Abstract
Background
The relationship between the levels of high-density lipoprotein (HDL) and bone mineral density (BMD) is controversial. Furthermore, the specific role of apolipoprotein A1 (APOA1), a primary HDL component, in regulating BMD remains unclear. This study aimed to elucidate the correlation between APOA1 levels and lumbar BMD in patients with osteoporotic fracture (OPF) for novel insights into potential therapeutic strategies against osteoporosis.
Methods
This study included 587 OPF patients enrolled at the Kunshan Hospital, Affiliated with Jiangsu University between January 2017 and July 2022. The patient’s serum APOA1 levels were determined, followed by the assessment of lumbar BMD and C-terminal telopeptide of type I collagen (β-CTX) as outcome variables. The association of APOA1 levels with lumbar BMD and β-CTX was assessed via Generalized Estimating Equations (GEE) and spline smoothing plot analyses. A generalized additive model (GAM) helped ascertain non-linear correlations. Moreover, a subgroup analysis was also conducted to validate the result’s stability.
Results
It was observed that APOA1 levels were positively correlated with lumbar BMD (β = 0.07, 95% CI: 0.02 to 0.11, p = 0.0045), indicating that increased APOA1 levels were linked with enhanced lumbar BMD. Furthermore, APOA1 levels were negatively related to β-CTX (β = −0.19, 95% CI: −0.29 to −0.09, p = 0.0003), suggesting APOA1 might reduce osteolysis. In addition, these findings were robustly supported by subgroup and threshold effect analyses.
Conclusion
This study indicated that increased APOA1 levels were correlated with enhanced lumbar BMD and decreased osteolysis in OPF patients. Therefore, APOA1 may inhibit osteoclast activity to prevent further deterioration in osteoporotic patients. However, further research I warranted to validate these conclusions and elucidate the underlying physiologies.
Keywords: osteoporosis, fracture, apolipoprotein A1 (APOA1), bone mineral density (BMD), C-terminal telopeptide of type I collagen (β-CTX), high-density lipoprotein (HDL) osteoporosis, high-density lipoprotein (HDL)
Introduction
Osteoporosis (OP) is a prevalent skeletal condition affecting nearly 200 million individuals worldwide (1). It is characterized by decreased bone mineral density (BMD) and increased bone structure degradation, thereby elevating the risk of fracture (2–4). Almost 20% to 30% of osteoporotic patients die within a year of suffering from osteoporotic fractures (OPFs) (5). Furthermore, OP is asymptomatic before an OPF occurs (6). Therefore, early diagnosis and a suitable treatment plan are critical for preventing fractures and re-fractures in OP and OPF patients, respectively.
Previous studies have indicated a correlation between serum HDL levels and bone mass (7). Apolipoprotein A1 (APOA1) is the primary (60–70% of all) apolipoproteins carried by high-density lipoprotein (HDL) (8). It has been observed that APOA1 has anti-atherosclerotic effects (9). Furthermore, recent studies have highlighted that APOA1 regulates bone metabolism homeostasis (10). Moreover, in diabetic individuals, it has been autonomously associated with OP and lumbar BMD but not with HDL (11).
Currently, the relationship between HDL and BMD remains controversial (12) as human epidemiological studies have revealed conflicting results. Some studies have indicated that higher HDL levels can improve bone quality and decrease the risk of OP. However, various studies have suggested an inverse relationship between HDL levels and bone mass (12–14). Therefore, this study aimed to clarify this relationship and determine the precise role of APOA1, a major component of HDL. This study also investigated alterations in the C-terminal telopeptide of type I collagen (β-CTX) in OP patients to assess the bone metabolism pathways and provide new insights into the treatment of OPFs and OP.
Materials and methods
Study subjects
This study included 3558 patients diagnosed with OPFs who underwent surgical treatment at Kunshan Hospital Affiliated with Jiangsu University between January 2017 and July 2022. OP is characterized by fragility-related fractures without different metabolic bone disorders despite a normal T-score in BMD. In specific areas such as the femoral neck, lumbar spine, 1/3 (33%) radius, and total hip, OP can be diagnosed without evident fractures with a T score of ≤ −2.5. From 3558 patients, 587 were selected and enrolled (Figure 1). Patients lacking data on β-CTX, lumbar BMD, or APOA1, as well as those who were diagnosed with other diseases that interfere with bone metabolism were excluded. This study acquired ethical clearance from the AKHJU (approval number: 2021-06-015-K01) and adhered to the guidelines of the Helsinki Declaration. The researchers analyzing the data were blinded to the patient-identifiable information. This is an observational study and informed permission as well as anonymized data was acquired from all the patients.
FIGURE 1.
Study flowchart.
