Abstract
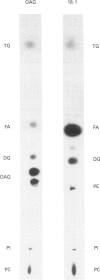
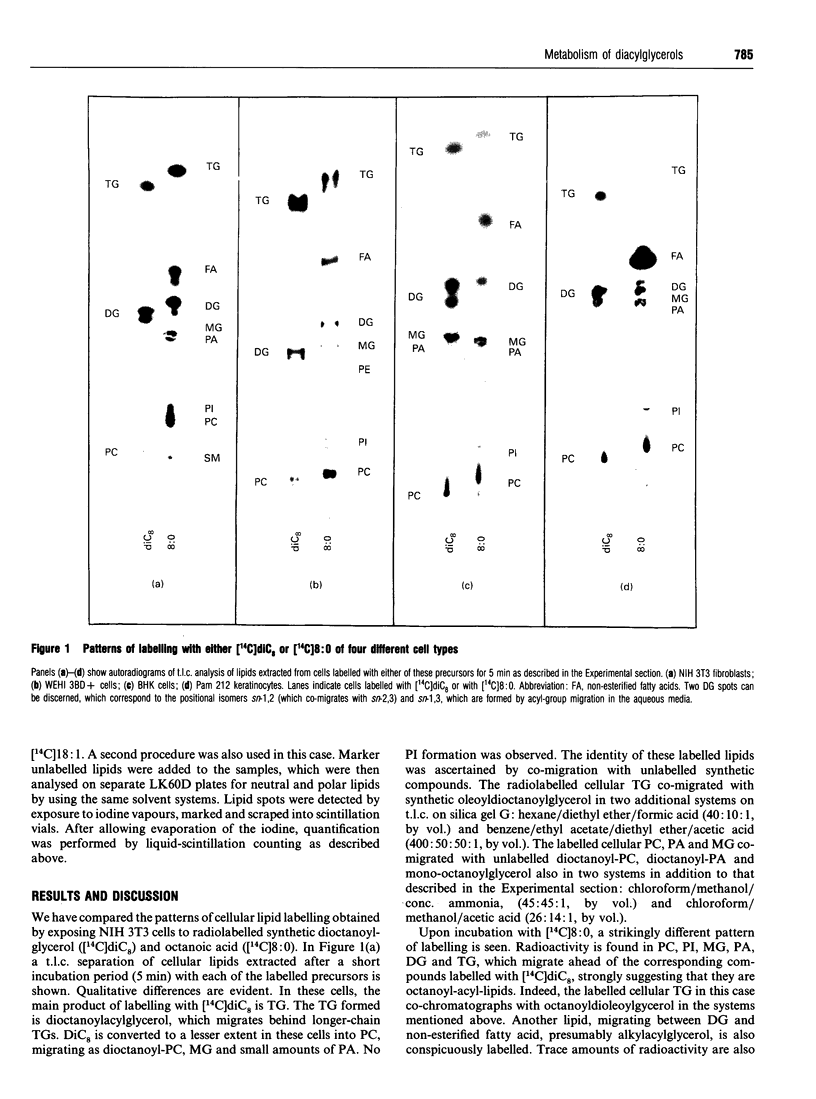
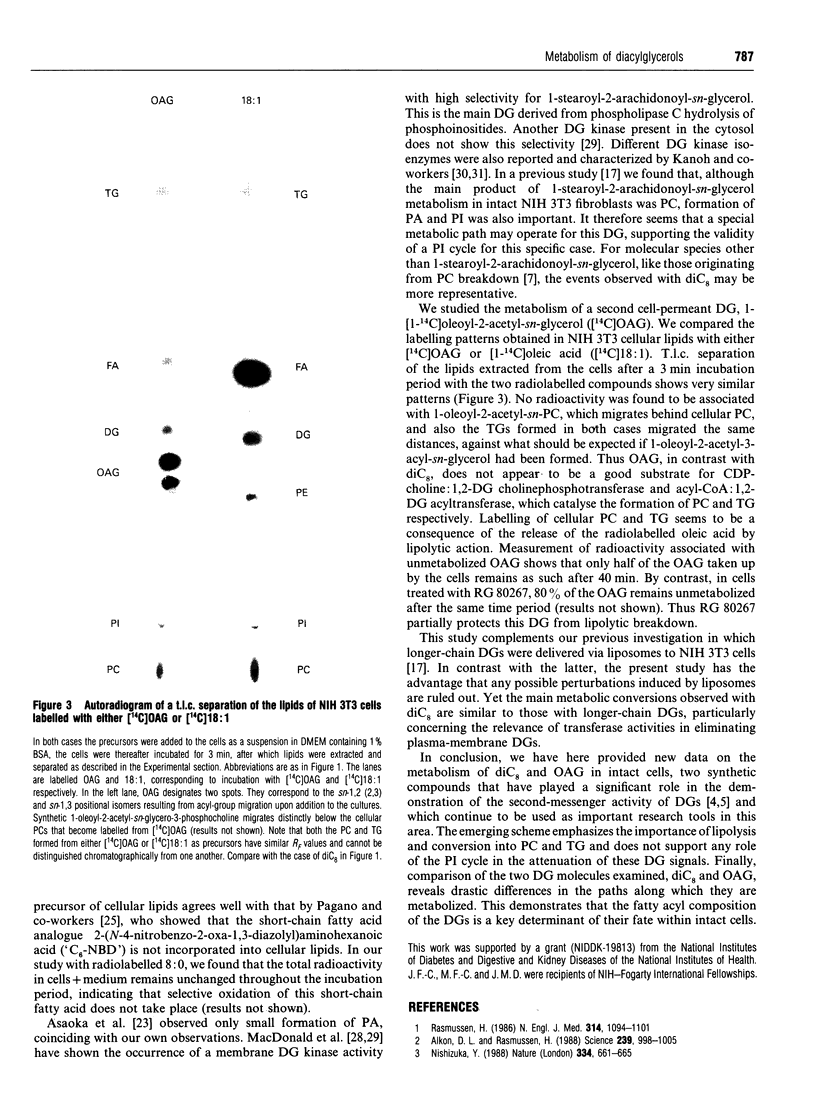
The metabolism of di[1-14C]octanoylglycerol metabolism was examined in four cell lines: NIH 3T3 fibroblasts, BHK cells, Pam 212 keratinocytes and WEHI 3BD+ cells. We found the direct conversion of 1,2-di[1-14C]octanoyl-sn-glycerol ([14C]diC8) into dioctanoylphosphatidylcholine and dioctanoylacylglycerol, but no formation of phosphatidylinositol. The [14C]diC8 also underwent lipolytic breakdown. In contrast, 1-[1-14C]oleoyl-2-acetyl-sn-glycerol was metabolized exclusively by lipolysis. Our findings support a new scheme for the metabolic termination of diacylglycerol signals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Metabolic rate of membrane-permeant diacylglycerol and its relation to human resting T-lymphocyte activation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8681–8685. doi: 10.1073/pnas.88.19.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Majerus P. W. Thrombin-induced hydrolysis of phosphatidylinositol in human platelets. J Biol Chem. 1980 Mar 10;255(5):1790–1792. [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers. Metabolism of exogenous diacylglycerols by human platelets. J Biol Chem. 1986 Sep 25;261(27):12513–12519. [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Florin-Christensen J., Florin-Christensen M., Delfino J. M., Stegmann T., Rasmussen H. Metabolic fate of plasma membrane diacylglycerols in NIH 3T3 fibroblasts. J Biol Chem. 1992 Jul 25;267(21):14783–14789. [PubMed] [Google Scholar]
- Gupta C. M., Radhakrishnan R., Khorana H. G. Glycerophospholipid synthesis: improved general method and new analogs containing photoactivable groups. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4315–4319. doi: 10.1073/pnas.74.10.