Abstract
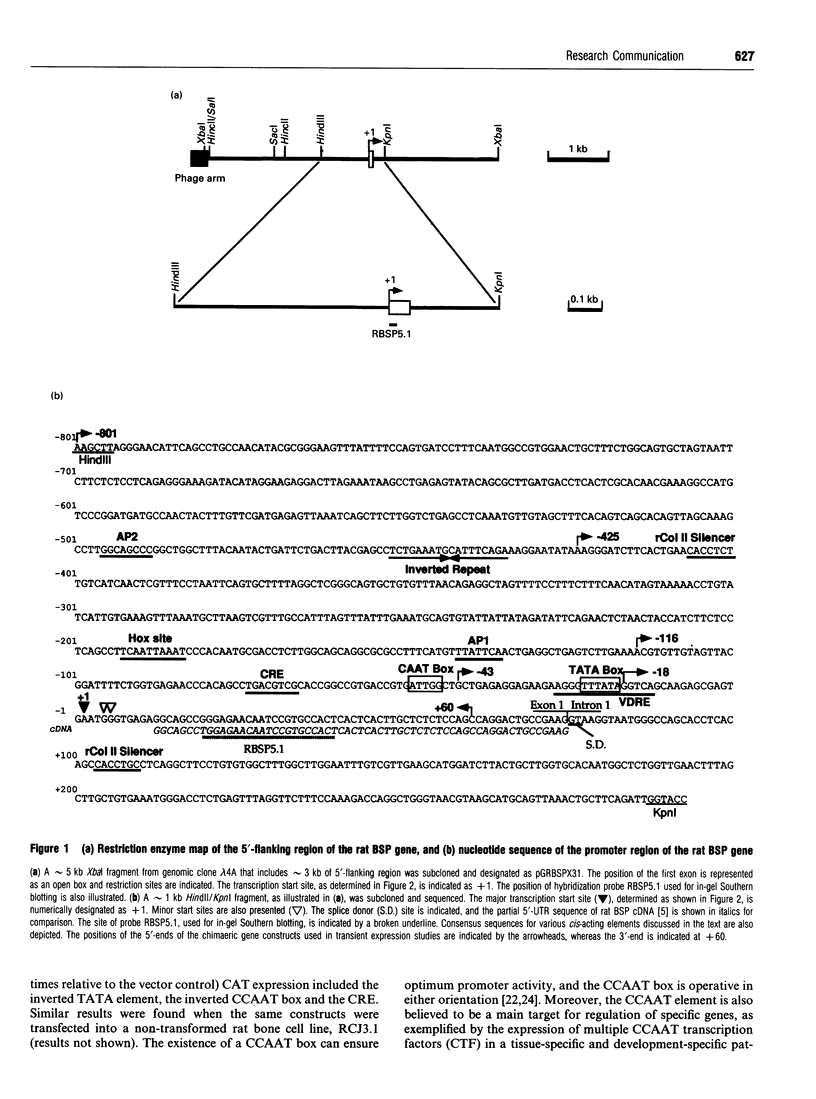
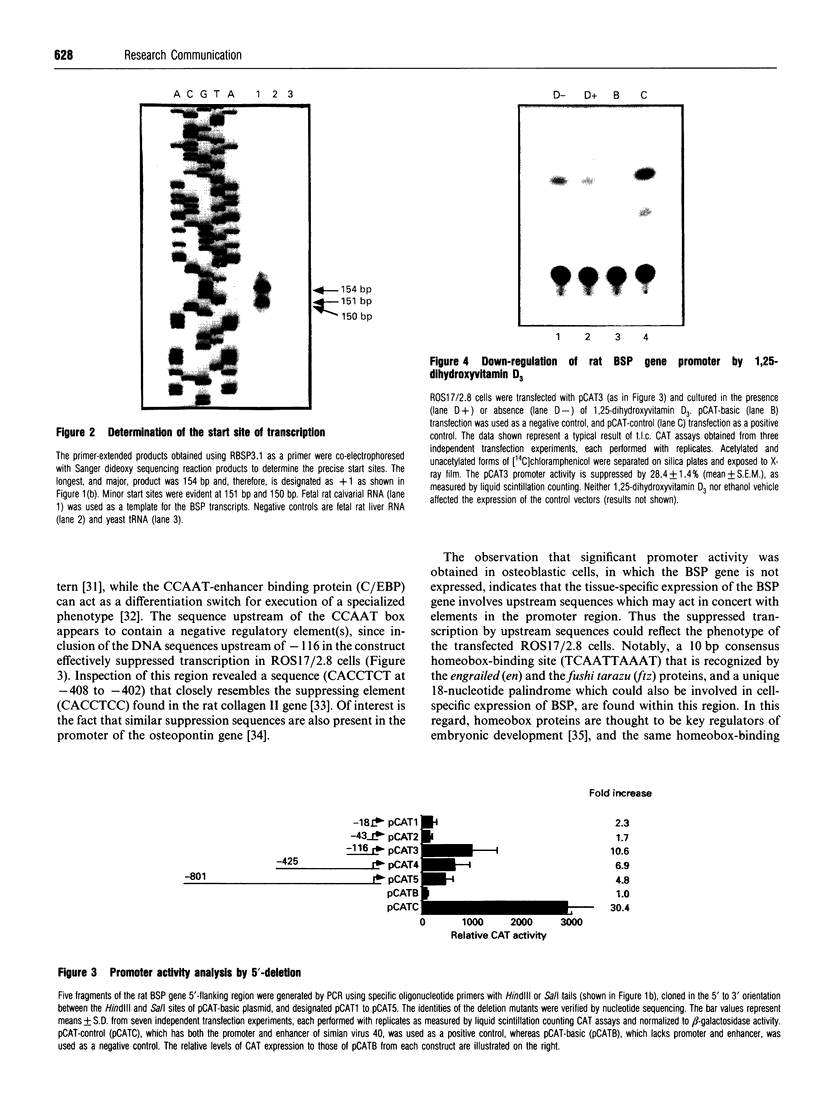
To study the transcriptional regulation of the rat bone sialoprotein (BSP) gene, the nucleotide sequence of a approximately 1 kb HindIII/KpnI subfragment from a genomic clone containing the 5' flanking sequence, exon 1 and part of intron 1 was determined and the transcription start site defined. This region includes an inverted TATA element (nt -24 to -19), an inverted CCAAT box, a homeobox-binding site, a putative 1,25-dihydroxyvitamin D3 response element (VDRE) sequence overlapping the inverted TATA sequence, and a novel 18 nt palindrome that may control the tissue-specific transcription of the BSP gene. The shortest promoter sequence capable of directing bacterial chloramphenicol acetyltransferase reporter gene expression included the inverted TATA element and the inverted CCAAT box. However, the promoter activity was down-regulated by 1,25-dihydroxyvitamin D3, indicating that the unique VDRE-like sequence overlapping the TATA element is functional. Thus the rat BSP gene promoter is characterized by novel cis-acting elements that may be involved in hormone- and tissue-specific regulation of transcription.
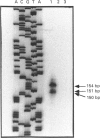
Full text
PDF


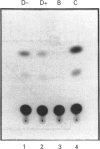
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Carcamo J., Maldonado E., Cortes P., Ahn M. H., Ha I., Kasai Y., Flint J., Reinberg D. A TATA-like sequence located downstream of the transcription initiation site is required for expression of an RNA polymerase II transcribed gene. Genes Dev. 1990 Sep;4(9):1611–1622. doi: 10.1101/gad.4.9.1611. [DOI] [PubMed] [Google Scholar]
- Chen J. K., Shapiro H. S., Wrana J. L., Reimers S., Heersche J. N., Sodek J. Localization of bone sialoprotein (BSP) expression to sites of mineralized tissue formation in fetal rat tissues by in situ hybridization. Matrix. 1991 Apr;11(2):133–143. doi: 10.1016/s0934-8832(11)80217-9. [DOI] [PubMed] [Google Scholar]