Measurement of relevant variables
In this observational research, the serum APOA1 levels were assessed as the independent variable using an electro-chemiluminescence immunoassay automated on the LABOSPECT 008AS platform (Hitachi High-Tech Co., Tokyo, Japan). The study outcome variables included lumbar BMD and β-CTX. The lumbar BMD was determined via dual-energy X-ray absorptiometry (DXA) using a hologic bone density instrument with dual-energy X-rays (Discovery Wi, Hologic Inc., USA). Furthermore, β-CTX was assessed using an automated electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics, Mannheim, Germany). The patient’s age and body mass index (BMI; overweight: 24–27.9 kg/m2, obese: ≥ 28 kg/m2 per the Chinese meta-analysis on obese individuals) (15) were assessed and documented. Alanine aminotransferase (ALT) and uric acid (UA) levels were determined with an enzymatic colorimetric method using the Beckman AU5800 biochemical analyzer pipeline. Moreover, triglyceride levels were assessed via the Guaiacol Peroxidase-Peroxidase Oxidation of Phenol (GPO-POD) method with the biochemical analyzer pipeline, Beckman AU5800. In addition, the Sysmex XN-10 (B4) hematology analyzer pipeline was employed to assess hemoglobin, monocyte, and platelet levels. The individuals’ parathyroid hormone (PTH) levels were determined with the Beckman DXI800 using a chemiluminescent assay. Furthermore, the patient’s HDL and LDL levels were assessed using the direct method with the biochemical analyzer pipeline, Beckman AU5800. Lastly, the automated coagulation analyzer CN-6000 and the Beckman AU5800 biochemical analyzer pipeline were employed to assess fibrinogen and albumin levels, respectively. FAR was calculated by dividing the fibrinogen and albumin levels for each patient. For the above analyses, the patient’s fasting blood samples were collected within 24 hours after admission. A skilled operator collected the samples using identical equipment according to established protocols.
Covariate analyses
Based on the previous literature (11, 16, 17), clinical guidelines (18), data within our database, and the results of previous clinical work by our team, following variables were selected as covariates: age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, PTH, HDL, LDL (low-density lipoprotein), neutrophil and FAR (fibrinogen/albumin ratio).
Statistical analyses
The demographic, laboratory, and clinical data are demonstrated as medians (25th and 75th percentile) or means with standard deviations (SD). Furthermore, the categorical data are presented as frequencies (percentages). Fisher’s exact or Pearson’s chi-square tests were performed for the univariate analysis of categorical data. Moreover, not-normally and regularly distributed continuous data were analyzed via Independent sample Mann-Whitney U tests and t-tests, respectively. In addition, univariate analyses were performed to determine the correlation of OPF patient’s characteristics with lumbar BMD and β-CTX.
The study utilized Generalized Estimating Equations (GEE) to elucidate the independent association between Lipoprotein A levels, lumbar BMD, and β-CTX in OPF patients while controlling for confounders. The models constructed comprised of unadjusted (Model 1), little adjusted (Model 2), and wholly adjusted (Model 3) models. Initially, the variance inflation factor (VIF) was utilized for assessing the covariance co-linearity. Then, these variables were corrected based on: (1) a substantial alteration within the matched odds ratio (OR) of at least 10%, after including or excluding covariates from the base or complete models, and (2) covariates either satisfied criteria 1 or demonstrated a p-value of < 0.1 within the univariate model. Models 2 and 3 were built using Criterion 1 and 2 to adjust for covariates. Three models were created, as outlined below: Model 1 was unadjusted; Model 2 (minimally adjusted model) accounted for age and BMI, while Model 3 accounted for age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, and PTH.
The probable non-linear correlations were assessed via a generalized additive model (GAM). A smoothing curve was generated using a two-piecewise linear regression model to determine the threshold effects. When an evident ratio was visible, the inflection point of these curves was determined using a recursive technique based on a model of maximum likelihood (19). The robustness and subgroup variability of the study patients were evaluated post-stratification according to specific variables. The subgroup interactions and modifications were employed for the likelihood ratio test (LRT).
All the statistical evaluations were performed using the R Foundation packages1 and EmpowerStats from X&Y Solutions, Inc., MA, USA.2 p < 0.05 was deemed as the significance level for a two-sided test.
Results
Features of patients
The baseline features of OPF patients (n = 3558) hospitalized within established APOA1 quartiles between January 2017 and July 2022 have been highlighted in Table 1. There were 31.93% males and 68.07% females with an average age of 69.18 ± 11.24 years, mean lumbar BMD of 0.74 ± 0.15 g/cm2, mean β-CTX of 0.53 ± 0.28 ng/mL and mean APOA1 of 1.22 ± 0.24 ng/mL. Patients were stratified into APOA1 quartiles (0.29–1.05, 1.06–1.19, 1.20–1.34, and 1.35–2.49 ng/mL), and interquartile differences within lumbar BMD, β-CTX, age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, PTH, HDL, LDL, neutrophil and FAR. It was observed that, higher APOA1 quartile patients had substantially increased lumbar BMD (Q1: 0.73 ± 0.15 g/cm2; Q2: 0.72 ± 0.15 g/cm2; Q3: 0.75 ± 0.17 g/cm2; Q4: 0.77 ± 0.15 g/cm2, p = 0.004), while those in elevated APOA1 quartiles had lower β-CTX (Q1: 0.58 ± 0.35 ng/mL; Q2: 0.55 ± 0.28 ng/mL; Q3: 0.53 ± 0.25 ng/mL; Q4: 0.51 ± 0.24 ng/mL).
