30 and 35 kDa) and was secreted less efficiently from infected cells as a 35-kDa protein. The fact that some was secreted suggested that the N-terminal signal sequence was present and that the C terminus might be missing.
FIG. 6.
Characterization of B18R encoded by VV Wyeth. (A) Identification of the B18R protein by immunoprecipitation. TK−143B cells were mock infected or infected with the indicated viruses and pulse-labeled with 35S-labeled Promix and [35S]cysteine from 1 to 5 h postinfection (early [E]) or from 4 to 8 h postinfection (late [L]). Cells and media were dissociated with RIPA buffer and immunoprecipitated with anti-B18R Ig. Immunoprecipitates were analyzed by SDS-PAGE and fluorography. The position of B18R is indicated by arrowheads. Molecular sizes in kilodaltons are shown on the left. The results shown are representative of two experiments. (B) Nucleotide and amino acid sequences of the B18R ORF from VV Wyeth. The diagram shows the location of ORFs B19R (B18R in VV WR) and C9L in the genome of VV Copenhagen, and the location of these ORFs in VV Wyeth. The arrow after nucleotide 836 in the DNA sequence marks the site where the 3′ region of the C9L ORF was inserted into the B18R ORF in VV Wyeth.
Sequence of the B18R gene encoded by VV Wyeth.
The immunoprecipitation experiment (Fig. 6A) indicated that the B18R protein encoded by VV Wyeth was approximately 35 kDa, smaller than the 60- to 65-kDa protein made by VV WR (3). This suggested that the Wyeth B18R ORF might be truncated. PCR analysis with oligonucleotides hybridizing to different locations of the B18R ORF suggested a deletion of the C-terminal region (data not shown). To determine the exact basis for the altered VV Wyeth B18R protein, the nucleotide sequence of the Wyeth B18R ORF was determined. This was achieved by a combination of direct sequencing of virus genomic DNA with specific oligonucleotide primers, and PCR amplification of the ORF using specific oligonucleotides and virus DNA as the template. Sequence analysis showed that the B18R protein was mutated after amino acid L256 between the second and third Ig domain and truncated because of a premature stop codon (Fig. 6B). The truncated B18R ORF in VV Wyeth showed a deletion of two amino acids in the signal peptide and two conservative amino acid substitutions compared with the VV WR ORF. The sequence immediately downstream of this region matched perfectly with the C-terminal region of the VV Copenhagen C9L ORF from the left end of the virus genome. This indicated that a terminal transposition event had occurred downstream of amino acid 246 of the VV Wyeth B18R ORF that had truncated the B18R ORF (named B19R in the Copenhagen strain).
B18R enhances replication of VV in tissue culture in the presence of IFN.
The antiviral effects of type I IFN on VV WR replication are relatively low compared to those for other viruses due to the expression of the intracellular VV proteins E3L and K3L. It was found that to block VV replication by approximately 95%, it was necessary to preincubate monolayers of human TK−143B and FS2 cells, monkey BSC-1 cells, and rabbit RK13 cells for 24 h with 100 to 500 U of IFN of the appropriate species per ml (data not shown).
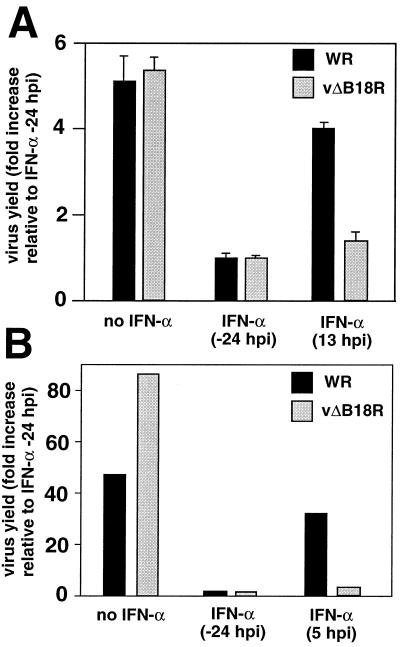
To determine the role of B18R in protecting VV from the antiviral effects mediated by type I IFN, the replication of wild-type VV WR or the B18R deletion mutant (vΔB18R) was determined in cell cultures treated with IFN. Figure 7 shows that both VV WR and vΔB18R replicated in human primary FS2 cells and rabbit RK13 cells in the absence of IFN. Preincubation of FS2 or RK13 cell monolayers for 24 h with human or rabbit natural IFN-α, respectively, induced an antiviral state that restricted the replication of both viruses. However, when IFN-α was added a few hours after virus infection, mimicking the induction of IFN following virus infection in an animal host, the expression of B18R conferred resistance to IFN in culture. Under these conditions, wild-type VV WR, but not vΔB18R, was able to replicate to levels similar to those observed in the absence of IFN.
