Abstract
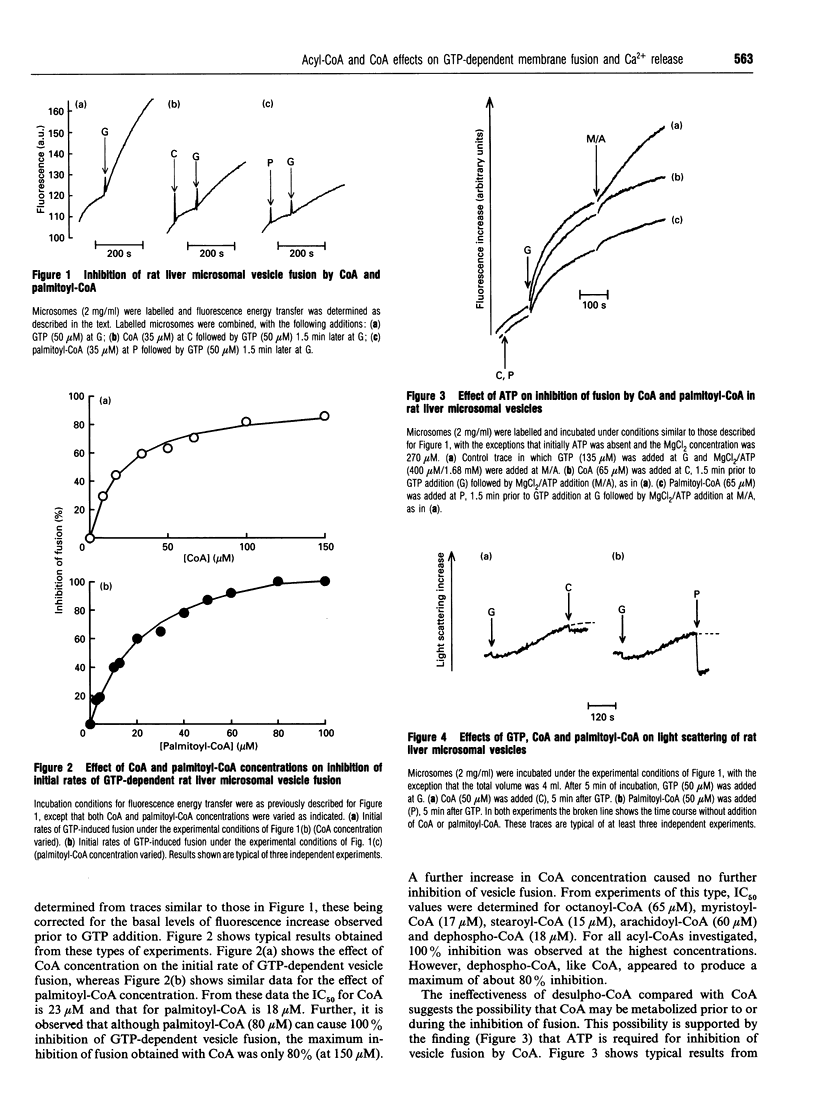
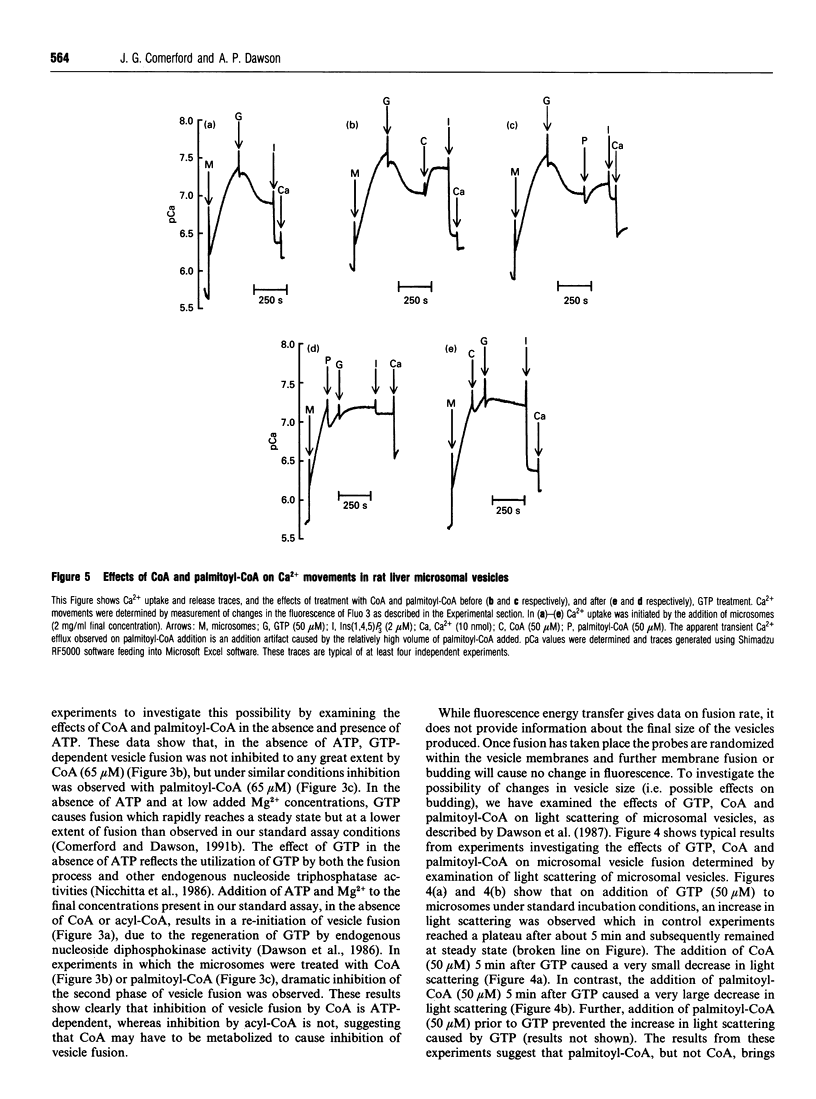
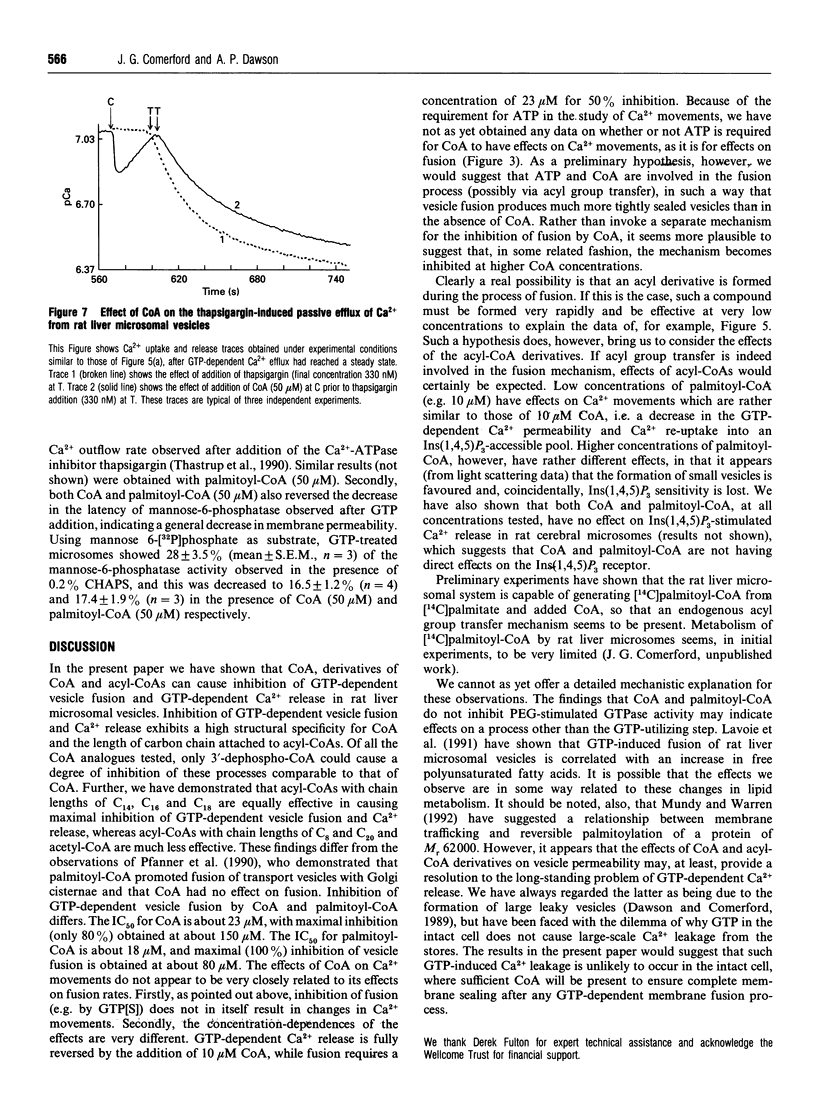
(1) CoA (IC50 23 microM) and acyl-CoAs (IC50 values 15-18 microM) inhibit GTP-dependent vesicle fusion in rat liver microsomal vesicles. Acyl-CoAs of carbon chain length C8 and C20 are much less effective than acyl-CoAs of carbon chain length C14-C18. The effect of CoA is mimicked by dephospho-CoA, but not by desulpho-CoA. High acyl-CoA concentrations (50 microM) appear to favour formation of small vesicles (budding), while 50 microM CoA does not. (2) Low concentrations of CoA (EC50 2 microM) and palmitoyl-CoA (10 microM) cause re-accumulation of Ca2+ released in response to GTP. This re-accumulation is into an Ins(1,4,5)P3-sensitive compartment. By investigation of the effects of CoA and palmitoyl-CoA on the thapsigargin-induced passive leak rate of Ca2+, and on the latency of the mannose-6-phosphatase of the vesicles, we conclude that CoA and palmitoyl-CoA cause decreased vesicle permeability rather than stimulation of Ca2+ pumping activity. (3) It is suggested that GTP-induced membrane fusion in rat liver microsomes involves an as yet uncharacterized acylation-deacylation reaction which is required to produce complete vesicle sealing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Wallin B. K., Carlson P. W., Lange A. J. The specificity of glucose 6-phosphatase of intact liver microsomes. J Biol Chem. 1972 Apr 25;247(8):2558–2565. [PubMed] [Google Scholar]