TABLE 1.
Patient characteristics based on apolipoprotein A quartiles.
| Apolipoprotein A1 quartile | Q1 | Q2 | Q3 | Q4 | P-value | P-value* |
| N | 535 | 568 | 542 | 563 | ||
| Lumber BMD, g/cm2 | 0.73 ± 0.15 | 0.72 ± 0.15 | 0.75 ± 0.17 | 0.77 ± 0.15 | 0.004 | 0.002 |
| β-CTX, ng/mL | 0.58 ± 0.35 | 0.55 ± 0.28 | 0.53 ± 0.25 | 0.51 ± 0.24 | 0.184 | 0.564 |
| Age, year | 70.17 ± 11.57 | 68.96 ± 11.22 | 68.76 ± 10.55 | 68.45 ± 11.25 | 0.061 | 0.053 |
| BMI categorical, N (%) | 22.82 ± 3.44 | 23.04 ± 3.43 | 23.17 ± 3.45 | 22.87 ± 3.33 | 0.304 | 0.432 |
| Triglycerides, mmol/L | 1.16 ± 0.79 | 1.22 ± 0.90 | 1.29 ± 0.87 | 1.26 ± 1.19 | 0.107 | 0.136 |
| ALT, IU/L | 21.59 ± 15.10 | 23.25 ± 30.04 | 23.33 ± 19.96 | 22.05 ± 13.30 | 0.411 | 0.066 |
| UA, μmol/L | 295.80 ± 95.95 | 284.30 ± 90.79 | 275.92 ± 88.16 | 271.41 ± 87.46 | <0.001 | <0.001 |
| Monocyte, × 109/L | 0.54 ± 0.26 | 0.52 ± 0.49 | 0.47 ± 0.24 | 0.46 ± 0.22 | <0.001 | <0.001 |
| Hemoglobin, g/L | 121.25 ± 20.68 | 126.69 ± 18.22 | 127.09 ± 16.94 | 128.63 ± 14.89 | <0.001 | <0.001 |
| Platelet, × 109/L | 172.35 ± 67.79 | 178.18 ± 61.20 | 175.37 ± 61.16 | 180.52 ± 55.88 | 0.146 | 0.010 |
| HDL, mmol/L | 1.07 ± 0.19 | 1.26 ± 0.19 | 1.40 ± 0.21 | 1.64 ± 0.30 | <0.001 | <0.001 |
| LDL, mmol/L | 2.26 ± 0.76 | 2.54 ± 0.75 | 2.66 ± 0.72 | 2.72 ± 0.75 | <0.001 | <0.001 |
| Neutrophil, × 109/L | 6.42 ± 3.24 | 6.29 ± 2.99 | 6.11 ± 2.83 | 6.48 ± 3.05 | 0.194 | 0.225 |
| FAR | 0.08 ± 0.03 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.02 | <0.001 | <0.001 |
| PTH, pg/mL | 16.29 ± 12.72 | 14.81 ± 11.42 | 14.30 ± 8.84 | 13.25 ± 7.44 | <0.001 | 0.002 |
| Diabetes | 0.153 | – | ||||
| No | 511 (95.51%) | 555 (97.71%) | 521 (96.13%) | 537 (95.38%) | ||
| Yes | 24 (4.49%) | 13 (2.29%) | 21 (3.87%) | 26 (4.62%) | ||
| Hypertension | 0.943 | – | ||||
| No | 465 (86.92%) | 495 (87.15%) | 469 (86.53%) | 484 (85.97%) | ||
| Yes | 70 (13.08%) | 73 (12.85%) | 73 (13.47%) | 79 (14.03%) |
*Kruskal-Wallis rank test for continuous variables, Fisher exact for categorical variables with expects < 10. APOA1, apolipoprotein A1; Lumber BMD, lumbar spine bone density; β-CTX, C-terminal telopeptide of type I collagen; BMI, body mass index; ALT, alanine aminotransferase; UA, uric acid; PTH, parathyroid hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FAR, fibrinogen/albumin ratio.
Factors correlated with lumbar BMD and β-CTX based on univariate analysis
The univariate analysis indicated a significant correlation between lumbar BMD, β-CTX, and other factors including age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, and PTH, particularly with APOA1 (Table 2).
TABLE 2.
Univariate analyses of factors associated with Lumber BMD and β-CTX.
