Abstract
Resolving the detailed structures of metal organic frameworks is of great significance for understanding their structure-property relation. Real-space imaging methods could exhibit superiority in revealing not only the local structure but also the bulk symmetry of these complex porous materials, compared to reciprocal-space diffraction methods, despite the technical challenges. Here we apply a low-dose imaging technique to clearly resolve the atomic structures of building units in a metal-organic framework, MIL-125. An unexpected node structure is discovered by directly imaging the rotation of Ti-O nodes, different from the unrotated structure predicted by previous X-ray diffraction. The imaged structure and symmetry can be confirmed by the structural simulations and energy calculations. Then, the distribution of node rotation from the edge to the center of a MIL-125 particle is revealed by the image analysis of Ti-O rotation. The related defects and surface terminations in MIL-125 are also investigated in the real-space images. These results not only unraveled the node symmetry in MIL-125 with atomic resolution but also inspired further studies on discovering more unpredicted structural changes in other porous materials by real-space imaging methods.
Subject terms: Metal-organic frameworks, Imaging techniques
Resolving the structures of MOFs is significant for understanding their structure-property relation. Here, authors apply a low-dose imaging technique to resolve the atomic structures of building units in MOF MIL-125. A low-symmetry structure of MIL-125 is discovered by directly imaging the rotated Ti-O nodes, different from the unrotated one predicted in previous works.
Introduction
Diffraction and imaging are two important types of characterization methods for atomically resolving material structure in reciprocal space and real space, respectively. The characterization methods based on X-ray diffraction are usually considered to be efficient for determining periodic crystal structures and even resolving the accurate position of each atom1–5. Although the real-space imaging methods have played an irreplaceable role in characterizing local structures, such as surfaces, interfaces, and defects6–9 they have not demonstrated any superiority over the diffraction methods in analyzing periodic bulk lattices. However, for complex porous and reticular structures, such as zeolites and metal organic frameworks (MOFs), accurate structural analysis by X-ray diffraction is becoming increasingly limited. On the one hand, the intrinsic local flexibility in framework enables various non-periodic restructuring under different conditions10–13, while the diffraction methods only provide averaged information over bulk materials instead of these local changes. On the other hand, some of these porous materials exist in the form of nanocrystals, which are difficult to use single-crystal X-ray diffraction to determine their fine structures. In these cases, the imaging methods represented by electron microscopy should inevitably rise to the challenge of accurately resolving the complex structures of porous materials.
Metal-organic frameworks (MOFs)14–16, formed by metal nodes and organic linkers via coordination, have flexible networks and complex local structures that may not be identified by powder X-ray diffraction (PXRD) and simulation theoretically. However, although electron microscopy has been one of the most powerful tools to image lattices and local structures with a resolution of at least 50 pm17–19, it is still challenging to apply it to materials that are sensitive to electron beam20–23, such as zeolites and MOFs. Thus, there seems to be no reported case to exhibit such superiority of real-space imaging methods in these materials to date. Recently, the integrated differential phase contrast scanning transmission electron microscopy (iDPC-STEM)24–26 was confirmed to be efficient for low-dose imaging of beam-sensitive materials and light elements with high resolution and high signal-to-noise ratio27–29. It has been used to achieve sub-unit-cell imaging of various MOF structures below the electron dose threshold30,31.
In this work, we find a MOF system, MIL-125, in which the real-space imaging method can help to discover a rotated node structure different from the one predicted by previous PXRD method. Based on the iDPC-STEM, the bulk (nodes and linkers) and local structures (surfaces and defects) in MIL-125 can be investigated with high resolution and signal-to-noise ratio. We can identify the unidirectional rotation of the Ti-O node and its distribution caused by the node rotation, which is a stable structure that prevails in MIL-125 nanocrystals. Such fine structural characteristics cannot be displayed in the PXRD results with the current level of accuracy. These results provide a case study to illustrate the superiority of real-space imaging methods in certain situations of structural analysis and bring insights into the local flexibility of MOF structures.
