Abstract
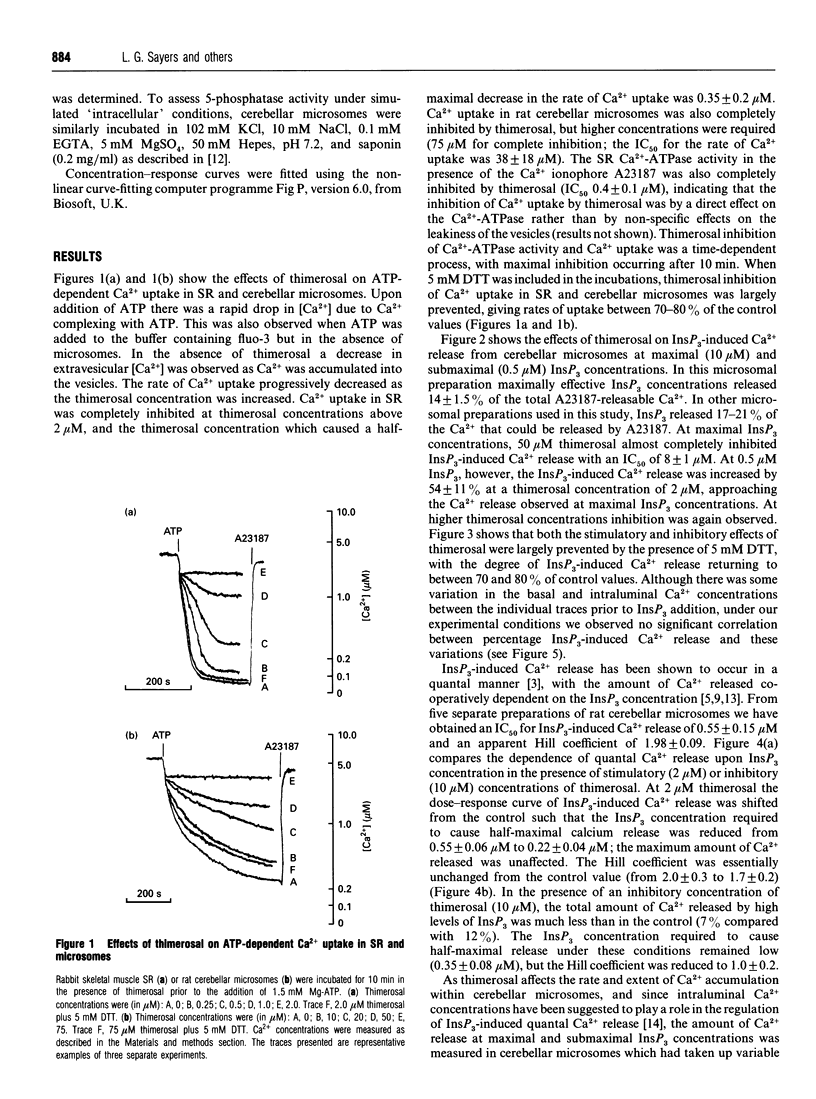
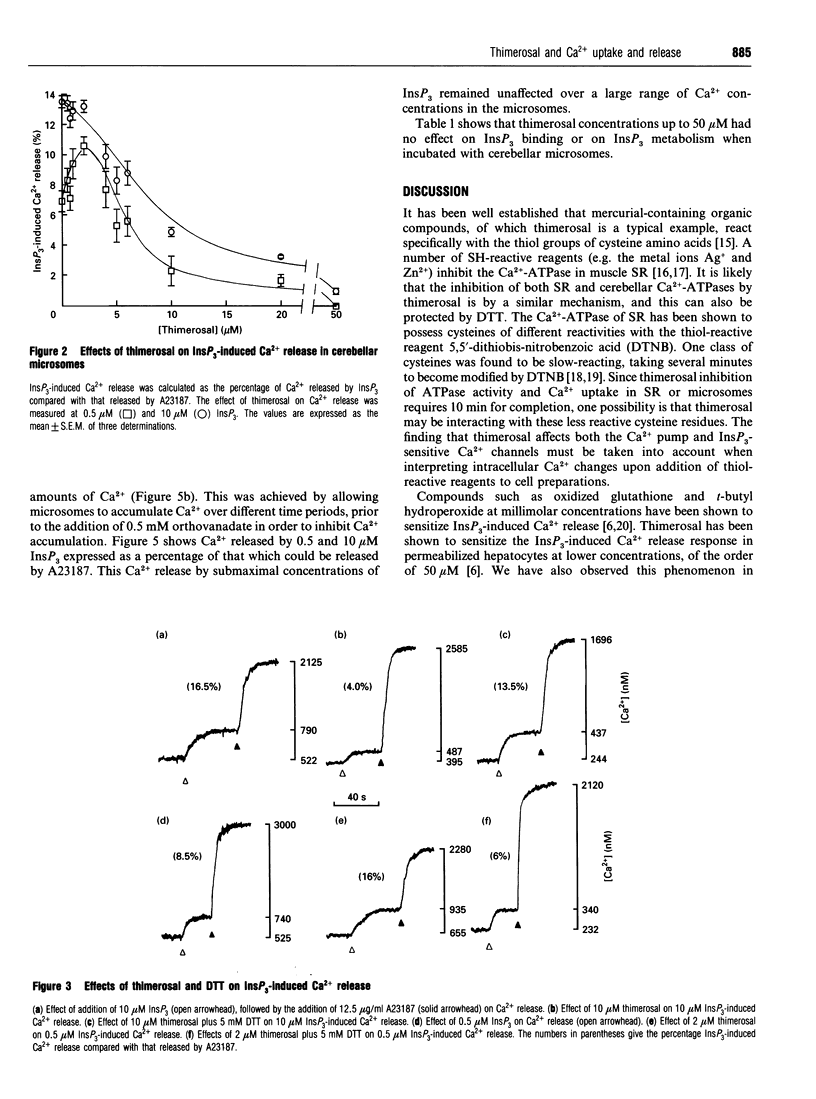
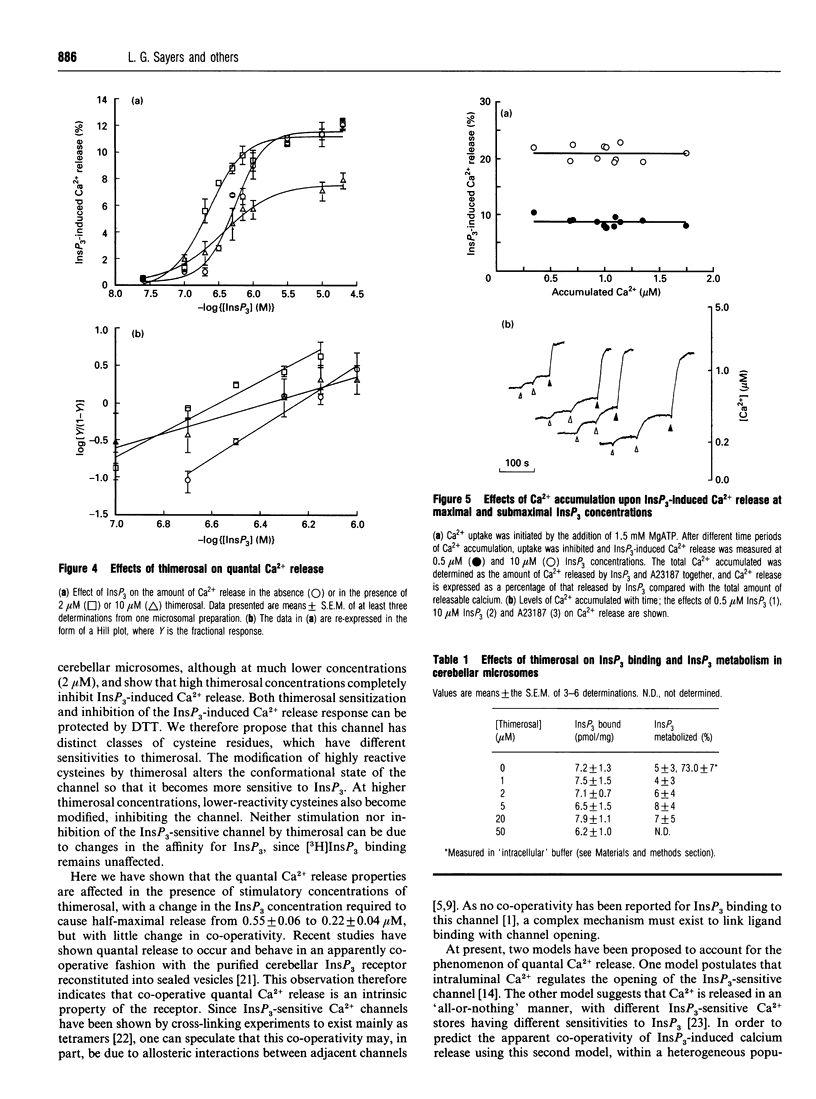
Thimerosal inhibits calcium uptake in skeletal muscle sarcoplasmic reticulum and rat cerebellar microsomes by inhibiting the Ca(2+)-ATPase. In the presence of 5 mM dithiothreitol (DTT), Ca2+ uptake and ATPase activity were not inhibited by thimerosal, indicating that thimerosal modifies cysteine residues of the Ca(2+)-ATPase. Low thimerosal concentrations (2 microM) sensitize the inositol 1,4,5-trisphosphate (InsP3)-sensitive Ca2+ channel, making it open at lower InsP3 concentrations. Higher concentrations of thimerosal, however, cause inhibition of InsP3-induced Ca2+ release. Both sensitization and inhibition of the InsP3 receptor by thimerosal can be prevented by DTT. The binding and metabolism of InsP3 by cerebellar microsomes is not affected by thimerosal. The amount of InsP3-induced Ca2+ release is co-operatively linked to the InsP3 concentration with a Hill coefficient of 2.0 +/- 0.3. This is decreased to 1.0 +/- 0.2 at inhibitory concentrations of thimerosal. Under our experimental conditions, we observed no dependence of quantal Ca2+ release on intraluminal Ca2+ concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. P., le Maire M., Møller J. V. Properties of detergent-solubilized and membranous (Ca2+ + Mg2+)-activated ATPase from sarcoplasmic reticulum as studied by sulfhydryl reactivity and ESR spectroscopy. Effect of protein-protein interactions. Biochim Biophys Acta. 1980 Dec 2;603(1):84–100. doi: 10.1016/0005-2736(80)90393-4. [DOI] [PubMed] [Google Scholar]