| Statistics | Lumber BMD β (95%CI) P-value |
β -CTX β (95%CI) P-value |
|
| APOA1, ng/mL | 1.22 ± 0.24 | 0.07 (0.02, 0.12) 0.0027 | −0.14 (−0.24, −0.04) 0.0080 |
| Age, year | 69.18 ± 11.24 | −0.00 (−0.00, −0.00) < 0.0001 | 0.00 (−0.00, 0.00) 0.5157 |
| BMI categorical, N (%) | 23.00 ± 3.38 | 0.01 (0.01, 0.01) < 0.0001 | 0.00 (−0.00, 0.01) 0.6053 |
| Triglycerides, mmol/L | 1.23 ± 0.95 | 0.01 (−0.00, 0.02) 0.1675 | −0.01 (−0.03, 0.02) 0.5471 |
| ALT, IU/L | 23.62 ± 22.32 | −0.00 (−0.00, 0.00) 0.8580 | −0.00 (−0.00, −0.00) 0.0040 |
| UA, μmol/L | 284.80 ± 92.36 | −0.00 (−0.00, 0.00) 0.3764 | −0.00 (−0.00, −0.00) 0.0014 |
| Monocyte, × 109/L | 0.51 ± 0.30 | 0.01 (−0.02, 0.04) 0.6119 | −0.17 (−0.26, −0.07) 0.0005 |
| Hemoglobin, g/L | 125.70 ± 18.45 | −0.00 (−0.00, 0.00) 0.6943 | −0.00 (−0.00, −0.00) 0.0243 |
| Platelet, × 109/L | 177.62 ± 62.32 | −0.00 (−0.00, 0.00) 0.4770 | 0.00 (0.00, 0.00) 0.0021 |
| Diabetes | |||
| No | 3413 (95.92%) | Reference | Reference |
| Yes | 145 (4.08%) | 0.04 (−0.01, 0.08) 0.0882 | 0.02 (−0.08, 0.13) 0.6342 |
| Hypertension | |||
| No | 3048 (85.67%) | Reference | Reference |
| Yes | 510 (14.33%) | 0.01 (−0.01, 0.04) 0.2196 | 0.05 (−0.01, 0.11) 0.1063 |
APOA1, apolipoprotein A1; Lumber BMD, lumbar spine bone density; β-CTX, C-terminal telopeptide of type I collagen; BMI, body mass index; ALT, alanine aminotransferase; UA, uric acid; PTH, parathyroid hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FAR, fibrinogen/albumin ratio.
The association of APOA1 levels with Lumbar BMD and β-CTX
Three models assessed the link between APOA1, lumbar BMD, and β-CTX in OPF patients (Table 3). The first analysis, Model 1, indicated a significant association of APOA1 with lumbar BMD (β = 0.07, 95% CI = 0.02 to 0.12, p = 0.0027), and β-CTX (β = −0.14, 95% CI = −0.24 to −0.04, p = 0.0080). After controlling for age and BMI, Model 2 indicated comparable correlations, specifically between APOA1 and lumbar BMD (β = 0.06, 95% CI = 0.02 to 0.11, p = 0.0050, and APOA1 and β-CTX (β = −0.14, 95% CI = −0.24 to −0.04, p = 0.0072). Model 3 showed comparable correlations between APOA1 and lumbar BMD (β = 0.07, 95% CI = 0.02 to 0.11, p = 0.0045), and APOA1 and β-CTX (β = −0.19, 95% CI = −0.29 to −0.09, p = 0.0003), after controlling for age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, and PTH.
TABLE 3.
Association between APOA1 and BMD and β-CTX in different models.
| Model 1aN = 718 β (95%CI) P-value | Model 1bN = 718 β (95%CI) P-value | Model 1cN = 718 β (95%CI) P-value | |
| BMD | 0.07 (0.02, 0.12) 0.0027 | 0.06 (0.02, 0.11) 0.0050 | 0.07 (0.02, 0.11) 0.0045 |
| β-CTX | −0.14 (−0.24, −0.04) 0.0080 | −0.14 (−0.24, −0.04) 0.0072 | −0.19 (−0.29, −0.09) 0.0003 |
aNo adjustment.
bAdjusted for age, BMI.
cAdjusted for age, BMI, triglycerides, ALT, UA, monocyte, hemoglobin, platelet, PTH, diabetes, and hypertension. APOA1, apolipoprotein A1; Lumber BMD, lumbar spine bone density; β-CTX, C-terminal telopeptide of type I collagen; BMI, body mass index; ALT, alanine aminotransferase; UA, uric acid; PTH, parathyroid hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FAR, fibrinogen/albumin ratio.
The strength and reliability of Model 3 were validated by additional subgroup analyses, which categorized OPF patients according to age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, and PTH levels. The analyses were adjusted to incorporate the remaining un-stratified covariates. Thus, similar patterns were observed without discernible interactions because of stratification (p > 0.05, Supplementary Table 1).
Spline smoothing graph and threshold exploration
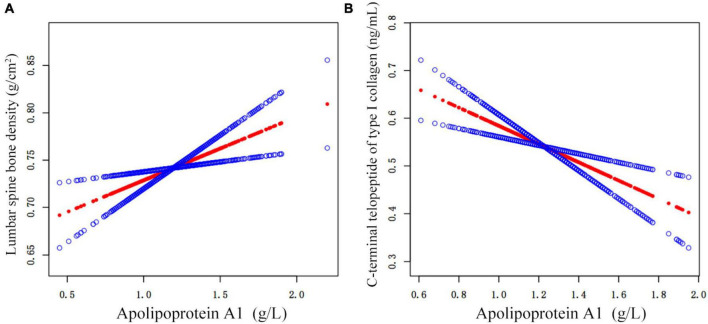
Different graphs assessed the linearity or non-linearity of the correlation between lumbar BMD-APOA1 and β-CTX-APOA1 for 769 and 587 individuals, respectively (Figure 2). The threshold effect analysis further involved age adjustments, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, and PTH (Table 4). However, a direct correlation of APOA1 levels with lumbar BMD and β-CTX could be observed. The final model adjusted for age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet, and PTH level counts indicated an impact size of 0.07 (95% CI = 0.02 to 0.12, p = 0.0027) and −0.19 (95% CI = −0.29 to −0.09, p = 0.0003). In addition, the curve fitting-based threshold effect analysis did not depict a non-linear correlation (Table 4) without proven inflection points (p > 0.05).