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Richardson C. N., Glomset J. A. Regulation of diacylglycerol kinase reaction in Swiss 3T3 cells. Increased phosphorylation of endogenous diacylglycerol and decreased phosphorylation of didecanoylglycerol in response to platelet-derived growth factor. J Biol Chem. 1988 Jan 25;263(3):1575–1583. [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Williams B. W., King W. C., Glomset J. A. A membrane-bound diacylglycerol kinase that selectively phosphorylates arachidonoyl-diacylglycerol. Distinction from cytosolic diacylglycerol kinase and comparison with the membrane-bound enzyme from Escherichia coli. J Biol Chem. 1988 Jan 25;263(3):1584–1592. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J., Martin O. C. Intracellular translocation and metabolism of a fluorescent phosphatidic acid analogue in cultured fibroblasts. J Biol Chem. 1983 Feb 10;258(3):2034–2040. [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J., Martin O. C., Struck D. K. Metabolism and intracellular localization of a fluorescently labeled intermediate in lipid biosynthesis within cultured fibroblasts. J Cell Biol. 1981 Dec;91(3 Pt 1):872–877. doi: 10.1083/jcb.91.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J. Phosphorylation, transbilayer movement, and facilitated intracellular transport of diacylglycerol are involved in the uptake of a fluorescent analog of phosphatidic acid by cultured fibroblasts. J Biol Chem. 1985 Feb 10;260(3):1909–1916. [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane F., Yamada K., Kanoh H. Different effects of sphingosine, R59022 and anionic amphiphiles on two diacylglycerol kinase isozymes purified from porcine thymus cytosol. FEBS Lett. 1989 Sep 25;255(2):409–413. doi: 10.1016/0014-5793(89)81134-2. [DOI] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Vance D. E. Boehringer Mannheim Award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem Cell Biol. 1990 Oct;68(10):1151–1165. doi: 10.1139/o90-172. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]