Abstract
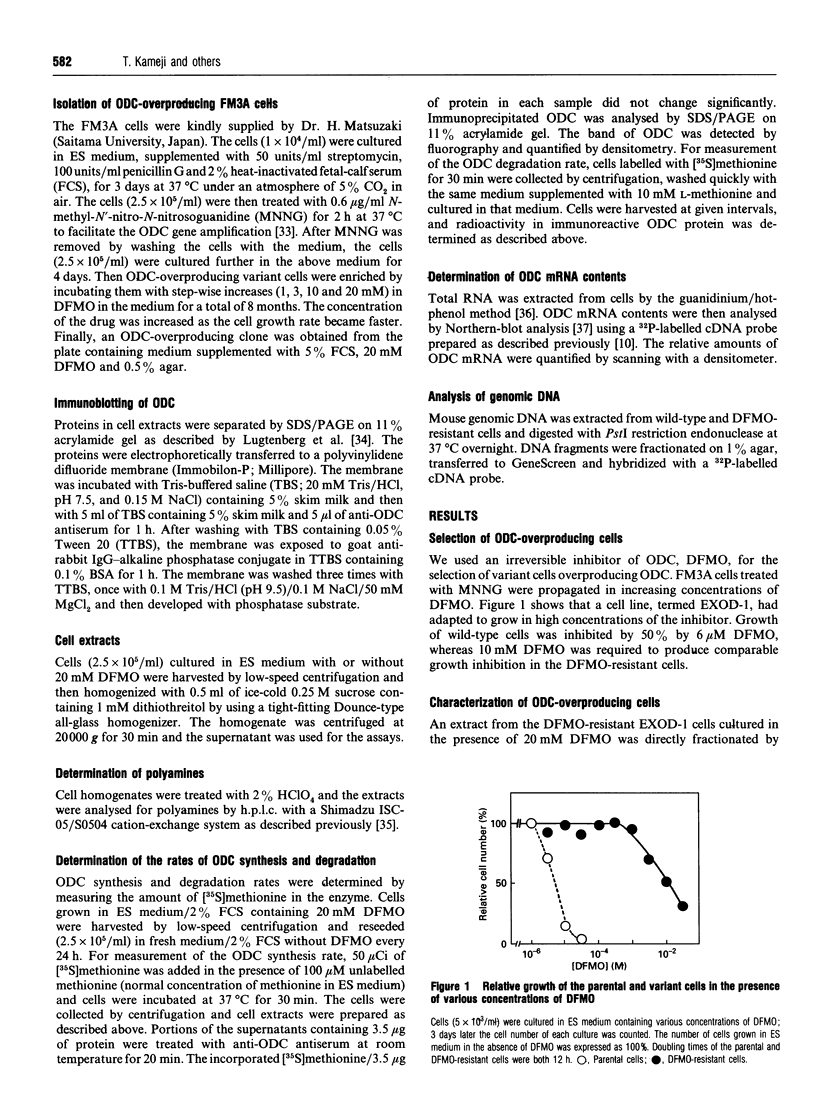
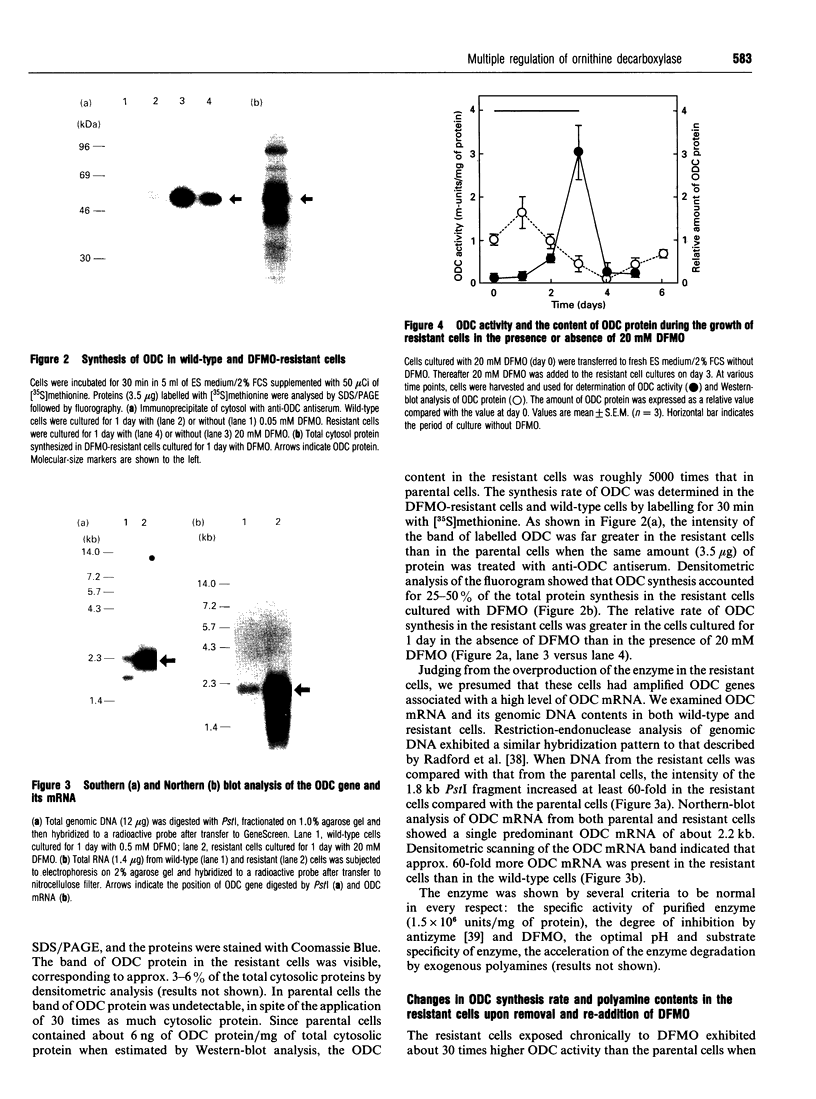
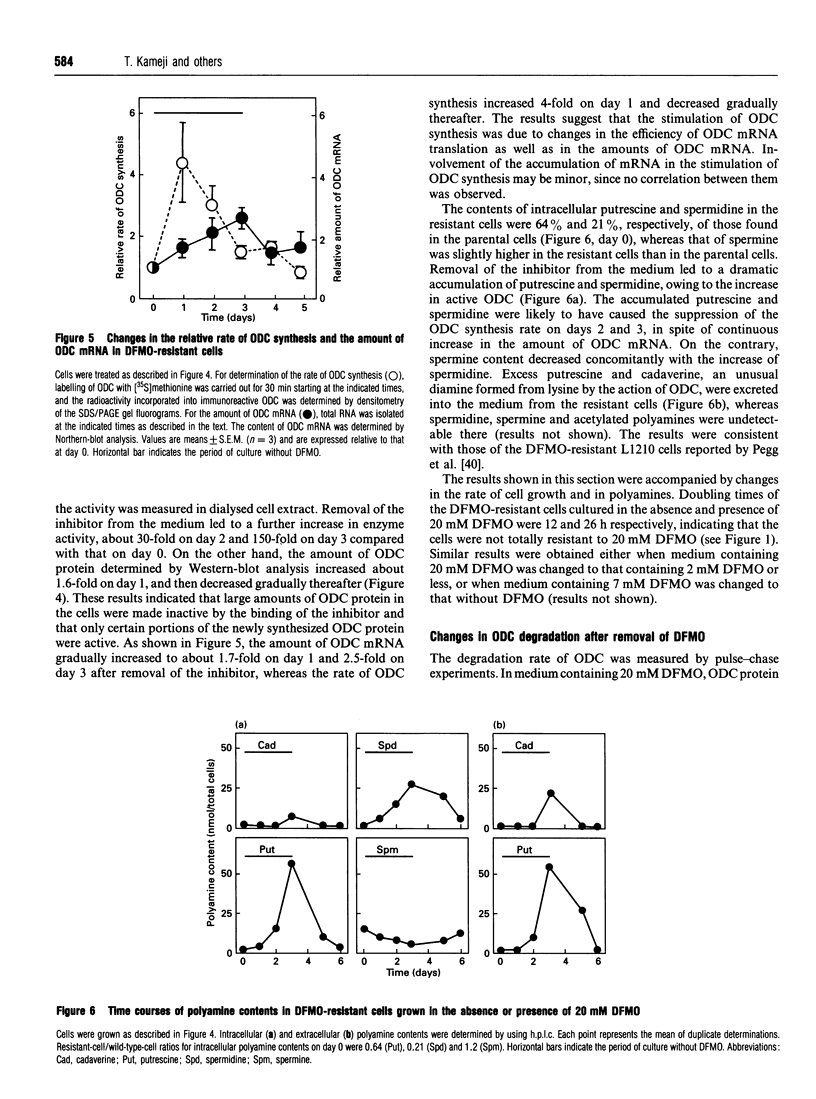
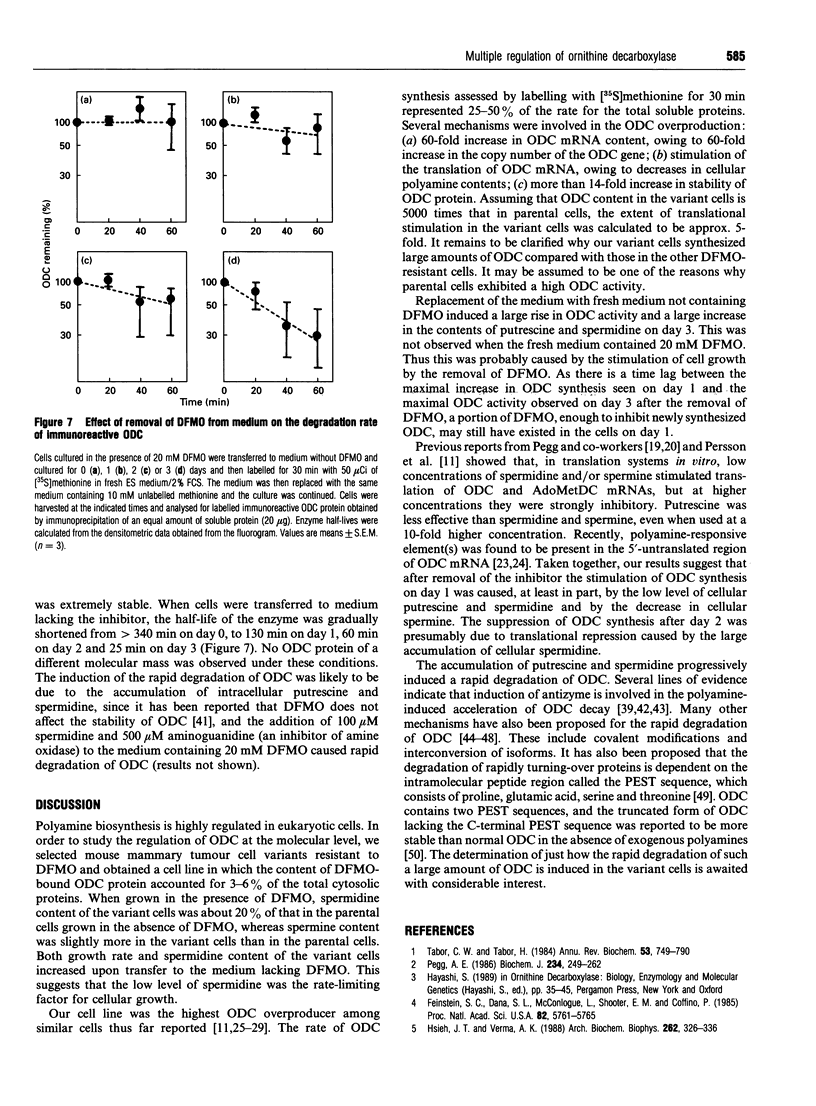
We have isolated from mouse FM3A cells a variant cell line, termed EXOD-1, that overproduces ornithine decarboxylase (ODC). The cells were resistant to alpha-difluoromethylornithine, an irreversible inhibitor of the enzyme, and produced the enzyme protein to the extent of approx. 3-6% of total cytosolic protein. The rate of ODC synthesis in this cell line accounted for 25-50% of the rate of total protein synthesis. The amounts of the ODC gene and its mRNA in the variant cells were both about 60 times as much as those in wild-type FM3A cells. Upon removal of the inhibitor, the growth of the ODC-overproducing cells was stimulated approx. 2-fold. Under these conditions, the rate of ODC synthesis increased about 4-fold on day 1 and then decreased to near the original level by day 3. The amount of ODC mRNA increased about 1.7-fold on day 1 and 2.5-fold on day 3. No correlation was observed between changes in ODC synthesis rate and in ODC mRNA content, suggesting a translational repression of ODC mRNA due to accumulation of polyamines. In fact, the cellular contents of putrescine and spermidine markedly increased and that of spermine inversely decreased during the same period. Pulse-chase experiments showed that the accumulation of putrescine and spermidine also elicited a rapid degradation of ODC. Excess amounts of newly synthesized putrescine and cadaverine were excreted into the medium, whereas spermidine, spermine and acetylated polyamines were undetectable there. We conclude that ODC regulation upon removal of the inhibitor is dependent on at least three steps, namely the level of mRNA, the translational efficiency of mRNA and the stability of the enzyme, the last two of which are involved in cellular polyamines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Kallio A., Sinervirta R., Seppänen P., Kontula K. K., Jänne O. A., Jänne J. Difluoromethylornithine-induced amplification of ornithine decarboxylase genes in Ehrlich ascites carcinoma cells. Biochem Biophys Res Commun. 