Results
Atomic imaging of MIL-125
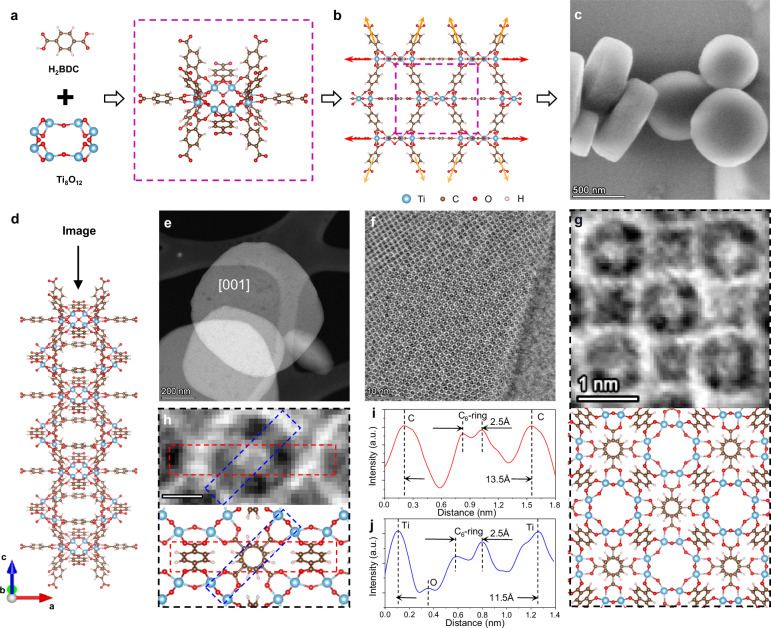
MIL-125, a class of titanium-based MOF formed by 1,4-benzenedicarboxylate (BDC) molecules and Ti-O nodes32, has the advantages of porous structure combined with the redox activity, photochemical property and biocompatibility33–35 of Ti species. Fig. 1a, b and Supplementary Fig. 1 show the structural model of the MIL-125 framework from building units to reticular structures, obtained by the Rietveld refinement of PXRD results (containing free oxygens). During a solvothermal process, the MIL-125 crystals show a higher growth rate along the plane direction of Ti-O octamers (the [100] and [010] directions, red arrows in Fig. 1b) than that along the vertical direction (the [001] direction, yellow arrows in Fig. 1b). Therefore, they have a plate-like shape as shown in the scanning electron microscopic (SEM) images in Fig. 1c and Supplementary Fig. 2.
Fig. 1. High resolution iDPC-STEM imaging of MIL-125.
a, b Structural models of MIL-125 framework. c SEM image of plate-like MIL-125 crystals. d Imaging direction in the model, along the short-c-axis. e HAADF-STEM image of viewed from the [001] direction. f IDPC-STEM image of MIL-125 viewed from the [001] direction. g Comparison between the magnified iDPC-STEM image and the model. h Magnified iDPC-STEM image and model of benzene-ring structure in BDC for detailed analysis. Scale bar, 0.5 nm. i, j Intensity profiles extracted from the red and blue dash-line frames in (h).
Such plate-like shape determines that we can observe the MIL-125 crystals by the STEM imaging from the [001] direction, where we can not only image the Ti-O nodes, but also the horizontal BDC linkers between them (as shown in Fig. 1d). Figure. 1e, f show the high angle annular dark field (HAADF) STEM and iDPC-STEM images of the thin MIL-125 plates viewed from the [001] direction (see more images in Supplementary Fig. 3). The magnified iDPC-STEM image in Fig. 1g exhibits the bulk lattice structure of MIL-125 with linked Ti-O nodes and BDC molecules, compared with the predicted structural model. And the clear benzene-ring structure in BDC can be further identified in Fig. 1h and analyzed by the intensity profiles in Fig. 1i, j.
The red and blue intensity profiles in Figs. 1i and 1j were extracted from the dash-boxed regions in Fig. 1h, and the intensity peaks were used to measure the projected distances between atoms in the iDPC-STEM image. As it corresponds to the structural model, Fig. 1i illustrates the diameter of benzene rings in the horizontal BDCs (2.5 Å) and the projected distance between the vertical BDCs on both sides (13.5 Å). Fig. 1j illustrates the diameter of benzene rings in the horizontal BDCs (2.5 Å) and the projected distance between the Ti atoms on both sides (11.5 Å). More profiles are given in Supplementary Fig. 4 to support these measured values, which are all consistent with the theoretical values in the model. These results confirm that with the help of iDPC-STEM we have achieved an ultra-high for the real-space imaging of MIL-125 lattice, demonstrating the effectiveness of this technique for further structural analysis from the perspectives of atoms and molecules in MOFs.
Rotated node structure in MIL-125
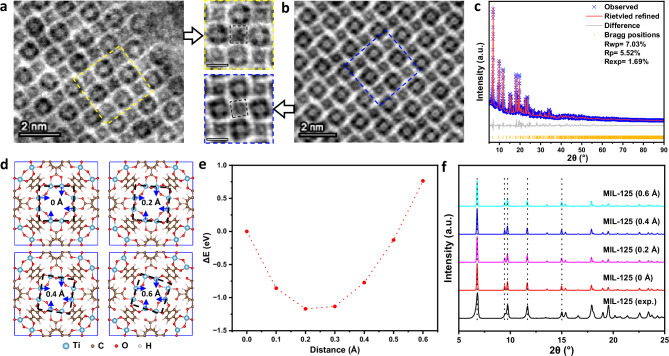
In addition to the unrotated nodes perfectly matched with the known structure (Fig. 2a), we also find another widely existing node structure caused by the rotation of Ti-O nodes (Fig. 2b), and the symmetry of framework changes from I4/mmm to P4. Such rotated node structure can be indicated by comparing the black dash frames in the magnified images of Fig. 2a, b. After observing various MIL-125 crystals, it is noticed that such rotated nodes are indeed dominant, and even nearly unrotated structures also contain some slight rotation of Ti-O nodes, which has not been ever found by previous diffraction methods. To explain the reason for the existence of node rotation in terms of energy, we conducted a more in-depth analysis by combining energy calculations with PXRD results. In Fig. 2c, we used the Rietveld refinement of PXRD results to obtain an initial unrotated model (Supplementary Fig. 1). Based on this refinement model, we manually adjust the positions of Ti atoms to obtain the rotated nodes (as shown in Fig. 2d and Supplementary Fig. 5) and calculate the energies of these structures using density functional theory (DFT) calculations. For example, in Fig. 2d, four Ti atoms were moved along the blue arrows with different displacements and then fixed to simulate the stable positions of other atoms. The obtained structures were referred to as MIL-125 (x), where x is the displacement of Ti atoms (representing different rotation angles of Ti-O nodes). The Ti-O nodes in MIL-125 (0 Å) can form an unrotated black dash frame, while those in MIL-125 (0.2 Å), MIL-125 (0.4 Å) and MIL-125 (0.6 Å) were adjusted to be rotated and disrupted the node symmetry (Fig. 2d). Based on these models, we simulated the corresponding iDPC-STEM images in Supplementary Figs. 6 and 7 to confirm that these models are consistent with the imaged results in Fig. 2a, b. The structure from lateral view was studied by electron diffraction in Supplementary Fig. 8.
