Highlights
-
•
Aldosterone analysis is not easy given to its low physiologic concentration.
-
•
Numerous potential interferences are described for aldosterone immunoassays.
-
•
LC-MS/MS method development for plasma and urinary aldosterone.
-
•
Compared with LC-MS/MS, RIA showed a marked positive bias in plasma and modest positive bias in urine.
-
•
We report revised aldosterone reference intervals on a well-characterized population.
Keywords: Aldosterone, Primary aldosteronism, Liquid chromatography, Mass spectrometry, Immunoassay
Abstract
Background
Aldosterone measurement is critical for diagnosis of primary aldosteronism and disorders of the renin-angiotensin system. We developed an LC-MS/MS method for plasma and urinary aldosterone and compared it to our RIA method. We present a reference interval study for a Belgian population.
Methods
68 plasma and 23 urine samples were assayed for as part of a method comparison. For the reference interval study, we enrolled 282 healthy Caucasian volunteers (114 Male: mean age 35 ± 11 y and 168 Female: mean age 42 ± 13 y). A subset of 139 healthy volunteers agreed to a 24-h urine collection. For the method validation, 5 plasma and 8 urine pools were run in triplicate and quadruplicate, respectively, on 3 different days.
Results
Between-run imprecision (CV) was 2.8–5.1% for plasma and 4.5–8.6% for urine, except at the low urine concentration of 2.99 nmol/L where a CV of 15.4% was observed. The limit of quantitation was 0.04 nmol/L for plasma and 6.65 nmol/L for urine. Recoveries, based on spiking experiments into natural matrix, did not differ significantly from 100%. Regression comparisons showed that, on average, RIA generated results were 59% and 11% higher than LC-MS/MS for plasma and urine, respectively. The MS reference interval we propose for plasma aldosterone is 0.07 nmol/L–0.73 nmol/L for women and 0.04 nmol/L–0.41 nmol/L for men. No gender difference was observed for urine aldosterone. The reference interval was determined to be <60.94 nmol/day.
Conclusions
The LC-MS/MS method was validated and reference intervals for plasma and urine were established. A significant bias between RIA and LC-MS/MS was noted.
1. Introduction
Primary aldosteronism (PA) was first described by Jerome Conn in 1954 [1] and is the most common form of secondary hypertension, representing approximately 10% of all cases of hypertension, a condition affecting at least 970 million people [2], [3]. The World Health Organization (WHO) has recently classified hypertension as one of the most important causes of premature death worldwide [4]. With estimates that there will be 1.56 billion adults living with high blood pressure in 2025 [5], it is a problem that is continuing to grow. In clinical practice, screening for PA is primarily performed using the aldosterone-renin ratio (ARR), which is calculated from plasma aldosterone measurement and either the concentration or activity of renin in a morning blood sample obtained from a seated ambulatory patient [6], [7]. Another confirmatory test for PA is the measurement of urinary aldosterone [8]. In effort to assist diagnosis of PA in Europeans, a number of recent articles have presented reference intervals for aldosterone, renin and the ARR for gender and age groups representative of different European regions [9], [10], [11].
Unfortunately, clinical analysis of aldosterone (molecular weight: 360.44 g/mol; CAS number: 52-39-1; formula: C21H28O5) is not easy given its relatively low concentration in plasma and the inherent difficulty in identification from biological matrices. From an analytical standpoint, the need for accurate, precise, and standardized measurement of aldosterone presents a significant challenge for routine clinical laboratories. According to the JCTLM database no aldosterone reference material was available prior to October 2017; and only had a reference method for the analysis of aldesterone via GCMS. Since October 2017, a reference material from the NMIJ has been available on this database in concentrations between 0.55 and 2.22 nmol/L (0.2–0.8 ng/mL).
