Abstract
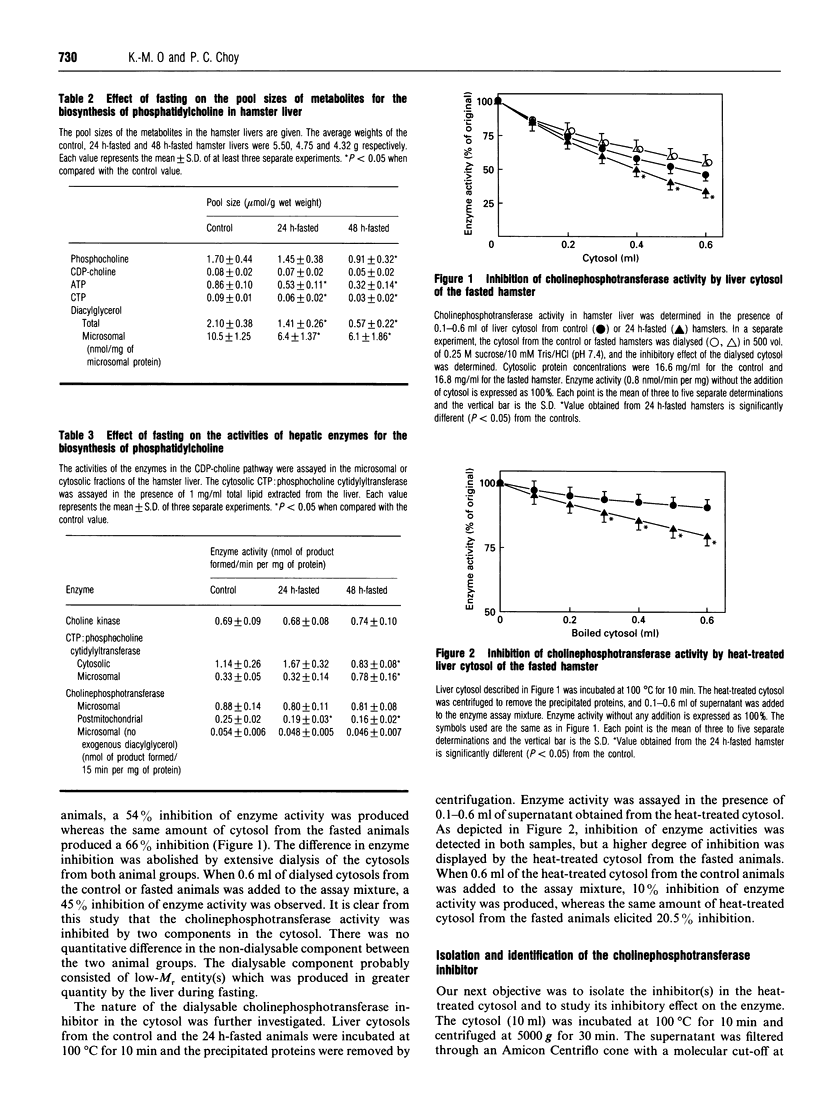
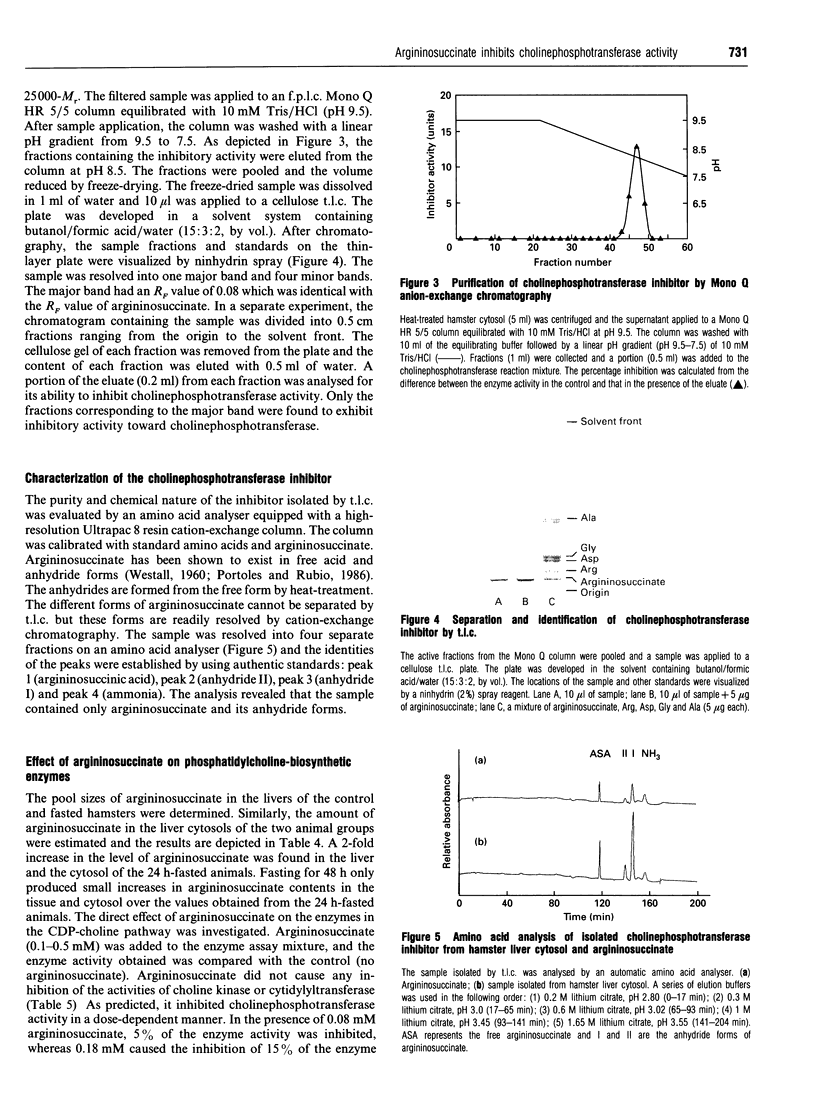
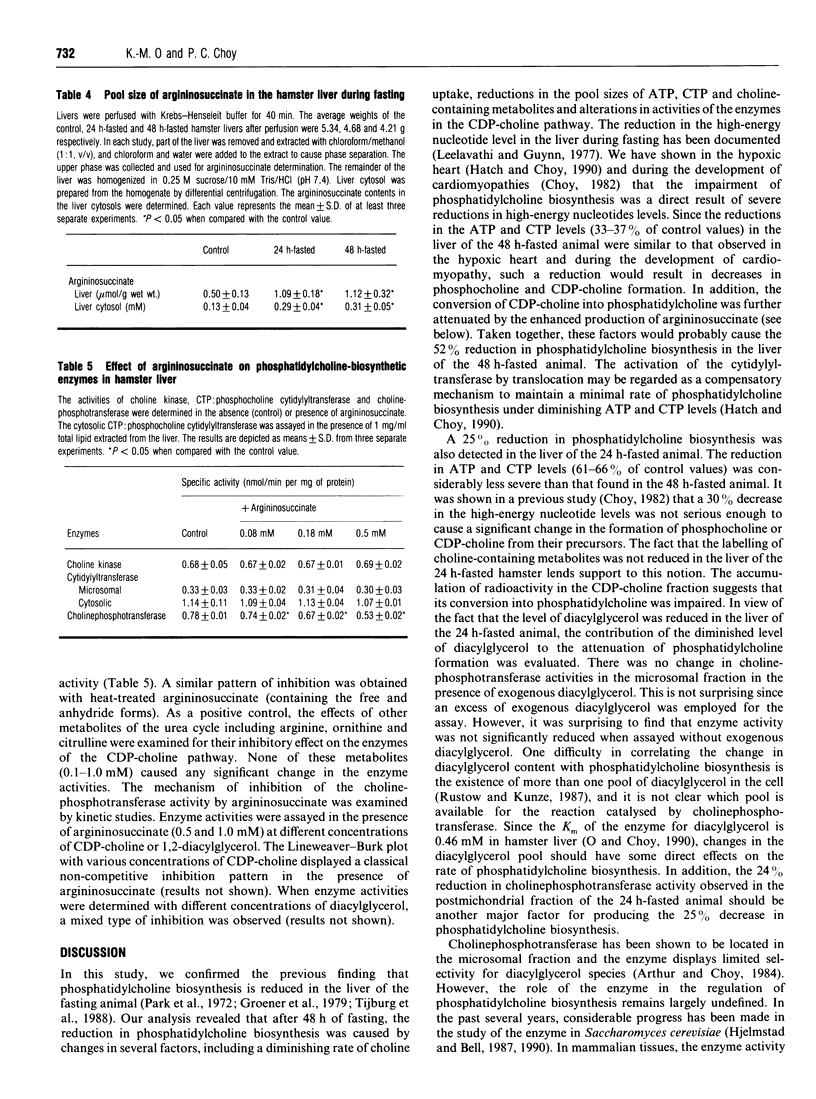
The control of phosphatidylcholine biosynthesis in the hamster liver was examined. Livers of hamsters fasted for 24 and 48 h were perfused with labelled choline. Under both fasting conditions, the incorporation of labelled choline into phosphatidylcholine was reduced. After 48 h of fasting, the 52% reduction in phosphatidylcholine biosynthesis was caused by changes in several factors including a diminishing rate of choline uptake and severe reductions in the pool sizes of ATP and CTP (to 33-37% control values) which resulted in a decrease in the pools of choline-containing metabolites. The activation of cytidylyltransferase after 48 h of fasting might be regarded as a compensatory mechanism for the maintenance of phosphatidylcholine biosynthesis. After 24 h of fasting, a 25% reduction in phosphatidylcholine biosynthesis was observed. The ATP and CTP levels were decreased but the reduction was not severe enough to affect the choline uptake or the labelling of the phosphocholine fraction. The activities of the cytidylyltransferase remained unchanged but an accumulation of labelled CDP-choline was detected. Although choline-phosphotransferase activity was not changed in the microsomes, the enzyme activity was attenuated in the postmitochondrial fraction. Further analysis revealed that cholinephosphotransferase in the liver was inhibited by an endogenous inhibitor in the cytosol which was later identified as argininosuccinate. The level of argininosuccinate was elevated during fasting and the change quantitatively accounted for the attenuation of cholinephosphotransferase activity. The inhibition of choline-phosphotransferase by argininosuccinate, coupled with a substantial decrease in the diacylglycerol level, would provide the hamster liver with an immediate mechanism for the transient modulation of phosphatidylcholine biosynthesis during short-term fasting.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G., Choy P. C. Acyl specificity of hamster heart CDP-choline 1,2-diacylglycerol phosphocholine transferase in phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Sep 12;795(2):221–229. doi: 10.1016/0005-2760(84)90069-9. [DOI] [PubMed] [Google Scholar]
