Abstract
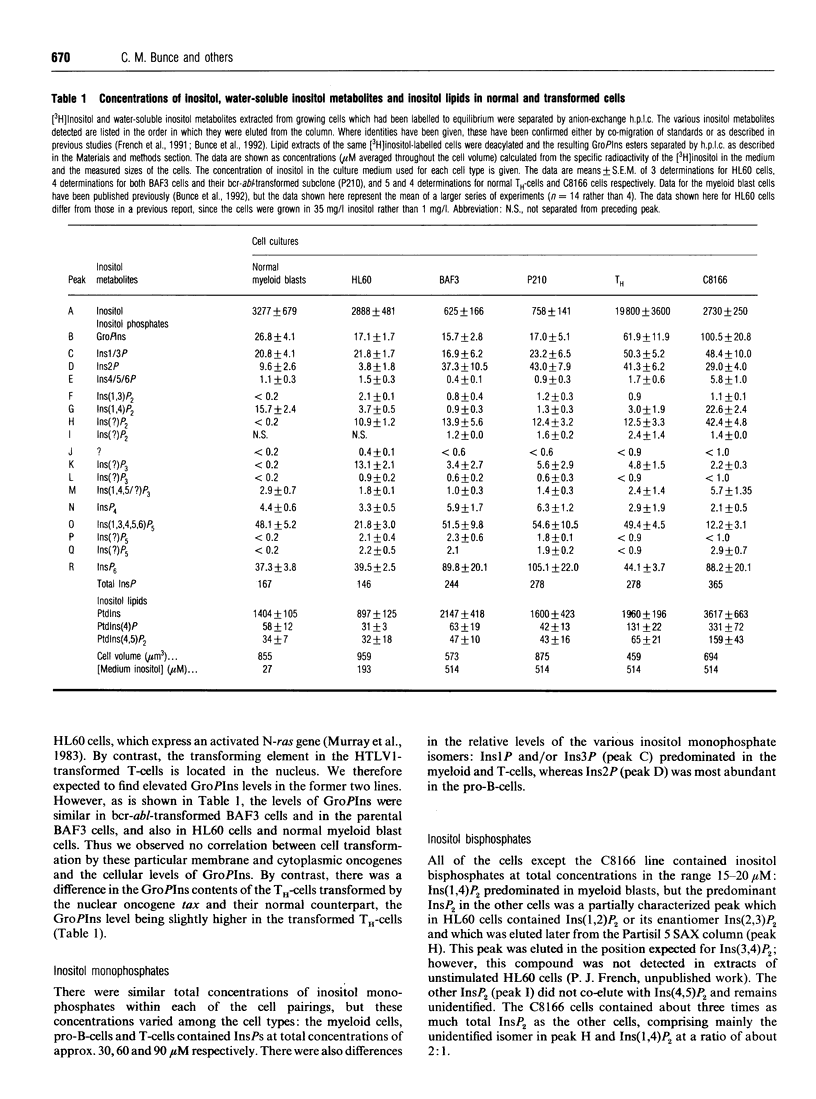
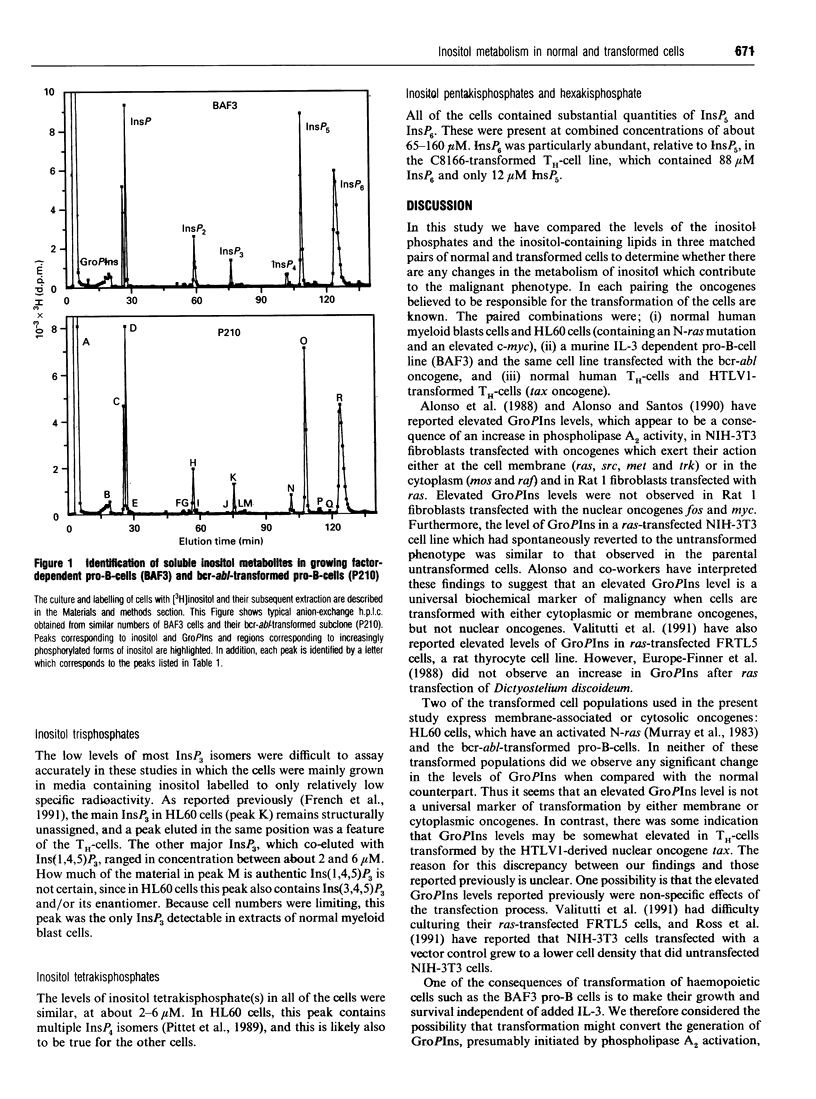
We have compared the levels of inositol metabolites in three pairs of normal and transformed cells which have been matched with respect to their cell lineage, differentiation and proliferation status: (i) normal human myeloid blast cells and the human promyelocytic leukaemic cell line, HL60; (ii) human umbilical-cord T-helper cells and C8166 cells, a HTLV-1-transformed T-helper cell line; and (iii) an interleukin 3-dependent long-term culture of murine pro-B-cells (BAF3) and BAF3 cells transformed by transfection with the bcr-abl oncogene. Complex patterns of inositol metabolites were present in each of the cell populations. Although there were a number of differences in the levels of certain inositol metabolites between individual cell populations in the paired groups, we did not observe any consistent difference in the levels of inositol metabolites between the proliferating normal and transformed cells. In particular, our data do not support the reported correlation between elevated glycerophosphoinositol (GroPIns) levels and transformation of cells by membrane and cytoplasmic oncogenes which has been reported by other workers. All the cells contained high concentrations of Ins(1,3,4,5,6)P5 (between 12 and 55 microM) and InsP6 (between 37 and 105 microM). The HTLV1-transformed T-helper cells had particularly high levels of total inositol phosphates (predominantly GroPIns, an unidentified inositol bisphosphate and InsP6). The observations are discussed with reference to cell transformation and to the differentiation status of the paired populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso T., Morgan R. O., Marvizon J. C., Zarbl H., Santos E. Malignant transformation by ras and other oncogenes produces common alterations in inositol phospholipid signaling pathways. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4271–4275. doi: 10.1073/pnas.85.12.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso T., Santos E. Increased intracellular glycerophosphoinositol is a biochemical marker for transformation by membrane-associated and cytoplasmic oncogenes. Biochem Biophys Res Commun. 1990 Aug 31;171(1):14–19. doi: 10.1016/0006-291x(90)91349-w. [DOI] [PubMed] [Google Scholar]
- Bizub D., Heimer E. P., Felix A., Chizzonite R., Wood A., Skalka A. M., Slater D., Aldrich T. H., Furth M. E. Antisera to the variable region of ras oncogene proteins, and specific detection of H-ras expression in an experimental model of chemical carcinogenesis. Oncogene. 1987 May;1(2):131–142. [PubMed] [Google Scholar]