Fig. 2. Discovering rotated node structure in MIL-125.
a, b Magnified iDPC-STEM images of MIL-125 showing unrotated (a) and rotated (b) node structures, respectively. Scale bars, 1 nm. c Rietveld PXRD refinement results of the MIL-125 sample. d Structural models of MIL-125 with the Ti displacements of 0 Å, 0.2 Å, 0.4 Å and 0.6 Å respectively. e Calculated potential energy differences varying with the Ti displacement. f Simulated PXRD results of the calculated MIL-125 (0 Å, 0.2 Å, 0.4 Å and 0.6 Å) structures compared with the experimentally measured results of the as-synthesized MIL-125 sample, the dashed lines indicats the consistent positions of the main peaks.
Then, the potential energies of these structures were calculated using the DFT method to study the stability of MIL-125 (x) structures. The relation between x and potential energy difference (ΔE, referenced to the initial unrotated node structure) was evaluated in Fig. 2e. It is surprisingly found that the rotated node structures are at energy-preferred states rather than metastable states. The rotated node structures at small Ti displacements (x < 0.5 Å) show lower potential energies than the unrotated node structure. That means the rotation of Ti-O nodes in MIL-125 is thermodynamically allowed and preferred. That is why we can generally find such rotated nodes in nearly all unit cells. We also changed the conditions within the synthesis window of MIL-125 samples, and different samples were obtained, all of which exhibited the rotated node structure (as shown in Supplementary Fig. 9), further confirming the universality of this structure energetically. To better understand why the rotated node structure cannot be identified by using diffraction methods, on the one hand, the strain analysis based on the iDPC-STEM image (as shown in Supplementary Fig. 10) exhibits that the strain distribution along the different axial directions in the MIL-125 crystal is quite uniform and the values of these strains only fluctuate up and down within a small range near the zero point, indicating that the strains were only induced by spontaneous node rotation rather than external stresses. On the other hand, we simulated the theoretical PXRD patterns of the rotated node structures with different Ti displacements (Fig. 2f and Supplementary Fig. 11) and compared them with the measured results. No shift of diffraction peaks can be observed in these models from x = 0 Å to x = 0.6 Å, which perfectly matches the measured ones. Therefore, it is impossible to directly distinguish the models by the PXRD peaks within such accuracy of PXRD measurement, which explains why real-space imaging is necessary for resolving such a complex porous framework, especially for the details in building units.
Distribution of node rotation in MIL-125
According to the rotated characteristics of Ti-O nodes discovered by the iDPC-STEM, we further exploited the advantages of this real-space imaging method to reveal the spatial distribution of such node rotation in a large range of view (Fig. 3a). First of all, the Ti-O nodes in the same projection show a coherent rotation chirality, that means they all rotate in one direction (counter-clockwise). Then, we found that the rotation angle changes with position (distance from the edge). In Fig. 3b, c, three different regions with different distance away from the edge (named as edge 1, 2 and 3, marked by different frames with corresponding colors in Fig. 3a). In the magnified images in Fig. 3c, the degrees of node rotation in these three regions are different and the Ti displacement should increase gradually away from the edge. Meanwhile, as shown in Fig. 3d, the region from the crystal center (about 200–300 nm away from the edge) show even higher degrees of node rotation. Based on the further analysis in Supplementary Figs. 2, 6, such distribution (different rotation angles) may not result from the sample thickness and imaging defocus, since the thickness variation from the edge to center of MIL-125 nanocrystals is only about 5% and the identification of node rotation is still efficient in the images even with a defocus of ±15 nm. And the lattice constant did not change in these three regions (Supplementary Fig. 12), indicating the local flexibility of the building units while the whole framework remains rigid. We also introduced the fast Fourier transform (FFT, representing diffraction information) patterns of these images from the edge to center of the MIL-125 nanocrystal and the simulated electron diffraction patterns of the models with different Ti-O rotation angles as shown in Supplementary Fig. 13. As we can find in these results, the position and symmetry of reflections in the diffraction patterns remain almost unchanged after Ti displacement (Ti-O rotation). However, such difference is difficult to identify in the diffraction experiments at current information transfer.