FIGURE 2.
APOA1 levels are connected with lumbar BMD (A) β-CTX (B) on adjusted smoothed curves. The top and bottom curves demonstrate the span of the 95% confidence interval. In contrast, the center curve highlights the linkage between APOA1 and lumbar BMD and β-CTX. Age, BMI, triglycerides, ALT, UA, monocyte, hemoglobin, platelet, diabetes, and hypertension were adjusted within the model. APOA1, apolipoprotein A1; Lumber BMD, lumbar spine bone density; β-CTX, C-terminal telopeptide of type I collagen; BMI, body mass index; ALT, alanine aminotransferase; UA, uric acid; PTH, parathyroid hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FAR, fibrinogen/albumin ratio.
TABLE 4.
Threshold analyses examining the relationship between APOA1 levels and BMD, β-CTX.
| Model 3a | ||
| Lumber BMD β (95% CI) P-value | β -CTX β (95% CI) P-value | |
| Model Ab | ||
| One line slope | 0.07 (0.02, 0.11) 0.0045 | −0.19 (−0.29, −0.09) 0.0003 |
| Model Bc | ||
| APOA1 turning point (K), g/L | 1.17 | 0.91 |
| <K | 0.01 (−0.09, 0.11) 0.8542 | 0.32 (−0.64, 1.29) 0.5133 |
| >K | 0.10 (0.03, 0.17) 0.0058 | −0.21 (−0.32, −0.10) 0.0002 |
| Slope 2–Slope 1 | 0.09 (−0.05, 0.24) 0.2180 | −0.53 (−1.53, 0.46) 0.2948 |
| BMD value at K, g/cm2 or β-CTX value at K, ng/mL | 0.73 (0.72, 0.75) | 0.60 (0.56, 0.64) |
| LRT testd | 0.214 | 0.289 |
aAdjusted for age, BMI, triglycerides, ALT, UA, diabetes, hypertension, monocyte, hemoglobin, platelet and PTH.
bLinear analysis, P-value < 0.05 indicates a linear relationship.
cNon-linear analysis.
dP-value < 0.05 means Model B is significantly different from Model A, which indicates a non-linear relationship APOA1, apolipoprotein A1; Lumber BMD, lumbar spine bone density; β-CTX, C-terminal telopeptide of type I collagen; BMI, body mass index; ALT, alanine aminotransferase; UA, uric acid; PTH, parathyroid hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FAR, fibrinogen/albumin ratio.
Discussion
The OP is an increasing public health concern (20–23). A recent cross-sectional research on Chinese individuals indicates an OP prevalence of 5.0% in males and 20.6% in women aged 40 years and above (24). OP significantly increases the financial burden of patients who endure severe bone fractures and physical pain (25). OPFs are common causes of morbidity and death among older adults (26–28), with vertebral fractures being the most common OPF (29). Therefore, in this study, lumbar BMD was selected as the outcome variable. Emerging evidence has depicted that aberrant lipid metabolic pathways can affect osteoblast differentiation, leading to bone mass loss (11). Furthermore, it has been indicated that serum HDL is associated with BMD; however, it remains controversial (12). Several studies have suggested that HDL is inversely associated with BMD (13, 14, 30). However, some studies reported that HDL is positively linked with BMD (13, 31). Recently, APOA1, the main HDL component, has been independently associated with BMD (11).
Therefore, this study used APOA1 as an exposure variable within a Chinese osteoporotic population. APOA1, a 243-amino acid polypeptide (32), is the primary apolipoprotein HDL component and a common protein in human plasma (33). Most of the ApoA1 protein (nearly 80%) is secreted by hepatocytes, while only a small amount (20%) is secreted by the intestinal epithelial cells (34). Similar to HDL, APOA1 could have antioxidant and anti-inflammatory properties (35). It has been observed to play a role in atherosclerotic diseases, including anti-atherosclerotic, anti-inflammatory, anti-apoptotic, and anti-thrombotic effects (36, 37). In vivo mice experiments have revealed that APOA1 deficiency affects bone metabolism via multiple mechanisms (10, 38, 39). Moreover, it is a versatile protein that positively influences various diseases (40).
The current study indicated that APOA1 is autonomously linked with lumbar BMD. Furthermore, its levels were positively correlated with lumbar BMD after adjusting for relevant variables (β = 0.07, 95% CI = 0.02 to 0.11, p = 0.005). The curve-fitting between APOA1 and lumbar BMD indicated a high linear relationship (Figure 2). These results indicate a significantly reliable arrangement with no evidence of stratification-linked exchanges (p > 0.05, Supplementary Table 1). Based on these findings, it was inferred that as APOA1 enhances, lumbar BMD also increases in OPF patients.