- Bunce C. M., French P. J., Patton W. N., Turnell A. S., Scott S. A., Michell R. H., Kirk C. J., Brown G. Levels of inositol metabolites within normal myeloid blast cells and changes during their differentiation towards monocytes. Proc Biol Sci. 1992 Jan 22;247(1318):27–33. doi: 10.1098/rspb.1992.0005. [DOI] [PubMed] [Google Scholar]
- Bunce C. M., Patton W. N., Pound J. D., Lord J. M., Brown G. Phorbol myristate acetate treatment of normal human myeloid blast cells promotes monopoiesis and inhibits granulopoiesis. Leuk Res. 1990;14(11-12):1007–1017. doi: 10.1016/0145-2126(90)90114-o. [DOI] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., McDuffie E., Braverman R. Human peripheral lymphocyte growth regulation and response to phorbol esters is linked to synthesis and phosphorylation of the cytosolic protein, prosolin. J Immunol. 1989 Aug 1;143(3):956–963. [PubMed] [Google Scholar]
- Downes C. P., Macphee C. H. myo-inositol metabolites as cellular signals. Eur J Biochem. 1990 Oct 5;193(1):1–18. doi: 10.1111/j.1432-1033.1990.tb19297.x. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Ludérus M. E., Small N. V., Van Driel R., Reymond C. D., Firtel R. A., Newell P. C. Mutant ras gene induces elevated levels of inositol tris- and hexakisphosphates in Dictyostelium. J Cell Sci. 1988 Jan;89(Pt 1):13–20. doi: 10.1242/jcs.89.1.13. [DOI] [PubMed] [Google Scholar]
- French P. J., Bunce C. M., Stephens L. R., Lord J. M., McConnell F. M., Brown G., Creba J. A., Michell R. H. Changes in the levels of inositol lipids and phosphates during the differentiation of HL60 promyelocytic cells towards neutrophils or monocytes. Proc Biol Sci. 1991 Sep 23;245(1314):193–201. doi: 10.1098/rspb.1991.0109. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Nerurkar L. S. Human retroviruses: their role in neoplasia and immunodeficiency. Ann N Y Acad Sci. 1989;567:82–94. doi: 10.1111/j.1749-6632.1989.tb16461.x. [DOI] [PubMed] [Google Scholar]
- Graham R. A., Meyer R. A., Szwergold B. S., Brown T. R. Observation of myo-inositol 1,2-(cyclic) phosphate in a Morris hepatoma by 31P NMR. J Biol Chem. 1987 Jan 5;262(1):35–37. [PubMed] [Google Scholar]
- Gudermann T. W., Cooper T. G. A sensitive bioluminescence assay for myo-inositol. Anal Biochem. 1986 Oct;158(1):59–63. doi: 10.1016/0003-2697(86)90588-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Wasilenko W. J., Mattingly R. R., Weber M. J., Garrison J. C. Fibroblasts transformed with v-src show enhanced formation of an inositol tetrakisphosphate. Science. 1989 Oct 6;246(4926):121–124. doi: 10.1126/science.2506643. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Ross T. S., Cunningham T. W., Caldwell K. K., Jefferson A. B., Bansal V. S. Recent insights in phosphatidylinositol signaling. Cell. 1990 Nov 2;63(3):459–465. doi: 10.1016/0092-8674(90)90442-h. [DOI] [PubMed] [Google Scholar]