1985 Jan 31;126(2):734–740. doi: 10.1016/0006-291x(85)90246-3. [DOI] [PubMed] [Google Scholar]
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. G., Loose D., Meisner H., Watson G. Androgen induction of messenger RNA concentrations in mouse kidney is posttranscriptional. Biochemistry. 1986 Mar 11;25(5):1170–1175. doi: 10.1021/bi00353a034. [DOI] [PubMed] [Google Scholar]
- Choi J. H., Scheffler I. E. Chinese hamster ovary cells resistant to alpha-difluoromethylornithine are overproducers of ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12601–12608. [PubMed] [Google Scholar]
- Feinstein S. C., Dana S. L., McConlogue L., Shooter E. M., Coffino P. Nerve growth factor rapidly induces ornithine decarboxylase mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5761–5765. doi: 10.1073/pnas.82.17.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science. 1989 Mar 17;243(4897):1493–1495. doi: 10.1126/science.2928784. [DOI] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm I., Persson L., Stjernborg L., Thorsson L., Heby O. Feedback control of ornithine decarboxylase expression by polyamines. Analysis of ornithine decarboxylase mRNA distribution in polysome profiles and of translation of this mRNA in vitro. Biochem J. 1989 Mar 1;258(2):343–350. doi: 10.1042/bj2580343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. T., Verma A. K. Involvement of protein kinase C in the transcriptional regulation of ornithine decarboxylase gene expression by 12-O-tetradecanoylphorbol-13-acetate in T24 human bladder carcinoma cells. Arch Biochem Biophys. 1988 Apr;262(1):326–336. doi: 10.1016/0003-9861(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Ito K., Igarashi K. Polyamine regulation of the synthesis of thymidine kinase in bovine lymphocytes. Arch Biochem Biophys. 1990 Apr;278(1):277–283. doi: 10.1016/0003-9861(90)90260-6. [DOI] [PubMed] [Google Scholar]