Moreover, a bone resorption (β-CTX) indicator was selected as the other outcome variable to assess the mechanisms affecting BMD changes. B -CTX is an indicator of bone metabolism (41–43). Clinical studies have indicated that enhanced β-CTX levels are linked with increased bone resorption, higher risk of fractures, and accelerated bone loss (44). The International Federation of Clinical Chemistry (IFCC) and the International Osteoporosis Foundation (IOF) have suggested that serum β-CTX could become a reference bone turnover marker (BTM) (45).
Therefore, in this study, it was observed that APOA1 levels were significantly correlated with β-CTX and indicated a linear negative relationship (β = −0.19, 95% CI = −0.29 to −0.09, p < 0.001). These outcomes delivered a significantly reliable array with no proof of stratification-linked interrelationships (p > 0.05, Supplementary Table 1). These findings suggest that β-CTX decreases with enhancing APOA1, highlighting osteolysis decline, and validating the conclusion that lumbar BMD increases with APOA1, indicating a potential mechanism that elevates lumbar BMD.
A recent in vivo mice study confirmed that APOA1 deficiency can influence bone metabolism by altering the phenotypic and molecular characteristics of bone marrow adipocytes (38, 39). Furthermore, APOA1 deficiency modified the bone cell precursor population, thereby increasing adipoblast and decreasing osteoblast syntheses (10).
Overall, these data indicated that APOA1 elevates BMD and prevents further deterioration of OP by decreasing osteoclast activity, depicting clinical implications. Therefore, based on the results of this study, clinicians should detect and maintain APOA1 in osteoporotic patients to decrease osteolysis and enhance BMD.
The study screened OPF patients via accurate statistical models and adjustment of essential confounding factors. Per our knowledge, this is the first Chinese investigation to investigate APOA1 in OPF patients to evaluate the relationship between APOA1 and lumbar BMD. Furthermore, this study not only provides novel insights into the association between HDL and BMD but also highlights innovative treatment strategies for patients.
However, the current study has limitations. (1) Several studies have identified the connection between HDL and BMD; however, many co-founding variables, such as age, sex, and ethnicity, were not focused. (2) This study only included OPF patients, and whether the current findings apply to the general population requires further exploration. (3) Due to the study’s cross-sectional design, the correlation between APOA1, lumbar BMD, and β-CTX as well as a causal relationship between APOA1 levels and variations in BMD and β-CTX could not be assessed. Without longitudinal data, it is not possible to monitor changes in bone density over time or evaluate the long-term effects of APOA1 levels on bone health. (4) The results suggested that the enhanced lumbar BMD was correlated with APOA1, which requires further investigation. Increased APOA1 reduced β-CTX, indicating that decreased osteolysis is a possible cause. Further studies are warranted to confirm these findings and illustrate the potential mechanisms.
Conclusion
In summary, the correlation between HDL and BMD has been controversial; however, this study indicated a positive correlation between APOA1 levels, a major HDL component, and lumbar BMD, indicating that APOA1 prevents further deterioration in OPF patients. Moreover, it was observed that APOA1 may produce an anti-OP effect by suppressing osteoclast action. Further investigations are warranted to validate these results and highlight the underlying physiologies.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the IRB of Affiliated Kunshan Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Ll-F: Writing−original draft, Writing−review and editing. KL: Writing−original draft, Writing−review and editing. CL: Writing−original draft, Writing−review and editing. H-qS: Writing−review and editing. M-zX: Writing−review and editing. Y-wY: Writing−review and editing. YY: Writing−review and editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. The study was supported by the Suzhou City Major Disease Multicenter Clinical Research Project (CN) (DZXYJ202312), Elderly Health Research Project of Jiangsu Province (CN) (LKZ2022020), Special Funding for Jiangsu Province Science and Technology Plan (Key Research and Development Program for Social Development) (CN) (BE2023738), Suzhou Collaborative Innovation Research Project of Medical and Industrial Integration (CN) (SLJ2022023), and Gusu Health Talent Plan Scientific Research Project (CN) (GSWS2022105).