Fig. 3. Distribution of the node rotation in MIL-125 crystal from the edge to the center.
a Three selected regions (edge 1, 2 and 3) with different distances away from a MIL-125 crystal edge. b, c Magnified images of three regions in a, showing different rotation angles of Ti-O nodes in these regions. Scale bars, 1 nm. d Selected region of a MIL-125 crystal center in the iDPC-STEM image. e Definition of long spacing D1 and short spacing D2 in the MIL-125 (0.3 Å) model, the difference between which can be used to describe the rotation of Ti-O nodes. f Statistical results of the D1-D2 values in the four selected regions. g Adsorption capacities of the MIL-125 samples with different crystal sizes for CO2 and H2O at different partial pressures.
Therefore, the real-space imaging is the only way to quantitatively describe the rotation of Ti-O nodes or the displacement of Ti atoms, and we introduced a distance difference, D1-D2, that can be measured directly in the iDPC-STEM images. Two distances, D1 and D2, are defined as shown in Fig. 3e. It is not difficult to deduce that the value of D1-D2 should be zero in unrotated node structure. As the displacement of Ti atoms increases, the value of D1 becomes higher, while that of D2 becomes lower, making the value of D1-D2 increasing. To obtain a more comprehensive distribution of the node rotation, we made a statistic of the D1-D2 values in four regions (30 sets of data for each region). As shown in the histogram of the D1-D2 values in Fig. 3f, the closer to the crystal center, the higher the D1-D2 value, indicating a larger Ti displacement. It can also be confirmed by the fact that the unrotated structures in our sample were usually observed near the crystal edges instead of the crystal center. These imaging and analysis results on rotated node structures with high spatial resolution help to unravel the coherence of such node rotation. On the one hand, the strong linker-node coordination network makes the Ti-O nodes rotate in one direction to form a coherent chirality. On the other hand, unbalanced interactions on the edges (weak binding from the outside of the crystal) allow a gradual approach to a higher-energy unrotated node structure, especially for the Ti-O nodes on surfaces (further analyzed in Fig. 4).
Fig. 4. Effects of the node rotation on missing linkers.
a, b IDPC-STEM images of unrotated (a) and rotated (b) regions. c, d Intensity profiles extracted from the white frames in (a, b). The peak intensities of Ti-O nodes and C6-rings are marked by the blue and red bands, respectively. e Statistics of the normalized intensities of C6-ring peaks in the unrotated and rotated regions.
Moreover, these results mean that we can achieve different averaged rotation angles in the samples with different crystal sizes (diameters), which is important for us to explore the difference in properties of different rotated nodes structures. For example, a short distance away from the crystal edge usually demonstrates a small Ti displacement from x = 0 Å to x = 0.3 Å, while the crystal center shows consistent larger Ti displacement (x = 0.6 Å). Thus, for small crystals (with a diameter of about 0.5 μm), the averaged Ti displacement is usually near 0.3 Å, while for the larger ones (with a diameter of over 1.5 μm), the averaged Ti displacement may be much larger, approaching the highest value of x = 0.6 Å. Such node rotation could change the adsorption capacity of MOFs for specific molecules, especially for the molecules showing special interactions with nodes. Thus, we choose the adsorption performances to evaluate the structure-property relation of the rotated node structures. The adsorption results of the MIL-125 samples with different crystal diameters for H2O and CO2 are given in Fig. 3g (more data in Supplementary Fig. 15). As the crystal size increases, the adsorption capacities of both two gas molecules tend to decrease. Especially for 30% H2O, the adsorption capacity surprisingly decreases by over 35% when the crystal diameter changes from 0.5 to 1.5 μm, while the specific surface areas of these MIL-125 samples are almost the same based on the N2 adsorption results in Supplementary Fig. 15. Such large difference in H2O adsorption capacity should be due to its interaction with rotated nodes, which is further supported by our energy calculations on gas adsorption also in Supplementary Fig. 15.
Revealing local structures in MIL-125
In addition to macroscopic adsorption performance, more exploration of local structural properties of MOF samples can also help us deeply understand the significance of using real-space imaging to characterize their atomic structures. For example, we selected two regions with relatively unrotated (Fig. 4a) and rotated (Fig. 4b) node structures, respectively, and extracted two intensity profiles along the white frames marked in Figs. 4a and 4b. The normalized intensity results are given in Fig. 4c, d, respectively. All the peaks in these profiles can be divided into two parts, Ti-O peaks and C6-ring peaks, marked by colored bands. It is obvious that the normalized intensity of C6-rings in the unrotated region is stronger than that in the rotated region, even close to the intensity of Ti-O nodes. This indicates that the BDC molecules in the rotated region are more likely to dissociate from the framework (perhaps by the thermal vibration or electron beam irradiation), and the histograms of the C6-ring intensity also confirm such difference (Fig. 4e). Comparing the iDPC-STEM images before and after filtering (Supplementary Fig. 14), we find that this conclusion does not change with the filtering process of these images. This is because that the coordination bonds between nodes and linkers were stretched by the Ti atom displacements, which may lead to the missing of BDC molecules and produce more defects during our imaging process (as shown in the simulated models in Fig. 2d and Supplementary Fig. 5).
