Abstract
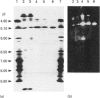
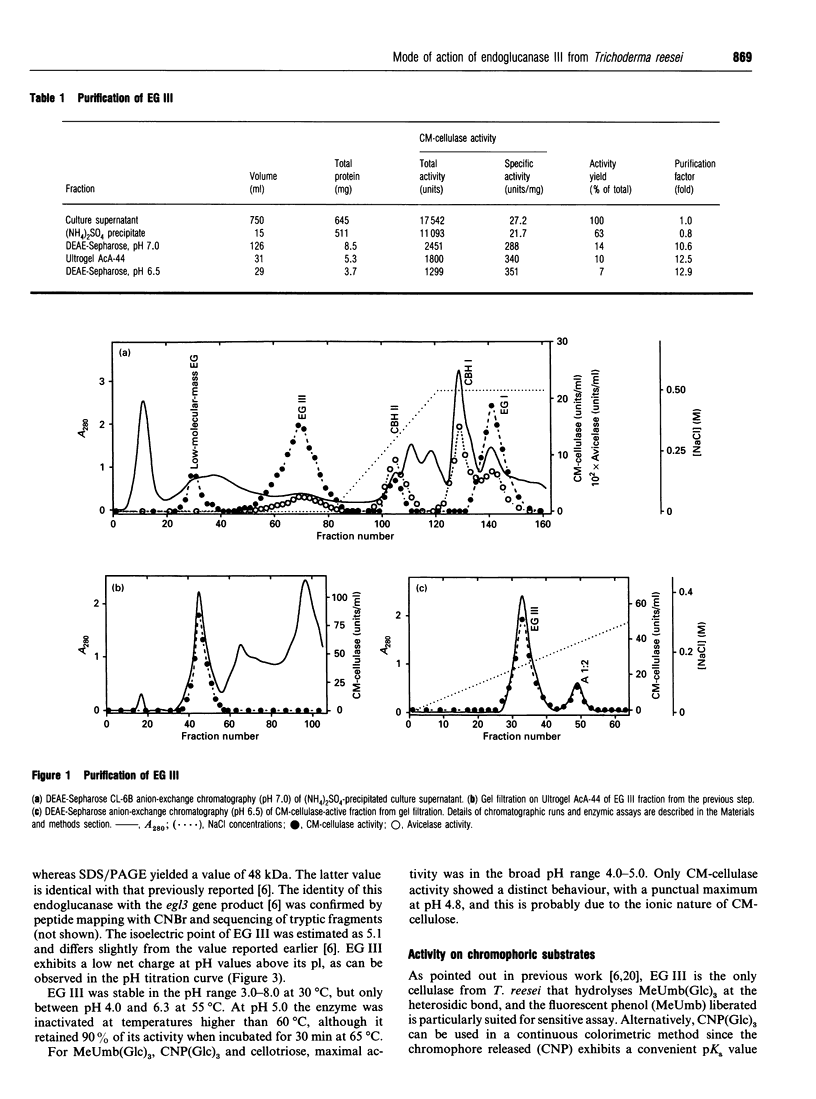
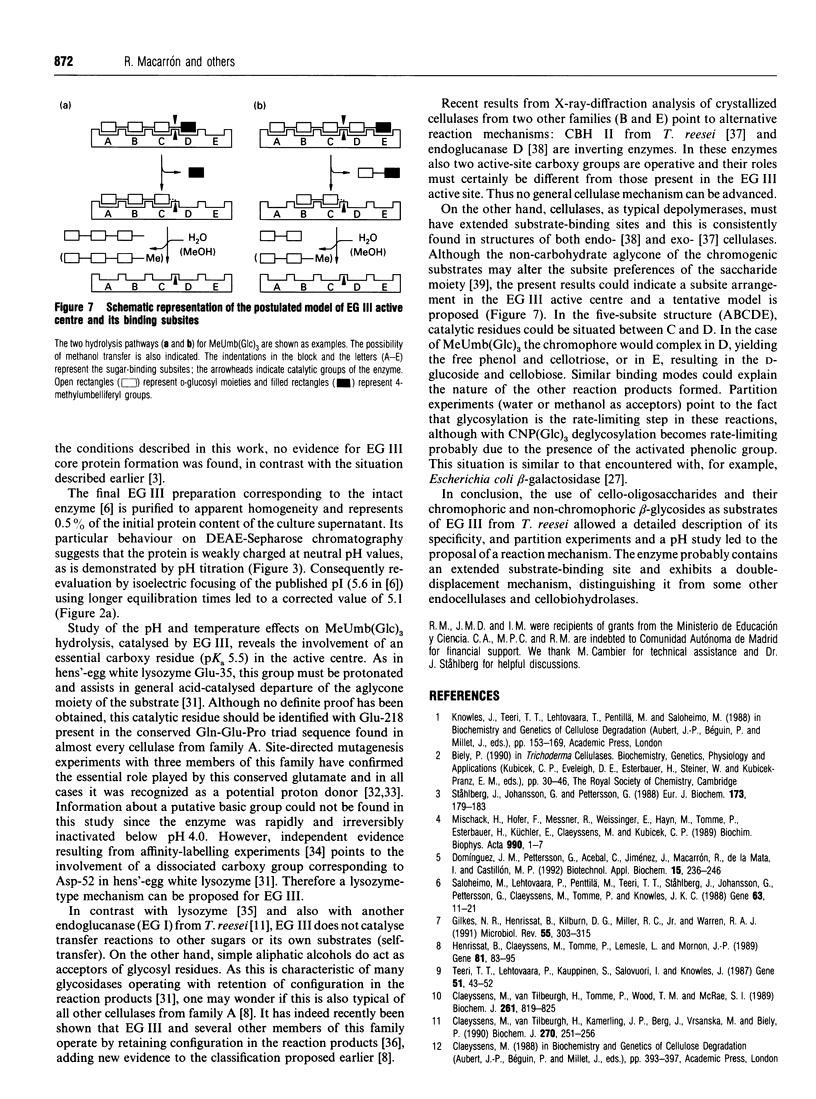
Endoglucanase III (EG III) was purified to homogeneity from the culture medium of Trichoderma reesei QM 9414. It has a molecular mass of 48 kDa, and an isoelectric point of 5.1. Maximal activity was observed between pH4 and 5. Celloligosaccharides and their chromophoric derivatives were used as substrates, and the reaction products were analysed by quantitative h.p.l.c. Nucleophilic competition experiments (between methanol and water) allowed unequivocal assessment of cleavage sites. EG III preferentially released cellobiose (or the corresponding glycoside) from the reducing end of the higher cellodextrins. A putative binding model containing five subsites is proposed. The pH-dependence of 4'-methylumbelliferyl beta-cellotrioside hydrolysis indicates the presence of a protonated group with a pK 5.5 in the reaction mechanism, and the possible involvement of a carboxy group is corroborated by a temperature study (delta Hion = -15.9 J/mol). This, together with independent evidence from affinity-labelling experiments [Tomme, Macarrón and Claeyssens (1991) Cellulose '91, New Orleans, Abstr. 32] and n.m.r. studies [Gebbler, Gilkes, Claeyssens, Wilson, Béguin, Wakarchuk, Kilburn, Miller, Warren and Withers (1992) J. Biol. Chem. 267, 12559-12561], favours the assumption of a lysozyme-type (retention of configuration, two essential carboxy groups) mechanism for this family A cellulase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird S. D., Hefford M. A., Johnson D. A., Sung W. L., Yaguchi M., Seligy V. L. The Glu residue in the conserved Asn-Glu-Pro sequence of two highly divergent endo-beta-1,4-glucanases is essential for enzymatic activity. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1035–1039. doi: 10.1016/0006-291x(90)91998-8. [DOI] [PubMed] [Google Scholar]