FIG. 7.
Replication of VV in tissue culture in the presence of IFN. Cultures of human FS2 cells (A) or rabbit RK13 cells (B) were infected with 200 PFU of VV WR or vΔB18R/well, and virus production was determined 48 h postinfection (hpi). Cultures were also treated with 500 U of human natural IFN-α (FS2 cells) or rabbit type I IFN (RK13 cells), added 24 h before infection or at the indicted time after infection. Virus yield is expressed as the fold increase relative to the virus production in cultures pretreated with IFN (duplicate samples, means ± standard deviations in the case of FS2 cells). The results shown are representative of two experiments.
VV replication in RK13 cells produces larger amounts of extracellular virus, and secondary plaques form, giving rise to comet-shaped virus plaques. This effect is seen most clearly with the VV strain IHD-J but can also be detected to a lesser extent with the WR strain. Comet formation by VV WR and vΔB18R was inhibited in cells preincubated with IFN. However, addition of IFN 11 h postinfection prevented comet formation with vΔB18R but not with wild-type VV WR (data not shown). This experiment illustrated that expression of the B18R protein during the first 11 h of infection enhanced replication of VV in cell monolayers in the presence of IFN, resulting in increased spread of VV.
Addition of soluble B18R is sufficient to protect cell monolayers from the antiviral effects of IFN.
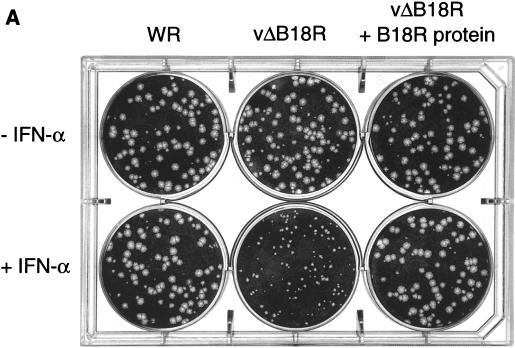
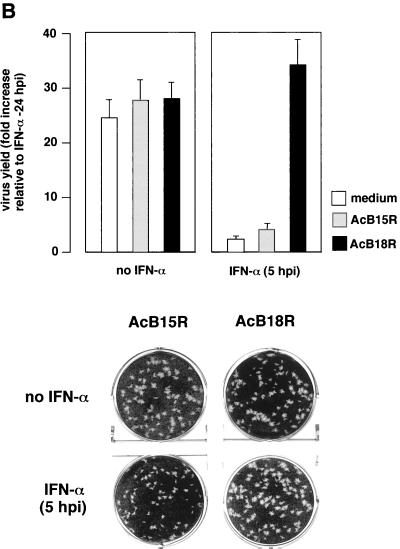
To determine whether the protective effect was mediated by soluble or cell surface B18R protein, we examined VV plaque formation in BSC-1 cells (Fig. 8A). Preincubation of BSC-1 cell monolayers with IFN prevented the development of the normal plaque size by both VV WR and vΔB18R, indicating inhibition of VV replication and/or spread (data not shown). However, addition of IFN at 4 h postinfection allowed sufficient B18R expression to restore normal replication of wild-type VV WR but not of vΔB18R. The IFN-induced restriction of vΔB18R replication was overcome if cultures were incubated first with baculovirus-produced recombinant B18R, followed by washing to remove excess soluble B18R protein.