- Choy P. C. Control of phosphatidylcholine biosynthesis in myopathic hamster hearts. J Biol Chem. 1982 Sep 25;257(18):10928–10933. [PubMed] [Google Scholar]
- Groener J. E., Klein W., Van Golde L. M. The effect of fasting and refeeding on the composition and synthesis of triacylglycerols, phosphatidylcholines, and phosphatidylethanolamines in rat liver. Arch Biochem Biophys. 1979 Nov;198(1):287–295. doi: 10.1016/0003-9861(79)90421-1. [DOI] [PubMed] [Google Scholar]
- Hatch G. M., Choy P. C. Effect of hypoxia on phosphatidylcholine biosynthesis in the isolated hamster heart. Biochem J. 1990 May 15;268(1):47–54. doi: 10.1042/bj2680047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. M., O K., Choy P. C. Regulation of phosphatidylcholine metabolism in mammalian hearts. Biochem Cell Biol. 1989 Feb-Mar;67(2-3):67–77. doi: 10.1139/o89-011. [DOI] [PubMed] [Google Scholar]
- Hjelmstad R. H., Bell R. M. Mutants of Saccharomyces cerevisiae defective in sn-1,2-diacylglycerol cholinephosphotransferase. Isolation, characterization, and cloning of the CPT1 gene. J Biol Chem. 1987 Mar 15;262(8):3909–3917. [PubMed] [Google Scholar]
- Hjelmstad R. H., Bell R. M. The sn-1,2-diacylglycerol cholinephosphotransferase of Saccharomyces cerevisiae. Nucleotide sequence, transcriptional mapping, and gene product analysis of the CPT1 gene. J Biol Chem. 1990 Jan 25;265(3):1755–1764. [PubMed] [Google Scholar]
- Ishidate K., Tsuruoka M., Nakazawa Y. Alteration in enzyme activities of de novo phosphatidylcholine biosynthesis in rat liver by treatment with typical inducers of microsomal drug-metabolizing system. Biochim Biophys Acta. 1980 Oct 6;620(1):49–58. [PubMed] [Google Scholar]