- Ito K., Kashiwagi K., Watanabe S., Kameji T., Hayashi S., Igarashi K. Influence of the 5'-untranslated region of ornithine decarboxylase mRNA and spermidine on ornithine decarboxylase synthesis. J Biol Chem. 1990 Aug 5;265(22):13036–13041. [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Effect of putrescine on the synthesis of S-adenosylmethionine decarboxylase. Biochem J. 1987 Apr 1;243(1):285–288. doi: 10.1042/bj2430285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Kanamoto R., Boyle S. M., Oka T., Hayashi S. Molecular mechanisms of the synergistic induction of ornithine decarboxylase by asparagine and glucagon in primary cultured hepatocytes. J Biol Chem. 1987 Oct 25;262(30):14801–14805. [PubMed] [Google Scholar]
- Kashiwagi K., Ito K., Igarashi K. Spermidine regulation of ornithine decarboxylase synthesis by a GC-rich sequence of the 5'-untranslated region. Biochem Biophys Res Commun. 1991 Aug 15;178(3):815–822. doi: 10.1016/0006-291x(91)90964-9. [DOI] [PubMed] [Google Scholar]
- Kopitz J., Rist B., Bohley P. Post-translational arginylation of ornithine decarboxylase from rat hepatocytes. Biochem J. 1990 Apr 15;267(2):343–348. doi: 10.1042/bj2670343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitz J., Rist B., Bohley P. Post-translational arginylation of ornithine decarboxylase from rat hepatocytes. Biochem J. 1990 Apr 15;267(2):343–348. doi: 10.1042/bj2670343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H., Kodama H. Adenine phosphoribosyltransferase deficiency in cultured mouse mammary tumor FM3A cells resistant to 4-carbamoylimidazolium 5-olate. Cancer Res. 1982 Oct;42(10):4210–4214. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legraverend C., Potter A., Hölttä E., Alitalo K., Andersson L. C. Interleukin-2 induces a rapid increase in ornithine decarboxylase mRNA in a cloned murine T lymphocytic cell line. Exp Cell Res. 1989 Mar;181(1):273–281. doi: 10.1016/0014-4827(89)90201-2. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Manzella J. M., Blackshear P. J. Regulation of rat ornithine decarboxylase mRNA translation by its 5'-untranslated region. J Biol Chem. 1990 Jul 15;265(20):11817–11822. [PubMed] [Google Scholar]
- McConlogue L., Coffino P. A mouse lymphoma cell mutant whose major protein product is ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12083–12086. [PubMed] [Google Scholar]
- McConlogue L., Coffino P. Ornithine decarboxylase in difluoromethylornithine-resistant mouse lymphoma cells. Two-dimensional gel analysis of synthesis and turnover. J Biol Chem. 1983 Jul 10;258(13):8384–8388. [PubMed] [Google Scholar]
- Meggio F., Flamigni F., Guarnieri C., Pinna L. A. Location of the phosphorylation site for casein kinase-2 within the amino acid sequence of ornithine decarboxylase. Biochim Biophys Acta. 1987 Jun 15;929(1):114–116. doi: 10.1016/0167-4889(87)90246-1. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Chen H. J. Conformational changes in ornithine decarboxylase enable recognition by antizyme. Biochim Biophys Acta. 1990 Jan 19;1037(1):115–121. doi: 10.1016/0167-4838(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Hoff J. A., Bareyal-Leyser A. Stable ornithine decarboxylase in a rat hepatoma cell line selected for resistance to alpha-difluoromethylornithine. Arch Biochem Biophys. 1991 Oct;290(1):143–152. doi: 10.1016/0003-9861(91)90600-n. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Qasba P., Stofko R. E., Franzen M. A. Ornithine decarboxylase modification and polyamine-stimulated enzyme inactivation in HTC cells. Biochem J. 1985 Jun 1;228(2):297–308. doi: 10.1042/bj2280297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Marumo M., Hayashi S. Ornithine decarboxylase antizyme in kidneys of male and female mice. Biochem J. 1988 Sep 1;254(2):367–372. doi: 10.1042/bj2540367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Matsufuji S., Miyazaki Y., Hayashi S. Destabilization of ornithine decarboxylase by transfected antizyme gene expression in hepatoma tissue culture cells. J Biol Chem. 1992 Jul 5;267(19):13138–13141. [PubMed] [Google Scholar]
- Murakami Y., Nishiyama M., Hayashi S. Involvement of antizyme in stabilization of ornithine decarboxylase caused by inhibitors of polyamine synthesis. Eur J Biochem. 1989 Mar 1;180(1):181–184. doi: 10.1111/j.1432-1033.1989.tb14630.x. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Tanaka K., Matsufuji S., Miyazaki Y., Hayashi S. Antizyme, a protein induced by polyamines, accelerates the degradation of ornithine decarboxylase in Chinese-hamster ovary-cell extracts. Biochem J. 1992 May 1;283(Pt 3):661–664. doi: 10.1042/bj2830661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Madhubala R., Kameji T., Bergeron R. J. Control of ornithine decarboxylase activity in alpha-difluoromethylornithine-resistant L1210 cells by polyamines and synthetic analogues. J Biol Chem. 1988 Aug 5;263(22):11008–11014. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Secrist J. A., 3rd, Madhubala R. Properties of L1210 cells resistant to alpha-difluoromethylornithine. Cancer Res. 1988 May 15;48(10):2678–2682. [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Regulation of ornithine decarboxylase mRNA translation by polyamines. Studies using a cell-free system and a cell line with an amplified ornithine decarboxylase gene. J Biol Chem. 1988 Mar 5;263(7):3528–3533. [PubMed] [Google Scholar]