FIG. 8.
Treatment of cell monolayers with recombinant B18R protein blocks the antiviral effects mediated by IFN. (A) Confluent BSC-1 cells were infected with approximately 100 PFU of VV strain WR or vΔB18R for 2 h at 37°C. After removal of the virus inoculum, cells were overlaid with either medium or medium containing 106 cell equivalents of supernatant from AcB18R-infected cells and were incubated at 37°C for 4 h. Cell monolayers were then washed three times with medium to remove soluble proteins and were then overlaid with medium containing 1.5% (wt/vol carboxymethyl cellulose with (+IFN) or without (−IFN) 100 U of human IFN-α (Wellferon)/ml. After incubation for 48 h, cells were stained with 0.1% (wt/vol) crystal violet in 15% ethanol. (B) Virus production and plaque formation in FS2 cells. Human FS2 cells were left untreated or treated with 100 U of human natural IFN-α/ml 24 h before infection with vΔB18R. At 3 h postinfection, cultures were also incubated with medium from an equivalent number of insect cell cultures infected with AcB15R or AcB18R expressing high levels of recombinant B15R or B18R proteins, respectively. Cell monolayers were washed twice with medium, and the infection was continued in the presence of IFN-α (100 U/ml). At 48 h postinfection, virus levels in infected cells were determined by plaque assay, and the virus plaques were stained with 0.1% (wt/vol) crystal violet in 15% ethanol. Virus yield is expressed as the fold increase (triplicate samples, means ± standard deviations) relative to the virus production in cultures pretreated with IFN.
The role of cell surface B18R in protecting cells from IFN effects was also investigated in a more quantitative way in FS2 cells by determining both plaque size and virus production. Figure 8B shows that addition of IFN to FS2 cell monolayers restricted vΔB18R replication and plaque formation. Preincubation of the cell monolayer with recombinant soluble B18R, but not B15R, followed by washing of the excess protein not bound to the cell surface, enabled replication of vΔB18R and production of plaques of normal sizes. These experiments suggested that soluble B18R protein bound to the cell surface is sufficient to protect cell monolayers from the effects of IFN.
DISCUSSION
VV encodes a soluble IFN-α/βR that is secreted from infected cells and blocks the activity of type I IFN. Because VV encodes two intracellular proteins that confer protection from the antiviral effects of type I IFN, the expression of a soluble IFN inhibitor by VV was unexpected. It is likely that the intracellular and extracellular inhibitors of IFN encoded by VV interfere with the function of IFNs at different levels, but the precise roles of these IFN inhibitors during VV replication have not been defined. In this report we propose a unique mechanism by which the VV IFN-α/βR blocks IFN activity for the benefit of the virus.
The VV B18R protein functions as an IFNR but has several properties that are distinct from those of the cellular counterpart. First, the B18R protein has three Ig domains, whereas the cellular type I IFNRs have fibronectin type III domains (24). Second, in contrast to the highly species-specific cellular IFNRs, the B18R protein binds IFNs from a variety of species, including rat, rabbit, and human IFNs (26). Third, the interaction of monoclonal antibodies with different regions of IFN shows that the cellular IFNRs interact with the C terminus of IFN, whereas the B18R protein interacts with both the N and C termini of IFN (15). Here we show another unique property of B18R, its ability to bind to the cell surface in order to protect cells from the antiviral effects of IFNs.