- Khan Z. U., Helmkamp G. M., Jr Stimulation of cholinephosphotransferase activity by phosphatidylcholine transfer protein. Regulation of membrane phospholipid synthesis by a cytosolic protein. J Biol Chem. 1990 Jan 15;265(2):700–705. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leelavathi D. E., Guynn R. W. Radiometric determination of cytidine 5'-triphosphate in biological materials. Anal Biochem. 1977 Nov;83(1):258–265. doi: 10.1016/0003-2697(77)90534-6. [DOI] [PubMed] [Google Scholar]
- O K. M., Choy P. C. Solubilization and partial purification of cholinephosphotransferase in hamster tissues. Lipids. 1990 Feb;25(2):122–124. doi: 10.1007/BF02562217. [DOI] [PubMed] [Google Scholar]
- O K. M., Siow Y. L., Choy P. C. Hamster liver cholinephosphotransferase and ethanolaminephosphotransferase are separate enzymes. Biochem Cell Biol. 1989 Oct;67(10):680–686. doi: 10.1139/o89-102. [DOI] [PubMed] [Google Scholar]
- Park C. E., Marai E., Mookerjea S. Phospholipid metabolism in endoplasmic reticular membranes in rats fed high-carbohydrate diet. Biochim Biophys Acta. 1972 May 23;270(1):50–59. doi: 10.1016/0005-2760(72)90176-2. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Baumann W. J. Lysolecithin as regulator of de novo lecithin synthesis in rat liver microsomes. Biochem Biophys Res Commun. 1979 Nov 28;91(2):637–642. doi: 10.1016/0006-291x(79)91569-9. [DOI] [PubMed] [Google Scholar]
- Portolés M., Rubio V. High-performance liquid chromatographic assay of argininosuccinate: its application in argininosuccinic aciduria and in normal man. J Inherit Metab Dis. 1986;9(1):31–38. doi: 10.1007/BF01813899. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Kunze D. Further evidence for the existence of different diacylglycerol pools of the phosphatidylcholine synthesis in microsomes. Biochim Biophys Acta. 1987 Oct 17;921(3):552–558. doi: 10.1016/0005-2760(87)90083-x. [DOI] [PubMed] [Google Scholar]
- SAKAMI W., HARRINGTON H. AMINO ACID METABOLISM. Annu Rev Biochem. 1963;32:355–398. doi: 10.1146/annurev.bi.32.070163.002035. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T. Differential effects of fasting and protein-free diets on levels of urea cycle enzymes in rat liver. J Biol Chem. 1962 Jun;237:1921–1924. [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in mammalian cells. III. Effects of alterations in the phospholipid compositions of Chinese hamster ovary and LM cells on the activity and distribution of CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1983 Jan 25;258(2):836–839. [PubMed] [Google Scholar]