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1415739/full#supplementary-material
References
- 1.Zhang Y, Xie N, Sun X, Nice E, Liou Y, Huang C, et al. Insights and implications of sexual dimorphism in osteoporosis. Bone Res. (2024) 12:8. 10.1038/s41413-023-00306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichikawa S, Koller D, Padgett L, Lai D, Hui S, Peacock M, et al. Replication of previous genome-wide association studies of bone mineral density in premenopausal american women. J Bone Miner Res. (2010) 25:1821–9. 10.1002/jbmr.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abar O, Dharmar S, Tang S. The effect of aminoguanidine (AG) and pyridoxamine (PM) on ageing human cortical bone. Bone Jt Res. (2018) 7:105–10. 10.1302/2046-3758.71.BJR-2017-0135.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Chassaing B, Tyagi A, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. (2016) 126:2049–63. 10.1172/JCI86062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh B, Yu H, Kim H, Lee S, Kong S, Kim J, et al. Interpretable deep-learning approaches for osteoporosis risk screening and individualized feature analysis using large population-based data: Model development and performance evaluation. J Med Internet Res. (2023) 25:e40179. 10.2196/40179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiu S, Mu Z, Sun L, Zhao L, Fu J. Hemoglobin level and osteoporosis in Chinese elders with type 2 diabetes mellitus. Nutr Diabetes. (2022) 12:19. 10.1038/s41387-022-00198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papachristou D, Blair H. Bone and high-density lipoprotein: The beginning of a beautiful friendship. World J Orthop. (2016) 7:74–7. 10.5312/wjo.v7.i2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdoğan K, Sanlier N, Özen E, Erol S, Kahyaoğlu I, Neselioglu S, et al. Evaluation of dysfunctional HDL by myeloperoxidase/paraoxonase ratio in unexplained infertility patients undergoing IVF/ICSI. J Clin Med. (2023) 12:1506. 10.3390/jcm12041506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Xia X, Huang N, Qiu Y, Yang X, Mao H, et al. Association of ratio of apolipoprotein B to apolipoprotein A1 with survival in peritoneal dialysis. Front Nutr. (2022) 9:801979. 10.3389/fnut.2022.801979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair H, Kalyvioti E, Papachristou N, Tourkova I, Syggelos S, Deligianni D, et al. Apolipoprotein A-1 regulates osteoblast and lipoblast precursor cells in mice. Lab Investig J Tech Methods Pathol. (2016) 96:763–72. 10.1038/labinvest.2016.51 [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Chen Z, Lv F, Tu M, Guo X. Apolipoprotein A1 is associated with osteocalcin and bone mineral density rather than high-density lipoprotein cholesterol in Chinese postmenopausal women with type 2 diabetes mellitus. Front Med. (2023) 10:1182866. 10.3389/fmed.2023.1182866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papachristou N, Blair H, Kypreos K, Papachristou D. High-Density Lipoprotein (HDL) metabolism and bone mass. J Endocrinol. (2017) 233:R95–107. 10.1530/JOE-16-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong I, Cho S, Kim S, Choi H, Park K, Kim S, et al. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif Tissue Int. (2010) 87:507–12. 10.1007/s00223-010-9427-3 [DOI] [PubMed] [Google Scholar]
- 14.Li S, Guo H, Liu Y, Wu F, Zhang H, Zhang Z, et al. Relationships of serum lipid profiles and bone mineral density in postmenopausal Chinese women. Clin Endocrinol. (2015) 82:53–8. 10.1111/cen.12616 [DOI] [PubMed] [Google Scholar]
- 15.Zhou B. Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. [PubMed] [Google Scholar]
- 16.Sun X, Wu X. Association of apolipoprotein A1 with osteoporosis: A cross-sectional study. BMC Musculoskelet Disord. (2023) 24:157. 10.1186/s12891-023-06264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Y, Wang S, Yi Q, Xia Y, Geng B. High-density lipoprotein cholesterol is negatively correlated with bone mineral density and has potential predictive value for bone loss. Lipids Health Dis. (2021) 20:75. 10.1186/s12944-021-01497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts N, Camacho P, Lewiecki E, Petak S. Aace/Ace Postmenopausal Osteoporosis Guidelines Task Force. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. (2021) 27:379–80. 10.1016/j.eprac.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Wang X, Lu Y, Li T, Gong Z, Sheng T, et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int. (2013) 7:901–9. 10.1007/s12072-013-9457-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khosla S, Hofbauer L. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. (2017) 5:898–907. 10.1016/S2213-8587(17)30188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martiniakova M, Babikova M, Mondockova V, Blahova J, Kovacova V, Omelka R. The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients. (2022) 14:523. 10.3390/nu14030523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alajlouni D, Tran T, Bliuc D, Blank R, Cawthon P, Orwoll E, et al. Muscle strength and physical performance improve fracture risk prediction beyond Garvan and FRAX: The osteoporotic fractures in men (MrOS) study. J Bone Miner Res. (2022) 37:411–9. 10.1002/jbmr.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Z, Da W, Zhao Y, Wang T, Xu H, Shu B, et al. Role of skeletal muscle satellite cells in the repair of osteoporotic fractures mediated by β−catenin. J Cachexia Sarcopenia Muscle. (2022) 13:1403–17. 10.1002/jcsm.12938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Yu W, Yin X, Cui L, Tang S, Jiang N, et al. Prevalence of osteoporosis and fracture in China: The China osteoporosis prevalence study. JAMA Netw Open. (2021) 4:e2121106. 