Morikawa and Ueda proposed that the B18R protein is anchored at the cell membrane by its signal peptide (16). These authors used antisera raised against the N terminus or the C terminus of B18R and found that the N-terminal region was not detected when B18R was present at the cell surface. They proposed that the signal peptide was recognized by the specific anti-N terminus antiserum in the cytoplasm but not when the B18R was anchored at the plasma membrane. However, it is well established that signal peptides are inserted into the endoplasmic reticulum membrane before the full polypeptide chain is synthesized, and thus accessibility to the signal peptide should be similar both inside the cell and at the plasma membrane. Moreover, the antiserum specific for the N terminus of B18R described by Morikawa and Ueda was raised against the N-terminal 141 amino acids of the B18R polypeptide (16), and this includes most of the first Ig domain of B18R (amino acids 53 to 149) (24). The results reported by Morikawa and Ueda may suggest that the N-terminal Ig domain of B18R is masked when the protein is found at the cell surface. This would be consistent with our hypothesis that the N-terminal region of B18R may be involved in interaction with the cell surface, based on the expression of a truncated B18R in the VV Wyeth strain (see below).
The mechanism proposed by Morikawa and Ueda would explain the presence of B18R at the surface of infected cells, but not in solution or on the surface of uninfected cells. In contrast, the mechanism we propose would enable B18R to be expressed at the surface of both infected and uninfected cells, suggesting a different mechanism for the anti-IFN action of B18R. We show here that the B18R protein bound to the cell surface, like the soluble form, retains high affinity for IFN and a broad IFN species specificity.
The ability of B18R to block the antiviral effects of type I IFN had been tested on the replication of vesicular stomatitis virus or Cocal viruses but not VV (8, 26). The role of B18R in protecting VV from the IFN is relevant because VV encodes other proteins that protect the virus from the antiviral effects of IFN. We have addressed the role in vitro by mimicking the situation in vivo, where IFNs are produced after virus replication is initiated. Under these conditions, expression of B18R enabled replication of VV in the presence of IFN and virus spread in cell cultures. We also show that B18R expressed at the cell surface is sufficient to confer protection against the antiviral effects of IFN.
It is not known which Ig domains of the B18R protein contribute to IFN or cell surface binding. However, a C-terminal-truncated B18R molecule expressed by VV Wyeth has a lower affinity for human IFN-α2 but retains its ability to bind to the cell surface. This suggests that the N-terminal domain is involved in binding to cells, whereas the C terminus contributes to IFN binding. Colamonici et al. (8) showed that some amino acid sequence similarity exists between cellular type I IFNRs and B18R, which was mainly located in the first and third Ig domains of the predicted B18R polypeptide. Expression of recombinant versions of B18R Ig domains or truncated versions of the protein may identify in more detail the function of the domains. The results we obtained after ultracentrifugation in sucrose density gradients of B18R protein complexed or not with human IFN-α2 are consistent with a 1:1 stoichiometry for the B18R-IFN complex.
High levels of IFN binding activity were expressed at the cell surface in most orthopoxvirus-infected cells, whereas the amount present in the medium varied. This suggests that B18R is targeted first to the cell surface, and the presence of protein in the medium may represent excess protein that did not bind to cells. It is possible that the most important site of action for B18R is at the cell surface. The expression of the B18R protein at early times during infection and in relatively small amounts would be consistent with this model. By contrast, some other VV cytokine receptors, such as the IL-1β receptor, target systemic effects of the cytokine and are produced in larger amounts and synthesized later during infection (2, 3). Expression of relatively small amounts of B18R at early times of infection that remain in the area where VV replicates would be sufficient to protect the tissues from the antiviral effects of type I IFN so that VV could replicate to normal levels. This is consistent with pathogenesis studies in vivo showing restricted replication of vΔB18R in the tissues of infected mice (26).
All the cytokine receptors or binding proteins identified so far in poxviruses are secreted. The only exception is the expression of increased TNF receptor activity at the surfaces of cells infected with VV strains Lister, USSR, and Evans, but the gene has not been identified yet (1).
The best, and probably the only, way to express virus proteins at the surfaces of surrounding uninfected cells is to secrete a protein that then binds to cells. To our knowledge, this is the first time that such a mechanism has been described for a virus. Perhaps the B18R protein is designed to function at the cell surface, and the main reason to secrete the protein is to transport it from infected cells to uninfected cells.
ACKNOWLEDGMENTS
This work was supported by The Wellcome Trust.