- Bhat K. M., Hay A. J., Claeyssens M., Wood T. M. Study of the mode of action and site-specificity of the endo-(1----4)-beta-D-glucanases of the fungus Penicillium pinophilum with normal, 1-3H-labelled, reduced and chromogenic cello-oligosaccharides. Biochem J. 1990 Mar 1;266(2):371–378. doi: 10.1042/bj2660371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhikhabhai R., Johansson G., Pettersson G. Isolation of cellulolytic enzymes from Trichoderma reesei QM 9414. J Appl Biochem. 1984 Oct-Dec;6(5-6):336–345. [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Claeyssens M., Tomme P., Brewer C. F., Hehre E. J. Stereochemical course of hydrolysis and hydration reactions catalysed by cellobiohydrolases I and II from Trichoderma reesei. FEBS Lett. 1990 Apr 9;263(1):89–92. doi: 10.1016/0014-5793(90)80712-r. [DOI] [PubMed] [Google Scholar]
- Claeyssens M., Van Tilbeurgh H., Tomme P., Wood T. M., McRae S. I. Fungal cellulase systems. Comparison of the specificities of the cellobiohydrolases isolated from Penicillium pinophilum and Trichoderma reesei. Biochem J. 1989 Aug 1;261(3):819–825. doi: 10.1042/bj2610819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeyssens M., van Tilbeurgh H., Kamerling J. P., Berg J., Vrsanska M., Biely P. Studies of the cellulolytic system of the filamentous fungus Trichoderma reesei QM 9414. Substrate specificity and transfer activity of endoglucanase I. Biochem J. 1990 Aug 15;270(1):251–256. doi: 10.1042/bj2700251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. Determining the chemical mechanisms of enzyme-catalyzed reactions by kinetic studies. Adv Enzymol Relat Areas Mol Biol. 1977;45:273–387. doi: 10.1002/9780470122907.ch4. [DOI] [PubMed] [Google Scholar]
- Estrada P., Mata I., Dominguez J. M., Castillón M. P., Acebal C. Kinetic mechanism of beta-glucosidase from Trichoderma reesei QM 9414. Biochim Biophys Acta. 1990 Mar 26;1033(3):298–304. doi: 10.1016/0304-4165(90)90137-l. [DOI] [PubMed] [Google Scholar]
- Gebler J., Gilkes N. R., Claeyssens M., Wilson D. B., Béguin P., Wakarchuk W. W., Kilburn D. G., Miller R. C., Jr, Warren R. A., Withers S. G. Stereoselective hydrolysis catalyzed by related beta-1,4-glucanases and beta-1,4-xylanases. J Biol Chem. 1992 Jun 25;267(18):12559–12561. [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrissat B., Claeyssens M., Tomme P., Lemesle L., Mornon J. P. Cellulase families revealed by hydrophobic cluster analysis. Gene. 1989 Sep 1;81(1):83–95. doi: 10.1016/0378-1119(89)90339-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mischak H., Hofer F., Messner R., Weissinger E., Hayn M., Tomme P., Esterbauer H., Küchler E., Claeyssens M., Kubicek C. P. Monoclonal antibodies against different domains of cellobiohydrolase I and II from Trichoderma reesei. Biochim Biophys Acta. 1989 Jan 27;990(1):1–7. doi: 10.1016/s0304-4165(89)80003-0. [DOI] [PubMed] [Google Scholar]