- Brown G. R., Sayers L. G., Kirk C. J., Michell R. H., Michelangeli F. The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem J. 1992 Mar 1;282(Pt 2):309–312. doi: 10.1042/bj2820309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Claret M., Champeil P. Do submaximal InsP3 concentrations only induce the partial discharge of permeabilized hepatocyte calcium pools because of the concomitant reduction of intraluminal Ca2+ concentration? FEBS Lett. 1992 Apr 27;301(3):287–290. doi: 10.1016/0014-5793(92)80258-i. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Cameron A. M., Huganir R. L., Snyder S. H. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 1992 Mar 26;356(6367):350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Gould G. W., Colyer J., East J. M., Lee A. G. Silver ions trigger Ca2+ release by interaction with the (Ca2+-Mg2+)-ATPase in reconstituted systems. J Biol Chem. 1987 Jun 5;262(16):7676–7679. [PubMed] [Google Scholar]
- Gutiérrez Merino C. Gel to liquid crystalline phase transition promotes a conformational reorganization of Ca2+, Mg2+-ATPase from sarcoplasmic reticulum in dimyristoylphosphatidylcholine reconstituted systems. Arch Biochem Biophys. 1987 Jan;252(1):303–314. doi: 10.1016/0003-9861(87)90035-x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Di Virgilio F., Villa A., Podini P., Meldolesi J., Pozzan T. Identification, kinetic properties and intracellular localization of the (Ca(2+)-Mg2+)-ATPase from the intracellular stores of chicken cerebellum. Biochem J. 1991 May 1;275(Pt 3):555–561. doi: 10.1042/bj2750555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelangeli F., Munkonge F. M. Methods of reconstitution of the purified sarcoplasmic reticulum (Ca(2+)-Mg2+)-ATPase using bile salt detergents to form membranes of defined lipid to protein ratios or sealed vesicles. Anal Biochem. 