We thank Caroline Gubser for determining the sequence of the 3′ end of the Wyeth B18R gene.
REFERENCES
- 1.Alcamí A, Khanna A, Paul N L, Smith G L. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80:949–959. doi: 10.1099/0022-1317-80-4-949. [DOI] [PubMed] [Google Scholar]
- 2.Alcamí A, Smith G L. A mechanism for the inhibition of fever by a virus. Proc Natl Acad Sci USA. 1996;93:11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcamí A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 4.Alcamí A, Smith G L. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie E, Tartaglia J, Paoletti E. Vaccinia-virus encoded eIF-2α homolog abrogates the antiviral effect of interferon. Virology. 1991;183:419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- 6.Biron C A. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 7.Chang H-W, Watson J C, Jacobs B L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1992;89:4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colamonici O R, Domanski P, Sweitzer S M, Larner A, Buller R M L. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- 9.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc London B. 1957;147:258–267. [PubMed] [Google Scholar]
- 13.Karupiah G, Fredrickson T N, Holmes K L, Khairallah L H, Buller R M L. Importance of interferons in recovery from mousepox. J Virol. 1993;67:4214–4226. doi: 10.1128/jvi.67.7.4214-4226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karupiah G, Xie Q, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 15.Liptáková H, Kontseková E, Alcamí A, Smith G L, Kontsek P. Analysis of an interaction between the soluble vaccinia virus-coded type I interferon (IFN)-receptor and human IFN-alpha 1 and IFN-alpha 2. Virology. 1997;232:86–90. doi: 10.1006/viro.1997.8527. [DOI] [PubMed] [Google Scholar]
- 16.Morikawa S, Ueda Y. Characterization of vaccinia surface antigen expressed by recombinant baculovirus. Virology. 1993;193:753–761. doi: 10.1006/viro.1993.1184. [DOI] [PubMed] [Google Scholar]
- 17.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. New York, N.Y: Lippincott Raven Press; 1996. pp. 2637–2671. [Google Scholar]
- 18.Mossman K, Upton C, Buller R M L, McFadden G. Species specificity of ectromelia virus and vaccinia virus interferon-γ binding proteins. Virology. 1995;208:762–769. doi: 10.1006/viro.1995.1208. [DOI] [PubMed] [Google Scholar]
- 19.Müller U, Steinhoff U, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defence. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 20.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 21.Pestka S, Langer J A, Zoon K C, Samuel C E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez J R, Rodriguez D, Esteban M. Interferon treatment inhibits early events in vaccinia virus gene expression in infected mice. Virology. 1991;185:929–933. doi: 10.1016/0042-6822(91)90575-v. [DOI] [PubMed] [Google Scholar]
- 23.Samuel C E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 24.Smith G L, Chan Y S. Two vaccinia virus proteins structurally related to interleukin-1 receptor and the immunoglobulin superfamily. J Gen Virol. 1991;72:511–518. doi: 10.1099/0022-1317-72-3-511. [DOI] [PubMed] [Google Scholar]
- 25.Smith G L, Symons J A, Alcami A. Poxviruses: interfering with interferon. Semin Virol. 1998;8:409–418. [Google Scholar]
- 26.Symons J A, Alcamí A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 27.Ueda Y, Ito M, Tagaya I. A specific surface antigen induced by poxvirus. Virology. 1969;38:180–182. doi: 10.1016/0042-6822(69)90141-x. [DOI] [PubMed] [Google Scholar]
- 28.Ueda Y, Morikawa S, Matsuura Y. Identification and nucleotide sequence of the gene encoding a surface antigen induced by vaccinia virus. Virology. 1990;177:588–594. doi: 10.1016/0042-6822(90)90524-u. [DOI] [PubMed] [Google Scholar]
- 29.Ueda Y, Tagaya I, Amano H, Ito M. Studies on the early antigens induced by vaccinia virus. Virology. 1972;49:794–800. doi: 10.1016/0042-6822(72)90535-1. [DOI] [PubMed] [Google Scholar]