- Chen J., Shapiro H. S., Sodek J. Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res. 1992 Aug;7(8):987–997. doi: 10.1002/jbmr.5650070816. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang Q., McCulloch C. A., Sodek J. Immunohistochemical localization of bone sialoprotein in foetal porcine bone tissues: comparisons with secreted phosphoprotein 1 (SPP-1, osteopontin) and SPARC (osteonectin). Histochem J. 1991 Jun;23(6):281–289. doi: 10.1007/BF01045047. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., McBride O. W., Termine J. D., Young M. F. Human bone sialoprotein. Deduced protein sequence and chromosomal localization. J Biol Chem. 1990 Feb 5;265(4):2347–2351. [PubMed] [Google Scholar]
- Fisher L. W., Whitson S. W., Avioli L. V., Termine J. D. Matrix sialoprotein of developing bone. J Biol Chem. 1983 Oct 25;258(20):12723–12727. [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson R. M., Micheau M. R., Iwamoto A., Miyamoto N. G. 5' flanking and first intron sequences of the human beta-actin gene required for efficient promoter activity. Nucleic Acids Res. 1989 Jan 11;17(1):253–270. doi: 10.1093/nar/17.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. Roles of TFIID in transcriptional initiation by RNA polymerase II. Cell. 1991 Sep 20;66(6):1067–1070. doi: 10.1016/0092-8674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Helfrich M. H., Nesbitt S. A., Dorey E. L., Horton M. A. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J Bone Miner Res. 1992 Mar;7(3):335–343. doi: 10.1002/jbmr.5650070314. [DOI] [PubMed] [Google Scholar]
- Kasugai S., Todescan R., Jr, Nagata T., Yao K. L., Butler W. T., Sodek J. Expression of bone matrix proteins associated with mineralized tissue formation by adult rat bone marrow cells in vitro: inductive effects of dexamethasone on the osteoblastic phenotype. J Cell Physiol. 1991 Apr;147(1):111–120. doi: 10.1002/jcp.1041470115. [DOI] [PubMed] [Google Scholar]
- Kinne R. W., Fisher L. W. Keratan sulfate proteoglycan in rabbit compact bone is bone sialoprotein II. J Biol Chem. 1987 Jul 25;262(21):10206–10211. [PubMed] [Google Scholar]
- Levine M., Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988 Nov 18;55(4):537–540. doi: 10.1016/0092-8674(88)90209-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Midura R. J., McQuillan D. J., Benham K. J., Fisher L. W., Hascall V. C. A rat osteogenic cell line (UMR 106-01) synthesizes a highly sulfated form of bone sialoprotein. J Biol Chem. 1990 Mar 25;265(9):5285–5291. [PubMed] [Google Scholar]