- Bhullar R. P., Haslam R. J. Detection of 23-27 kDa GTP-binding proteins in platelets and other cells. Biochem J. 1987 Jul 15;245(2):617–620. doi: 10.1042/bj2450617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. Fluoroaluminate treatment of rat liver microsomes inhibits GTP-dependent vesicle fusion. Biochem J. 1991 Dec 1;280(Pt 2):335–340. doi: 10.1042/bj2800335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. The effect of limited proteolysis on GTP-dependent Ca2+ efflux and GTP-dependent fusion in rat liver microsomal vesicles. Biochem J. 1989 Mar 15;258(3):823–829. doi: 10.1042/bj2580823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Measurement of vesicle fusion by fluorescence energy transfer. Biochem J. 1988 Jan 1;249(1):89–93. doi: 10.1042/bj2490089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Comerford J. G. Effects of GTP on Ca2+ movements across endoplasmic reticulum membranes. Cell Calcium. 1989 Jul;10(5):343–350. doi: 10.1016/0143-4160(89)90060-2. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Comerford J. G., Fulton D. V. The effect of GTP on inositol 1,4,5-trisphosphate-stimulated Ca2+ efflux from a rat liver microsomal fraction. Is a GTP-dependent protein phosphorylation involved? Biochem J. 1986 Mar 1;234(2):311–315. doi: 10.1042/bj2340311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P. GTP enhances inositol trisphosphate-stimulated Ca2+ release from rat liver microsomes. FEBS Lett. 1985 Jun 3;185(1):147–150. doi: 10.1016/0014-5793(85)80759-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Hills G., Comerford J. G. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Biochem J. 1987 May 15;244(1):87–92. doi: 10.1042/bj2440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Glick B. S., Rothman J. E. Possible role for fatty acyl-coenzyme A in intracellular protein transport. Nature. 