In addition to the missing-linker defects, surface terminations are also important local structures that attract wide attention in material science. As shown in Supplementary Fig. 16, we can capture the iDPC-STEM images of the edges in different regions of a particle. These edges can be attributed to two surface structures in two directions, the (110) and (100) surfaces, respectively. Figure 5a–d shows the terminations of the (110) surface. As shown in Fig. 5a, b, the switching between two types of terminations on the (110) surface can be observed at the saw teeth or the steps. After a more detailed observation, the termination with incomplete nodes in Fig. 5c is defined as type A, while that with complete nodes in Fig. 5d is defined as type B. Then, the identification of A/B types in Fig. 5b illustrates the process of outward growth of MIL-125 crystals. First, the BDC molecules on surfaces coordinate with the incomplete nodes outside (type A). Then, the nodes gradually form the complete octamer rings (type B), and the complete nodes connect with the new BDC molecules to grow to the next layer. Meanwhile, the (100) surfaces are relatively flat and terminated mainly by the complete nodes and dangling BDC linkers (type C), as shown in Fig. 5e, f. Moreover, we used the desorption energies (ΔE) of O atoms bonded by surface Ti atoms to indicate the stability of each surface structure (as shown in Supplementary Fig. 17). The calculations explain why the type A and B terminations, which have similar ΔE, alternate simultaneously on the (110) surface, while only type C termination was observed on the (100) surface. And the coexistence of (110) and (100) surfaces lead to an irregular round shape of MIL-125 crystals viewed from the [001] direction.
Fig. 5. Imaging of the surface terminations in MIL-125 crystal.

a, b Imaging the (110) surface structure with the switching of two different types of terminations. c, d Definition of type A and B terminations on the (110) surface. Scale bars, 1 nm. e, f Definition of type C termination on the (100) surface. Scale bars, 1 nm.
In summary, this work exhibits the effectiveness of iDPC-STEM for beam-sensitive materials and the advantages of real-space imaging over diffraction method for studying porous materials through the imaging and analysis of MIL-125 lattice structures. The iDPC-STEM imaging helped us reveal the unexpected rotated node feature of MIL-125 lattice caused by the rotation of Ti-O nodes, which has not been realized by the previous structural analysis combining the PXRD and simulation. Then, the first-principle calculations explained why such rotated node structure exists and it cannot be identified in the PXRD patterns. From the edge to the center of MIL-125 particle, the rotation angles of Ti-O nodes show a clear trend of progressive increase, while the defects and surface terminations in MIL-125 are also related to such distribution of Ti-O node rotation. The discovery of rotated node structure in MIL-125 provides insights on local flexibility and node-linker coordination in reticular chemistry, which may guide our further study of the structure-property relation in MOFs at the molecular and atomic scales. Moreover, in this case, the real-space imaging methods represented by electron microscopy can contribute to a more accurate structural analysis on these complex porous materials than the diffraction methods, which may help us confirm the bulk lattice structures in these materials. It will encourage more researchers to reasonably question the previous diffraction analysis of complex structures and reveal more local structural changes in these materials through real-space imaging. Thus, the analysis of these local structures allows us to make a more accurate perception of crystals and better understand the node-linker coordination. All the results showed the promising application of iDPC-STEM with powerful low-dose, element-light imaging features for resolving complex structures of electron-sensitive materials.
Methods
Synthesis of MIL-125 crystals
The plate-like MIL-125 crystals were prepared by a solvothermal method. Typically, 1 mL of tetrabutyl titanate (TBT) and 1.75 g of 1,4-benzene dicarboxylate (BDC) were dissolved into a 24 mL mixture of N,N-dimethylformamide (DMF) and ethanol (7:1, v/v). The mixture was stirred for 5 min, then transferred to a 200 mL Teflon-line autoclave and heated at 130 °C for 15 h. Afterwards, the white solid product was collected by washing it with methanol three times. Then the product was dried under vacuum at 70 °C for 12 h.
Imaging conditions and simulations
The iDPC-STEM images were obtained using a Cs-corrected STEM (FEI Titan Cubed Themis G2 300) that was operated at 300 kV and equipped with a DCOR+ spherical aberration corrector. The convergence semi-angle is 15 mrad. The collection angle is 3–18 mrad. The beam current is about 0.1 pA (measured by a pixel array detector). The dwell time for each pixel is 16 μs. The pixel size is 0.5027 × 0.5027 Å2.
The strain distribution in the selected regions of MIL-125 was analyzed by means of geometric phase analysis (GPA), which is widely used to map the external strains, based on the same iDPC-STEM image (Fig. 3a). According to the method given in previous work36, the calculations of symmetric strain tensor were carried out in Gatan Digital Micrograph as a plug-in. The range of maximum and minimum strains were set from 5% to −5%. In reciprocal space, the strain maps at each area were plotted according to the shift of lattice positions of g1 = (200) and g2 = (020) through Lorentzian mask with a diameter of 0.5 nm−1. The reference region and corresponding FFT pattern are given in Supplementary Fig. 10.