- O'Shea-Greenfield A., Smale S. T. Roles of TATA and initiator elements in determining the start site location and direction of RNA polymerase II transcription. J Biol Chem. 1992 Jan 15;267(2):1391–1402. [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D., Pierschbacher M., Ruoslahti E. Identification of a bone sialoprotein receptor in osteosarcoma cells. J Biol Chem. 1988 Dec 25;263(36):19433–19436. [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988 Dec 25;263(36):19430–19432. [PubMed] [Google Scholar]
- Oldberg A., Jirskog-Hed B., Axelsson S., Heinegård D. Regulation of bone sialoprotein mRNA by steroid hormones. J Cell Biol. 1989 Dec;109(6 Pt 1):3183–3186. doi: 10.1083/jcb.109.6.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Diverse transcriptional functions of the multisubunit eukaryotic TFIID complex. J Biol Chem. 1992 Jan 15;267(2):679–682. [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Savagner P., Miyashita T., Yamada Y. Two silencers regulate the tissue-specific expression of the collagen II gene. J Biol Chem. 1990 Apr 25;265(12):6669–6674. [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Fisher L. W., Foster R. A., Sauk J. J. Human bone sialoprotein I and II enhance fibroblast attachment in vitro. Calcif Tissue Int. 1988 Jul;43(1):50–53. doi: 10.1007/BF02555169. [DOI] [PubMed] [Google Scholar]
- Strömstedt P. E., Poellinger L., Gustafsson J. A., Carlstedt-Duke J. The glucocorticoid receptor binds to a sequence overlapping the TATA box of the human osteocalcin promoter: a potential mechanism for negative regulation. Mol Cell Biol. 1991 Jun;11(6):3379–3383. doi: 10.1128/mcb.11.6.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Domenicucci C., Goldberg H. A., Wrana J. L., Sodek J. Characterization of fetal porcine bone sialoproteins, secreted phosphoprotein I (SPPI, osteopontin), bone sialoprotein, and a 23-kDa glycoprotein. Demonstration that the 23-kDa glycoprotein is derived from the carboxyl terminus of SPPI. J Biol Chem. 1990 May 5;265(13):7583–7589. [PubMed] [Google Scholar]
- Zhang Q., Wrana J. L., Sodek J. Characterization of the promoter region of the porcine opn (osteopontin, secreted phosphoprotein 1) gene. Identification of positive and negative regulatory elements and a 'silent' second promoter. Eur J Biochem. 1992 Jul 15;207(2):649–659. doi: 10.1111/j.1432-1033.1992.tb17092.x. [DOI] [PubMed] [Google Scholar]