- Py B., Bortoli-German I., Haiech J., Chippaux M., Barras F. Cellulase EGZ of Erwinia chrysanthemi: structural organization and importance of His98 and Glu133 residues for catalysis. Protein Eng. 1991 Feb;4(3):325–333. doi: 10.1093/protein/4.3.325. [DOI] [PubMed] [Google Scholar]
- Rouvinen J., Bergfors T., Teeri T., Knowles J. K., Jones T. A. Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990 Jul 27;249(4967):380–386. doi: 10.1126/science.2377893. [DOI] [PubMed] [Google Scholar]
- Saloheimo M., Lehtovaara P., Penttilä M., Teeri T. T., Ståhlberg J., Johansson G., Pettersson G., Claeyssens M., Tomme P., Knowles J. K. EGIII, a new endoglucanase from Trichoderma reesei: the characterization of both gene and enzyme. Gene. 1988;63(1):11–22. doi: 10.1016/0378-1119(88)90541-0. [DOI] [PubMed] [Google Scholar]
- Ståhlberg J., Johansson G., Pettersson G. A binding-site-deficient, catalytically active, core protein of endoglucanase III from the culture filtrate of Trichoderma reesei. Eur J Biochem. 1988 Apr 5;173(1):179–183. doi: 10.1111/j.1432-1033.1988.tb13982.x. [DOI] [PubMed] [Google Scholar]
- Teeri T. T., Lehtovaara P., Kauppinen S., Salovuori I., Knowles J. Homologous domains in Trichoderma reesei cellulolytic enzymes: gene sequence and expression of cellobiohydrolase II. Gene. 1987;51(1):43–52. doi: 10.1016/0378-1119(87)90472-0. [DOI] [PubMed] [Google Scholar]
- Ulker A., Sprey B. Characterization of an unglycosylated low molecular weight 1,4-beta-glucan-glucanohydrolase of Trichoderma reesei. FEMS Microbiol Lett. 1990 Jun 1;57(3):215–219. doi: 10.1111/j.1574-6968.1990.tb04232.x. [DOI] [PubMed] [Google Scholar]
- de la Mata I., Estrada P., Macarrón R., Dominguez J. M., Castillón M. P., Acebal C. Chemical mechanism of beta-glucosidase from Trichoderma reesei QM 9414. pH-dependence of kinetic parameters. Biochem J. 1992 May 1;283(Pt 3):679–682. [PMC free article] [PubMed] [Google Scholar]