The image simulations were conducted by a three-layered model of MIL-125, which is applied for simulation using the ToTEM software37. The parameters for simulations, such as the convergence angle, the collection angle and the applied dose were selected to be the same as those in our imaging experiments. Four images were separately collected by four segments of the DF4 detector and then integrated into the simulated iDPC-STEM images.
DFT simulations
All calculations were carried out using VASP (Vienna Ab-initial Simulation Package, VASP) software 5.4.438–40, the exchange function was described by PBE (Perdew, Burke and Ernzerhof) functional parameterization of GGA (Generalized Gradient Approximation, GGA) of DFT (Density of Functional Theory)41. And these calculations took DFT-D3 correction for London disperse with Becke-johnson damping into consideration42. The PAW (Projector Augmented Wave) method was used account for core-valence interactions43,44. The kinetic energy cutoff for plane wave expansions was set 400 eV, and reciprocal space was sampled by Γ-centered Monkhorst-Pack scheme with a grid of 1 1 1. The convergence criteria are 1 ×10−5 eV energy difference for solving the electronic wave function. All atomic coordinates were converged within 1 × 10−2 eV Å−1 for maximal components of force. Next, we obtained rotated MIL-125 models from the conventional cell, getting from refining XRD, and adjusted the position of part Ti node inward by a fixed distance (0.1 Å) forming a rotated angle as shown in Supplementary Fig. 5. During the structural optimization, most atoms are relaxed except Ti atoms. All PXRD calculations are obtained using Pyxtal45, where profiling function is gaussian. In terms of desorbed energy of O atom and absorbed energy of CO2 and H2O, small molecules were inserted into the hole of different rotated MIL-125 using python lib ase46 forming surf-CO2 and surf-H2O models, the calculated equations are as followed:
| 1 |
| 2 |
Esurf-O is the energy of MIL-125 surface subtracting an O atom, Esurf is the energy of MIL-125 surface, is the energy of O2 in gas; Esurf-mol is the whole energy including CO2 or H2O molecule absorbed into the tunnel of MIL-125 cell, Esurf is the energy of MIL-125 cell, Emol is the energy of CO2 or H2O.
Other characterizations
The PXRD results were obtained by an Empyrean diffractometer (PANalytical) using Cu Kα radiation at 40 kV and 40 mA. The Rietveld PXRD refinement results were obtained by TOPAS V5.0.
The N2 isotherm was measured using BSD-660M (Beishide Instrument Technology) at −196 °C. Before adsorption measurements, 50 mg of MIL-125 samples were activated at 150 °C under vacuum for 3 h. The CO2 and H2O isotherm were measured using BSD-DVS (Beishide Instrument Technology) at 25 °C. Before adsorption measurements, 50 mg of MIL-125 samples were activated at 150 °C under high-purity nitrogen for 3 h.
The HAADF-STEM imaging was also conducted in the same Cs-corrected STEM (FEI Titan Cubed Themis G2 300) operated at 300 kV.
The SEM images were captured by Zeiss G500 with an operating voltage of 3–5 kV.
Supplementary information
Source data
Acknowledgements
This work was supported by National Natural Science Foundation of China (22275133), Science Foundation of Jiangsu Province (BK20220484), Suzhou Key Laboratory of Functional Nano & Soft Materials, Collaborative Innovation Center of Suzhou Nano Science & Technology, the 111 Project, Joint International Research Laboratory of Carbon-Based Functional Materials and Devices.
Author contributions
B. Shen conceived this project and designed the studies; H.M., M.M. and L.W. prepared the MOF specimens; B. Shen, X.C. and F.W. performed the imaging experiments; L.X. and T.C. carried out the DFT simulations; B. Song, X.T. and S.D. helped on the experiments during the revision; All authors are involved in the analysis and discussion; J.F. and Z.F. analyzed the imaging data and wrote the manuscript with the help of others.
Peer review
Peer review information
Nature Communications thanks Lin Gu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that all relevant data supporting the findings of this study are available in the paper and its Supplementary Information files or from the corresponding authors upon request. Source Data file has also been deposited in Figshare under the accession link 10.6084/m9.figshare.2590639347. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiale Feng, Zhipeng Feng, Liang Xu, Haibing Meng.
Contributor Information
Xiao Chen, Email: chenx123@tsinghua.edu.cn.
Fei Wei, Email: wf-dce@tsinghua.edu.cn.
Tao Cheng, Email: tcheng@suda.edu.cn.
Boyuan Shen, Email: byshen@suda.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-51384-9.