Since the early 1970s steroids have been primarly analysed by radio-immuno assay (RIA), an antibody-based approach pioneered by Yalow and Berson in 1959 [12]. While antibody–based methods have demonstrated reasonable sensitivity, they lack specificity, particularly in the competitive formats required for small molecule analysis [13], [14], [15], [16]. The major challenge is the antibody’s potential for cross-reactivity or inability to recognize a single molecular structure to the exclusion of other related molecules present in the matrix [17], [18], [19], [20]. To overcome this limitation, which plagues the analysis of many small molecules, among other analytes, clinical laboratorians have increasingly turned to Liquid Chromatography coupled to Tandem Mass Spectrometry (LC-MS/MS) [21], [22], [23]. Besides high sensitivity, specificity, and excellent reproducibility, LC-MS/MS has the capacity for multiplexing, high-throughput, and significant cost savings. These features explain its increasing popularity, even in the face of its need for specialized and specifically-trained technicians and its own set of analytical challenges, such anion suppression (in electrospray mode) and coeluting isobaric compounds. Recently published articles dealing with the analysis of aldosterone analysis by LC-MS/MS describe a variety of extraction protocols, (e.g., liquid–liquid extraction (LLE) [24] and solid phase extraction (SPE) [25] as well as different ionization approaches (e.g., atmospheric pressure chemical ionization (APCI) [15], electrospray ionization (ESI) [25], [26], and atmospheric photospray ionization (APPI) [27]).
Our laboratory recently migrated from the DiaSorin RIA for aldosterone to an LC-MS/MS method employing LLE and ESI in negative mode. We adapted the method described by Holmes et al. [24] to a more sensitive Sciex TQ 5500 device, which allowed us to reduce the sample volume needed for the analysis from 500 µL to 250 µL.
Here, we report on the first part of this implementation involving (i) method validation and comparison to our previously employed RIA assay using human plasma and urine samples, and (ii) determination of a reference interval for plasma and urinary aldosterone using the LC-MS/MS method on a well-characterized population of healthy Belgian subjects.
2. Material and methods
2.1. Reagents and material
2.1.1. Reagents
Aldosterone at 2770 nmol/L in methanol, purchased from Cerilliant (Ref A-096; Sigma–Aldrich, St. Louis, MO, USA), was used as a reference standard. Deuterated aldosterone (d7-aldosterone, Ref 706035, Sigma–Aldrich, St. Louis, MO, USA) at 2.77 mmol/L in methanol was used as an internal standard. The natural stock solution was then diluted to create standard solutions of 277, 27.7 and 2.77 nmol/L using mobile phase (methanol:water (1:1)) and stored at −80 °C. The standard solutions were then used to create plasma calibrators at 0, 0.01, 0.03, 0.07, 0.14, 0.28, 0.69, 1.39, 2.77 nmol/L using blank plasma matrix (Double–Stripped Plasma, Golden West Biologicals, Tamecula, CA, USA). Urine calibrators at 0, 6.93, 13.85, 27.7, 69.25, 138.5 nmol/L were prepared using LCMS grade water. Labeled internal standard was added to calibrators, patient samples and quality controls at 35 nmol/L and 55 nmol/L for plasma and urine, respectively. LCMS grade water and methanol were purchased from Biosolve (Biosolve, Dieuze, France). Tertbutylmethyl ether HPLC grade, ammonium acetate, sodium hydroxide and chloride acid were purchased from Sigma–Aldrich (Sigma–Aldrich, St. Louis, MO, USA).
2.1.2. Quality controls
Internal quality control (IQC) was monitored using Lyphochek Immunoassay plus controls levels 1 and 2 (Biorad, Hercules, California, USA) for plasma, and two human pools of routinely leftover urine (i.e., low and high concentration). External quality assessment (EQA) was performed using control samples obtained from UK NEQAS (Sheffield, United Kingdom) and Referenzinstitut für Bioanalytik (Bonn, Germany) for plasma, and Instand (Düsseldorf, Germany) and the College of American Pathologists (Northfield, USA) for urine.
2.2. Sample preparation
2.2.1. Human plasma
A volume of 250 µL of calibrator, plasma sample or quality control sample was placed into a 5 mL PYREX® tube (Corning, NY, USA) and mixed with 10 µL of labeled internal standard at 34.625 nmol/L. 1.6 mL of tertbutylmethyl ether was then added, followed by 30 s of vortexing (Vortex Genius 3, IKA®, IKA-Werke GmbH & Co. KG, Staufen, Germany) and 5 min of centrifugation at 3000 rpm. A fixed volume (i.e.,1400 µL) of the organic layer was transferred to a vial, and the solvent was evaporated under a gentle stream of nitrogen. The dry extract was reconstituted with 65 µL of a mixture methanol and water (15: 50) before injection of 50 µL into the LC system.