1987 Mar 19;326(6110):309–312. doi: 10.1038/326309a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavoie C., Jolicoeur M., Paiement J. Accumulation of polyunsaturated free fatty acids coincident with the fusion of rough endoplasmic reticulum membranes. Biochim Biophys Acta. 1991 Nov 18;1070(1):274–278. doi: 10.1016/0005-2736(91)90175-8. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Mundy D. I., Warren G. Mitosis and inhibition of intracellular transport stimulate palmitoylation of a 62-kD protein. J Cell Biol. 1992 Jan;116(1):135–146. doi: 10.1083/jcb.116.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Joseph S. K., Williamson J. R. GTP-mediated Ca2+ release in rough endoplasmic reticulum. Correlation with a GTP-sensitive increase in membrane permeability. Biochem J. 1987 Dec 15;248(3):741–747. doi: 10.1042/bj2480741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Joseph S. K., Williamson J. R. Polyethylene glycol-stimulated microsomal GTP hydrolysis. Relationship to GTP-mediated Ca2+ release. FEBS Lett. 1986 Dec 15;209(2):243–248. doi: 10.1016/0014-5793(86)81120-6. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Glick B. S., Arden S. R., Rothman J. E. Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J Cell Biol. 1990 Apr;110(4):955–961. doi: 10.1083/jcb.110.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Orci L., Glick B. S., Amherdt M., Arden S. R., Malhotra V., Rothman J. E. Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell. 1989 Oct 6;59(1):95–102. doi: 10.1016/0092-8674(89)90872-6. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]