- Mattingly R. R., Stephens L. R., Irvine R. F., Garrison J. C. Effects of transformation with the v-src oncogene on inositol phosphate metabolism in rat-1 fibroblasts. D-myo-inositol 1,4,5,6-tetrakisphosphate is increased in v-src-transformed rat-1 fibroblasts and can be synthesized from D-myo-inositol 1,3,4-trisphosphate in cytosolic extracts. J Biol Chem. 1991 Aug 15;266(23):15144–15153. [PubMed] [Google Scholar]
- Murray M. J., Cunningham J. M., Parada L. F., Dautry F., Lebowitz P., Weinberg R. A. The HL-60 transforming sequence: a ras oncogene coexisting with altered myc genes in hematopoietic tumors. Cell. 1983 Jul;33(3):749–757. doi: 10.1016/0092-8674(83)90017-x. [DOI] [PubMed] [Google Scholar]
- Nunez G., Ugolini V., Capra J. D., Stastny P. Monoclonal antibodies against human monocytes. II. Recognition of two distinct cell surface molecules. Scand J Immunol. 1982 Dec;16(6):515–523. doi: 10.1111/j.1365-3083.1982.tb00753.x. [DOI] [PubMed] [Google Scholar]
- Ohmichi M., Decker S. J., Pang L., Saltiel A. R. Phospholipase C-gamma 1 directly associates with the p70 trk oncogene product through its src homology domains. J Biol Chem. 1991 Aug 15;266(23):14858–14861. [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pittet D., Schlegel W., Lew D. P., Monod A., Mayr G. W. Mass changes in inositol tetrakis- and pentakisphosphate isomers induced by chemotactic peptide stimulation in HL-60 cells. J Biol Chem. 1989 Nov 5;264(31):18489–18493. [PubMed] [Google Scholar]
- Ross T. S., Majerus P. W. Inositol-1,2-cyclic-phosphate 2-inositolphosphohydrolase. Substrate specificity and regulation of activity by phospholipids, metal ion chelators, and inositol 2-phosphate. J Biol Chem. 1991 Jan 15;266(2):851–856. [PubMed] [Google Scholar]
- Ross T. S., Whiteley B., Graham R. A., Majerus P. W. Cyclic hydrolase-transfected 3T3 cells have low levels of inositol 1,2-cyclic phosphate and reach confluence at low density. J Biol Chem. 1991 May 15;266(14):9086–9092. [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Takahashi C., Yamaoka S., Nosaka T., Maki M., Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S., Cucchi P., Colletta G., Di Filippo C., Corda D. Transformation by the k-ras oncogene correlates with increases in phospholipase A2 activity, glycerophosphoinositol production and phosphoinositide synthesis in thyroid cells. Cell Signal. 1991;3(4):321–332. doi: 10.1016/0898-6568(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Daley G. Q., Jackson P., Baltimore D., Cantley L. C. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991 Feb;11(2):1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]