2.2.2. Human urine
A volume of 100 µL of calibrator, urine sample or quality control sample was placed into a 5 mL PYREX® tube (Corning, NY, USA). Hydrolysis was performed by adding 1 mL of 0.1 M HCl to the sample followed by overnight incubation at 37 °C. After hydrolysis, the pH was adjusted to any point between 6 and 7 using 0.1 M NaOH. 10 µL of labeled internal standard at 55.40 nmol/L was then added, and, after vortexing for 30 s, 50 µL of sample was injected onto the LC system.
2.3. Liquid chromatography and electrospray tandem mass spectrometry
We used an HPLC system AD20XR (Shimadzu Co., Kyoto, Japan) consisting of a vacuum degasser, autosampler and a tertiary pump, equipped with a C18 Gemini NX analytical column (100 mm × 2.0 mm, 3 µm particle size) (Phenomenex, Torrance, USA). Separation was achieved with a time programmed gradient were mobile phases A and B were water and methanol, respectively. The flow rate was 0.3 mL/min. The gradient started with a mobile phase composition of 20% B which was held for one minute. The percentage of B was then linearly ramped from 20% to 70% over 1 to 5 min and then held constant up to 6 min. The percentage of B was then increased to 90% and held constant for 1 min. Finally, the column was re-equilibrated for 2 min at the initial conditions of 20% B. The retention and cycle times were 5.31 and 10.5 min, respectively. The HPLC system was connected to a triple quadrupole mass spectrometer TQ5500 (SCIEX, Framingham, Massachusetts, USA). Quadrupoles Q1 and Q3 were tuned to unit resolution and the MS parameters optimized for maximum signal intensity for each mass transition. An ESI source in the negative ionization mode was employed; ion spray voltage was −4500 V, gases 1, 2 and curtain gas were 40, 40 and 30, respectively; entrance potential (EP) and declustering potential (DP) were 9 V and 170 V, respectively. The ion source temperature was 600 °C. Collision energies (CE) and exit potentials (CXP) were 25 V and 24 V, respectively, for the two multiple reaction monitoring (MRM) transitions 359.2 → 189 (qualifier) and 359.2 → 331.1 (quantifier). The MRM transitions for the internal standard (d7-aldosterone) were 366.2 → 194 (qualifier) and 366.2 → 338.1 (quantifier). Data acquisition and processing was carried out using Analyst 1.6.2, and calibration curves were prepared using 1/x2-weighted linear regression.
The summarized analytical characteristics of our method, compared to previously published ones [20], [24], [25], [28], can be found in Table 1.
Table 1.
Comparison of previously reported LC-MS/MS methods for the quantification of aldosterone in human serum/plasma against our proposed methodology. SLE = Solid Phase Extraction, MTBE = Methyl tert-butyl ether, SPE = Solid Phase Extraction.