10.1001/jamanetworkopen.2021.21106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Y, Sun J, Zhao Y, Tang K, Zhu R, Zhao W, et al. Chinese patent medicine for osteoporosis: A systematic review and meta-analysis. Bioengineered. (2022) 13:5581–97. 10.1080/21655979.2022.2038941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu K, Liu K, Huang J, Weng X, Chen Q, Gao T, et al. Toxoplasma gondii infection as a risk factor for osteoporosis: A case–control study. Parasit Vectors. (2022) 15:151. 10.1186/s13071-022-05257-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Cui W, Zhou P, Li C, Wen Y, Xiao W. Comparison of a flexible versus rigid bone cement injection system in unilateral percutaneous vertebroplasty. Eur J Med Res. (2020) 25:36. 10.1186/s40001-020-00436-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz J, Akhavan N, Mullins A, Arjmandi B. Macrophage polarization and osteoporosis: A review. Nutrients. (2020) 12:2999. 10.3390/nu12102999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shim J, Kim K, Kim K, Choi U, Kyung J, Sohn S, et al. Safety and efficacy of Wharton’s jelly-derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: A phase I/IIa study. Stem Cells Transl Med. (2020) 10:554–67. 10.1002/sctm.20-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Dai J, Zhong W, Hu C, Lu S, Chai Y. Association between serum cholesterol level and osteoporotic fractures. Front Endocrinol. (2018) 9:30. 10.3389/fendo.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Lu K, Shi H, Shi Q, Gong Y, Wang J, et al. Association between lumbar endplate damage and bone mineral density in patients with degenerative disc disease. BMC Musculoskelet Disord. (2023) 24:762. 10.1186/s12891-023-06812-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deckmyn O, Poynard T, Bedossa P, Paradis V, Peta V, Pais R, et al. Clinical interest of serum alpha-2 macroglobulin, apolipoprotein A1, and haptoglobin in patients with non-alcoholic fatty liver disease, with and without type 2 diabetes, before or during COVID-19. Biomedicines. (2022) 10:699. 10.3390/biomedicines10030699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhale A, Venkataraman K. Leveraging knowledge of HDLs major protein ApoA1: Structure, function, mutations, and potential therapeutics. Biomed Pharmacother. (2022) 154:113634. 10.1016/j.biopha.2022.113634 [DOI] [PubMed] [Google Scholar]
- 34.Márquez A, Nazir S, van der Vorst E. High-density lipoprotein modifications: A pathological consequence or cause of disease progression? Biomedicines. (2020) 8:549. 10.3390/biomedicines8120549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang R, Chen Y, Tu M, Wang W. Monocyte to high-density lipoprotein and apolipoprotein A1 ratios are associated with bone homeostasis imbalance caused by chronic inflammation in postmenopausal women with type 2 diabetes mellitus. Front Pharmacol. (2022) 13:1062999. 10.3389/fphar.2022.1062999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chistiakov D, Orekhov A, Bobryshev Y. ApoA1 and ApoA1-specific self-antibodies in cardiovascular disease. Lab Investig J Tech Methods Pathol. (2016) 96:708–18. 10.1038/labinvest.2016.56 [DOI] [PubMed] [Google Scholar]
- 37.Yao Y, Li X, Wang Z, Ji Q, Xu Q, Yan Y, et al. Interaction of lipids, mean platelet volume, and the severity of coronary artery disease among chinese adults: A mediation analysis. Front Cardiovasc Med. (2022) 9:753171. 10.3389/fcvm.2022.753171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastrenopoulou A, Kypreos K, Papachristou N, Georgopoulos S, Mastora I, Papadimitriou-Olivgeri I, et al. ApoA1 deficiency reshapes the phenotypic and molecular characteristics of bone marrow adipocytes in mice. Int J Mol Sci. (2022) 23:4834. 10.3390/ijms23094834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouweneel A, Reiche M, Snip O, Wever R, van der Wel E, Schaftenaar F, et al. Apolipoprotein A1 deficiency in mice primes bone marrow stem cells for T cell lymphopoiesis. J Cell Sci. (2022) 135:jcs258901. 10.1242/jcs.258901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cochran B, Ong K, Manandhar B, Rye K. APOA1: A protein with multiple therapeutic functions. Curr Atheroscler Rep. (2021) 23:11. 10.1007/s11883-021-00906-7 [DOI] [PubMed] [Google Scholar]
- 41.Vasikaran S, Eastell R, Bruyère O, Foldes A, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. (2011) 22:391–420. 10.1007/s00198-010-1501-1 [DOI] [PubMed] [Google Scholar]
- 42.Cho S. New biological markers of bone metabolism in osteoporosis treatment. Endocrinol Metab. (2016) 31:400–1. 10.3803/EnM.2016.31.3.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheater G, Elshahaly M, Tuck S, Datta H, van Laar J. The clinical utility of bone marker measurements in osteoporosis. J Transl Med. (2013) 11:201. 10.1186/1479-5876-11-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eastell R, Vrijens B, Cahall D, Ringe J, Garnero P, Watts N. Bone turnover markers and bone mineral density response with risedronate therapy: Relationship with fracture risk and patient adherence. J Bone Miner Res. (2011) 26:1662–9. 10.1002/jbmr.342 [DOI] [PubMed] [Google Scholar]
- 45.Szulc P, Naylor K, Hoyle N, Eastell R, Leary E. National Bone Health Alliance Bone Turnover Marker Project. Use of CTX-I and PINP as bone turnover markers: National bone health alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. (2017) 28:2541–56. 10.1007/s00198-017-4082-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.