- Snodgrass P. J. Biochemical aspects of urea cycle disorders. Pediatrics. 1981 Aug;68(2):273-83, 295-7. [PubMed] [Google Scholar]
- Taniguchi S., Morikawa S., Hayashi H., Fujii K., Mori H., Fujiwara M., Fujiwara M. Effects of Ca2+ on ethanolaminephosphotransferase and cholinephosphotransferase in rabbit platelets. J Biochem. 1986 Aug;100(2):485–491. doi: 10.1093/oxfordjournals.jbchem.a121737. [DOI] [PubMed] [Google Scholar]
- Tardi P. G., Man R. Y., Choy P. C. The effect of methyl-lidocaine on the biosynthesis of phospholipids de novo in the isolated hamster heart. Biochem J. 1992 Jul 1;285(Pt 1):161–166. doi: 10.1042/bj2850161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijburg L. B., Houweling M., Geelen M. J., van Golde L. M. Effects of dietary conditions on the pool sizes of precursors of phosphatidylcholine and phosphatidylethanolamine synthesis in rat liver. Biochim Biophys Acta. 1988 Mar 4;959(1):1–8. doi: 10.1016/0005-2760(88)90143-9. [DOI] [PubMed] [Google Scholar]
- Vance D. E. Boehringer Mannheim Award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem Cell Biol. 1990 Oct;68(10):1151–1165. doi: 10.1139/o90-172. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Pelech S. D., Choy P. C. CTP: phosphocholine cytidylyltransferase from rat liver. Methods Enzymol. 1981;71(Pt 100):576–581. doi: 10.1016/0076-6879(81)71070-x. [DOI] [PubMed] [Google Scholar]
- WESTALL R. G. Argininosuccinic aciduria: identification and reactions of the abnormal metabolite in a newly described form of mental disease, with some preliminary metabolic studies. Biochem J. 1960 Oct;77:135–144. doi: 10.1042/bj0770135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski T. A., Savard J. D., Man R. Y., Choy P. C. Phosphatidylcholine biosynthesis in isolated hamster heart. J Biol Chem. 1980 Dec 10;255(23):11423–11428. [PubMed] [Google Scholar]