| Ray et al., J Chomatogr B 2014 (20) |
Meunier et al. CCA 2015 (28) | Hinchliffe et al., J Chomatogr B, 2013 (25) | Van der Gugten et al., JCP 2012 (24) | Our method | |
|---|---|---|---|---|---|
| Sample volume and matrix (µL) | 250 µL serum or plasma | 450 µL EDTA plasma | 250 µL EDTA plasma | 500 µL serum | 250 µL EDTA plasma or serum |
| Internal Standard | d7 | d7 | d7 | d7 | d7 |
| Instrument (LC + MS) | Agilent HPLC 1260 + TQ5500 (Sciex) | Agilent HPLC + API3000 (Sciex) | Waters Acquity UPLC + Xevo TQS | Shimadzu LC20 AD/CT + API 5000 (Sciex) | Shimadzu LC20 AD/XR + TQ5500 (Sciex) |
| Column | Agilent Zorbax Eclipse XDB CN 50mmx2.1 mm, 5 µm, + Poroshell 120 EC C18 50 mm × 2.2 mm, 2.7 µm + guard cartridge Poroshell EC C18 2.1 mm × 5 mm, 2.7 µm |
Phenomenex Luna C18 50 mm × 2.1 mm, 5 µm |
Phenomenex Krudcatcher UPLC In-Line Filter 0.5 × 0.004 + Kinetex PFP 100 mm × 2.1 mm, 2.6 µm |
Phenomenex Gemini-NX Secuity Guard C18 4X20 mm guard cartridge + Gemini- NX C18 110A 100 × 2mm, 3 µm | Phenomenex Gemini-NX Secuity Guard C18 4X20 mm guard cartridge + Gemini- NX C18 110A 100 × 2 mm, 3 µm |
| Extraction | LLE with MTBE | SLE with MTBE | SPE (HLB, Oasis Waters) | LLE + MTBE | LLE + MTBE |
| Mobile phase (initial condition) | A: 2 mM ammonium acetate in water B: methanol |
A: 65%water B: 35% methanol |
A: 70% water B: 30% methanol |
A: 80% ammonium acetate c in water B: 20% NH4Ac in MeOH |
A: 80% water B: 20% methanol |
| CV (range tested in nmol/L) | Within-run (2–6.5%) Between run(2.5–8.3%) Total imprecision (2.9–6.9%) (0, 13, 1, 38, 83, 3 nmol/L) |
Within-run (1.3–5.5%) (62, 514, 1800 pmol/L) Between run (<14.7%) (0.04, 0.40, 1.68 nmol/L) |
Within-run: 5.7% Between run: 7.8% (0.06, 0.35, 1.22 nmol/L) |
Within-run (1.5–6.1%) Between run (2.5–7.9) Total imprecision: 3.3–9.9% (0.05, 0.12, 0.22, 0.42, 0.95, 0.99, 1.64 nmol/L) |
Within-run (2.8–5.1%) Between run (2.8–5.1%) (0.06, 0.11, 0.31, 0.71, 2.20 nmol/L) |
| LOQ (nmol/L) | 0.04 nmol/L | 0.04 nmol/L | 0.03 nmol/L | 0.05 nmol/L | 0.038 nmol/L |
2.4. Radioimmunoassay method (RIA)
All samples were analyzed using the commercial RIA method, ALDOCTK-2 (DiaSorin, Saluggia, Italy). The kit is based on a competitive binding assay where the aldosterone contained in the samples competes with a fixed amount of 125I-aldosterone for antibody binding sites on the assay tube walls. After overnight incubation, the tube is washed and the radioactivity remaining within the tube is measured to determine the amount of aldosterone present in the sample.
2.5. LC–MS/MS validation and comparison with DiaSorin RIA
The R statistical programming language (R Foundation for Statistical Computing, Boston, Massachusetts, USA) and Medcalc (MedCalc Software, Mariakerke, Belgium) were used to perform the more complex statistical calculations described below.
Method validation was performed in accordance with the guidelines of the Unitated States Food and Drug Administration (FDA) [29]. Two calibration curves were prepared using 7 and 6 points for plasma and urine, respectively, and response was determined by calculating the integrated peak area ratio between endogenous aldosterone to d7-aldosterone. To evaluate within- and between-run CVs, 5 plasma pools were run in triplicate, and 8 urine pools were run in quadruplicate on 3 different days. Calibration curve linearity was assessed by performing linear, quadratic and cubic non-linear least squares regression. Additionally, the best polynomial fit obtained was compared to the linear fit by way of difference plot [30]. A predefined tolerance of 15% non-linearity, corresponding to the desirable bias according to the biological variation of aldosterone, was used to define unacceptable non-linearity for samples having low aldosterone concentration.