References
- 1.Bragg, W. L. & Bragg, W. H. The structure of some crystals as indicated by their diffraction of X-rays. Proc. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character89, 248–277 (1997). [Google Scholar]
- 2.Harris, K. D. M., Tremayne, M., Lightfoot, P. & Bruce, P. G. Crystal Structure Determination from Powder Diffraction Data by Monte Carlo Methods. J. Am. Chem. Soc.116, 3543–3547 (1994). 10.1021/ja00087a047 [DOI] [Google Scholar]
- 3.Harris, K. D. M. & Cheung, E. Y. How to determine structures when single crystals cannot be grown: opportunities for structure determination of molecular materials using powder diffraction data. Chem. Soc. Rev.33, 526–538 (2004). 10.1039/b409059b [DOI] [PubMed] [Google Scholar]
- 4.Easun, T. L., Moreau, F., Yan, Y., Yang, S. & Schröder, M. Structural and dynamic studies of substrate binding in porous metal–organic frameworks. Chem. Soc. Rev.46, 239–274 (2017). 10.1039/C6CS00603E [DOI] [PubMed] [Google Scholar]
- 5.Feyand, M. et al. Automated Diffraction Tomography for the Structure Elucidation of Twinned, Sub-micrometer Crystals of a Highly Porous, Catalytically Active Bismuth Metal–Organic Framework. Angew. Chem. Int. Ed.51, 10373–10376 (2012). 10.1002/anie.201204963 [DOI] [PubMed] [Google Scholar]
- 6.Ma, M. et al. Atomically Unraveling the Structural Evolution of Surfaces and Interfaces in Metal Halide Perovskite Quantum Dots. Adv. Mater.35, 2300653 (2023). 10.1002/adma.202300653 [DOI] [PubMed] [Google Scholar]
- 7.Shen, B. et al. Atomic Spatial and Temporal Imaging of Local Structures and Light Elements inside Zeolite Frameworks. Adv. Mater.32, 1906103 (2020). 10.1002/adma.201906103 [DOI] [PubMed] [Google Scholar]
- 8.Li, Q. et al. Local Structure Insight into Hydrogen Evolution Reaction with Bimetal Nanocatalysts. J. Am. Chem. Soc.144, 20298–20305 (2022). 10.1021/jacs.2c07844 [DOI] [PubMed] [Google Scholar]
- 9.Zhou, J. et al. Cryogenic Focused Ion Beam Enables Atomic-Resolution Imaging of Local Structures in Highly Sensitive Bulk Crystals and Devices. J. Am. Chem. Soc.144, 3182–3191 (2022). 10.1021/jacs.1c12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong, H. et al. In situ imaging of the sorption-induced subcell topological flexibility of a rigid zeolite framework. Science376, 491–496 (2022). 10.1126/science.abn7667 [DOI] [PubMed] [Google Scholar]
- 11.Feng, T. et al. A Robust Mixed-Lanthanide PolyMOF Membrane for Ratiometric Temperature Sensing. Angew. Chem. Int. Ed.59, 21752–21757 (2020). 10.1002/anie.202009765 [DOI] [PubMed] [Google Scholar]
- 12.Liu, X.-L. et al. An Organophilic Pervaporation Membrane Derived from Metal–Organic Framework Nanoparticles for Efficient Recovery of Bio-Alcohols. Angew. Chem. Int. Ed.50, 10636–10639 (2011). 10.1002/anie.201104383 [DOI] [PubMed] [Google Scholar]
- 13.Denny, M. S. Jr. & Cohen, S. M. In Situ Modification of Metal–Organic Frameworks in Mixed-Matrix Membranes. Angew. Chem. Int. Ed.54, 9029–9032 (2015). 10.1002/anie.201504077 [DOI] [PubMed] [Google Scholar]
- 14.Li, H., Eddaoudi, M., O’Keeffe, M. & Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature402, 276–279 (1999). 10.1038/46248 [DOI] [Google Scholar]
- 15.Yaghi, O. M. & Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc.117, 10401–10402 (1995). 10.1021/ja00146a033 [DOI] [Google Scholar]
- 16.Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science341, 1230444 (2013). 10.1126/science.1230444 [DOI] [PubMed] [Google Scholar]
- 17.Haider, M. et al. Electron microscopy image enhanced. Nature392, 768–769 (1998). 10.1038/33823 [DOI] [Google Scholar]
- 18.Erni, R., Rossell, M. D., Kisielowski, C. & Dahmen, U. Atomic-Resolution Imaging with a Sub-50-pm Electron Probe. Phys. Rev. Lett.102, 096101 (2009). 10.1103/PhysRevLett.102.096101 [DOI] [PubMed] [Google Scholar]
- 19.Hage, F. S., Radtke, G., Kepaptsoglou, D. M., Lazzeri, M. & Ramasse, Q. M. Single-atom vibrational spectroscopy in the scanning transmission electron microscope. Science367, 1124–1127 (2020). 10.1126/science.aba1136 [DOI] [PubMed] [Google Scholar]
- 20.Zhang, D. et al. Atomic-resolution transmission electron microscopy of electron beam–sensitive crystalline materials. Science359, 675–679 (2018). 10.1126/science.aao0865 [DOI] [PubMed] [Google Scholar]
- 21.Wang, L. et al. Real-Space Imaging of the Molecular Changes in Metal–Organic Frameworks under Electron Irradiation. ACS Nano17, 4740–4747 (2023). 10.1021/acsnano.2c11110 [DOI] [PubMed] [Google Scholar]
- 22.Egerton, F. R. Mechanisms of radiation damage in beam-sensitive specimens, for TEM accelerating voltages between 10 and 300 kV. Microsc. Res. Tech.75, 1550–1556 (2012). 10.1002/jemt.22099 [DOI] [PubMed] [Google Scholar]
- 23.Susi, T. et al. Atomistic Description of Electron Beam Damage in Nitrogen-Doped Graphene and Single-Walled Carbon Nanotubes. ACS Nano6, 8837–8846 (2012). 10.1021/nn303944f [DOI] [PubMed] [Google Scholar]