1991 May 1;194(2):231–236. doi: 10.1016/0003-2697(91)90223-g. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992 Jun 18;357(6379):599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Taylor C. W., Berridge M. J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991 Jul 18;352(6332):241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Ryou Y., Yoshikawa S., Nakagawa T., Saitoh T., Mikoshiba K. Structure-function relationships of the mouse inositol 1,4,5-trisphosphate receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol Pharmacol. 1992 Jan;41(1):115–119. [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Renard D. C., Sass E. J., Thomas A. P. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991 Jul 5;266(19):12272–12282. [PubMed] [Google Scholar]
- Shears S. B., Parry J. B., Tang E. K., Irvine R. F., Michell R. H., Kirk C. J. Metabolism of D-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J. 1987 Aug 15;246(1):139–147. doi: 10.1042/bj2460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T. J. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+]. J Biol Chem. 1992 Feb 25;267(6):3573–3576. [PubMed] [Google Scholar]
- Strupish J., Wojcikiewicz R. J., Challiss R. A., Safrany S. T., Willcocks A. L., Potter B. V., Nahorski S. R. Is decavanadate a specific inositol 1,4,5-trisphosphate receptor antagonist? Biochem J. 1991 Jul 1;277(Pt 1):294–294. doi: 10.1042/bj2770294a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Volpe P., Alderson-Lang B. H. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release. II. Effect of cAMP-dependent protein kinase. Am J Physiol. 1990 Jun;258(6 Pt 1):C1086–C1091. doi: 10.1152/ajpcell.1990.258.6.C1086. [DOI] [PubMed] [Google Scholar]