- Radford D. M., Nakai H., Eddy R. L., Haley L. L., Byers M. G., Henry W. M., Lawrence D. D., Porter C. W., Shows T. B. Two chromosomal locations for human ornithine decarboxylase gene sequences and elevated expression in colorectal neoplasia. Cancer Res. 1990 Oct 1;50(19):6146–6153. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Rincke G., Marks F. The induction of ornithine decarboxylase by the tumor promoter TPA is controlled at the post-transcriptional level in murine Swiss 3T3 fibroblasts. Biochem Biophys Res Commun. 1987 Aug 31;147(1):219–225. doi: 10.1016/s0006-291x(87)80109-2. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Manen C. A. Posttranslationally modified ornithine decarboxylase may regulate RNA polymerase I activity. Biochem Pharmacol. 1982 Nov 1;31(21):3373–3378. doi: 10.1016/0006-2952(82)90614-1. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Effect of androgens on turnover of ornithine decarboxylase in mouse kidney. Studies using labeling of the enzyme by reaction with [14C] alpha-difluoromethylornithine. J Biol Chem. 1982 Jul 10;257(13):7549–7553. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- White M. W., Kameji T., Pegg A. E., Morris D. R. Increased efficiency of translation of ornithine decarboxylase mRNA in mitogen-activated lymphocytes. Eur J Biochem. 1987 Dec 30;170(1-2):87–92. doi: 10.1111/j.1432-1033.1987.tb13670.x. [DOI] [PubMed] [Google Scholar]
- van Daalen Wetters T., Brabant M., Coffino P. Regulation of mouse ornithine decarboxylase activity by cell growth, serum and tetradecanoyl phorbol acetate is governed primarily by sequences within the coding region of the gene. Nucleic Acids Res. 1989 Dec 11;17(23):9843–9860. doi: 10.1093/nar/17.23.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daalen Wetters T., Macrae M., Brabant M., Sittler A., Coffino P. Polyamine-mediated regulation of mouse ornithine decarboxylase is posttranslational. Mol Cell Biol. 1989 Dec;9(12):5484–5490. doi: 10.1128/mcb.9.12.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]