- 24.Lazić, I., Bosch, E. G. T. & Lazar, S. Phase contrast STEM for thin samples: Integrated differential phase contrast. Ultramicroscopy160, 265–280 (2016). 10.1016/j.ultramic.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 25.Yücelen, E., Lazić, I. & Bosch, E. G. T. Phase contrast scanning transmission electron microscopy imaging of light and heavy atoms at the limit of contrast and resolution. Sci. Rep.8, 2676 (2018). 10.1038/s41598-018-20377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majert, S. & Kohl, H. High-resolution STEM imaging with a quadrant detector—Conditions for differential phase contrast microscopy in the weak phase object approximation. Ultramicroscopy148, 81–86 (2015). 10.1016/j.ultramic.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Shen, B. et al. Atomic imaging of zeolite-confined single molecules by electron microscopy. Nature607, 703–707 (2022). 10.1038/s41586-022-04876-x [DOI] [PubMed] [Google Scholar]
- 28.Shen, B. et al. Resolving atomic SAPO-34/18 intergrowth architectures for methanol conversion by identifying light atoms and bonds. Nat. Commun.12, 2212 (2021). 10.1038/s41467-021-22438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, B. et al. A single-molecule van der Waals compass. Nature592, 541–544 (2021). 10.1038/s41586-021-03429-y [DOI] [PubMed] [Google Scholar]
- 30.Shen, B., Chen, X., Shen, K., Xiong, H. & Wei, F. Imaging the node-linker coordination in the bulk and local structures of metal-organic frameworks. Nat. Commun.11, 2692 (2020). 10.1038/s41467-020-16531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, M. et al. Real-Space Imaging of the Node–Linker Coordination on the Interfaces between Self-Assembled Metal–Organic Frameworks. Nano Lett.22, 9928–9934 (2022). 10.1021/acs.nanolett.2c03375 [DOI] [PubMed] [Google Scholar]
- 32.Dan-Hardi, M. et al. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc.131, 10857–10859 (2009). 10.1021/ja903726m [DOI] [PubMed] [Google Scholar]
- 33.Zhu, J., Li, P.-Z., Guo, W., Zhao, Y. & Zou, R. Titanium-based metal–organic frameworks for photocatalytic applications. Coord. Chem. Rev.359, 80–101 (2018). 10.1016/j.ccr.2017.12.013 [DOI] [Google Scholar]
- 34.Schneider, J. et al. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev.114, 9919–9986 (2014). 10.1021/cr5001892 [DOI] [PubMed] [Google Scholar]
- 35.Chen, X. et al. Recent advances in titanium metal–organic frameworks and their derived materials: Features, fabrication, and photocatalytic applications. Chem. Eng. J.395, 125080 (2020). 10.1016/j.cej.2020.125080 [DOI] [Google Scholar]
- 36.Hÿtch, M. J., Snoeck, E. & Kilaas, R. Quantitative measurement of displacement and strain fields from HREM micrographs. Ultramicroscopy74, 131–146 (1998). 10.1016/S0304-3991(98)00035-7 [DOI] [Google Scholar]
- 37.Yuan, P. J. et al. ToTEM: A software for fast TEM image simulation. J. Microsc.287, 93–104 (2022). 10.1111/jmi.13127 [DOI] [PubMed] [Google Scholar]
- 38.Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B47, 558–561 (1993). 10.1103/PhysRevB.47.558 [DOI] [PubMed] [Google Scholar]
- 39.Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B54, 11169–11186 (1996). 10.1103/PhysRevB.54.11169 [DOI] [PubMed] [Google Scholar]
- 40.Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci.6, 15–50 (1996). 10.1016/0927-0256(96)00008-0 [DOI] [PubMed] [Google Scholar]
- 41.Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett.77, 3865–3868 (1996). 10.1103/PhysRevLett.77.3865 [DOI] [PubMed] [Google Scholar]
- 42.Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys.132, 154104 (2010). 10.1063/1.3382344 [DOI] [PubMed] [Google Scholar]
- 43.Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953–17979 (1994). 10.1103/PhysRevB.50.17953 [DOI] [PubMed] [Google Scholar]
- 44.Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B59, 1758–1775 (1999). 10.1103/PhysRevB.59.1758 [DOI] [Google Scholar]
- 45.Fredericks, S., Parrish, K., Sayre, D. & Zhu, Q. PyXtal: A Python library for crystal structure generation and symmetry analysis. Comput. Phys. Commun.261, 107810 (2021). 10.1016/j.cpc.2020.107810 [DOI] [Google Scholar]
- 46.Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys.: Condens. Matter29, 273002 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Feng, J., Shen, B. Real-space imaging for discovering a rotated node structure in metal-organic framework. Figshare10.6084/m9.figshare.25906393 (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all relevant data supporting the findings of this study are available in the paper and its Supplementary Information files or from the corresponding authors upon request. Source Data file has also been deposited in Figshare under the accession link 10.6084/m9.figshare.2590639347. Source data are provided with this paper.