We evaluated recovery by spiking aldosterone from standard solutions into native low-aldosterone plasma to achieve three final concentrations; low: 0.07 nmol/L, medium: 0.28 nmol/L, and high: 1.39 nmol/L. A similar experiment spiking aldosterone into urine was performed to achieve final concentrations of 13.85, 27.70 and 138.50 nmol/L. Recoveries were calculated as the percentage difference between the quantity of aldosterone recovered from the spiked and unspiked sample divided by the quantity of the aldosterone added. Matrix effect was evaluated by measuring the peak area ratio of aldosterone to its IS in a plasma and urine pool enriched with water-based calibration at three different concentrations (i.e., 0.07 nmol/L, 0.28 nmol/L, 1.39 nmol/L for plasma, and 13.85 nmol/L, 27.70 nmol/L, 138.50 nmol/L for urine). The increases in peak area ratio of enriched plasma pools were compared with the respective peak area ratios measured in the nonspiked plasma and the calibrator solutions. Three determinations for each calibrator concentration were performed [31].
By measuring the peak area ratio of aldosterone relative to its internal standard in plasma, urine and water, matrix effect was calculated as follows: Matrix Effect (%) = 100 × [peak area ratio (spiked in matrix) − peak area ratio (endogenous matrix)]/[peak area ratio (spiked in water)].
The method linearity was assessed by analyzing high and low patient pools, mixed in proportions of 1:3, 1:1, 3:1.
The limit of detection (LOD) and limit of quantitation (LOQ) were calculated in plasma and urine samples containing low endogenous levels of aldosterone concentration. LOD and LOQ were defined as 3:1 and 10:1 signal-to-noise ratio (S/N), respectively.
Finally, for method comparison, results of aldosterone obtained by LC-MS/MS in 23 urine samples and 68 plasma samples were compared with the Diasorin RIA.
2.6. Reference interval
A cohort of 282 healthy, fasting caucasian Belgian volunteers (114 M: mean age 34 ± 10 y and 168 F: mean age 42 ± 13 y) were enrolled and gave informed consent. Exclusion criteria were: prescription of any medications (including oral contraceptives), a history of hypertension, abnormal plasma sodium, and body mass index (BMI) >30 kg/m2. Each volunteer was required to sit for 5–15 min before blood sampling from 8:00 to10:00 AM. All samples were centrifuged in less than 30 min after sampling, and were stored less than 6 months at −80 °C. A subset of 139 of these healthy volunteers (48 M: mean age 34 ± 11 y, 91 F: mean age 42 ± 13 y) agreed to collect a 24 h urine collection. Urine sodium concentrations were measured on a Cobas c501 (Roche Diagnostic, Manheim, Germany) and daily excretion of NaCl were calculated using: ExcNaCl = 58 × V24h × [NaU] where ExcNaCl is the 24 h urine excretion of NaCl in mg/day. The CLSI EP28AC3 guideline was used to establish the reference interval.
3. Results
3.1. Validation of the LC-MS/MS method
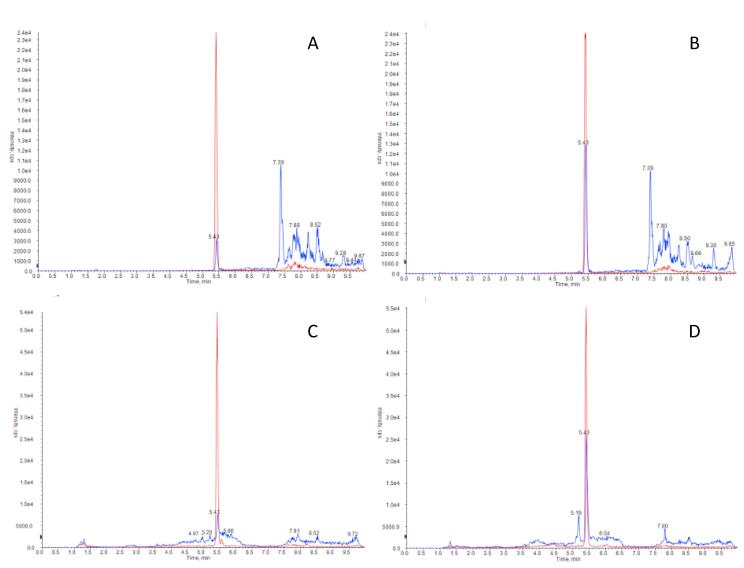
Selectivity of the proposed method was evaluated in routine samples. As an example, Fig. 1 shows the chromatograms for aldosterone in plasma (A, B) and urine samples (C,D) at low and high concentrations. A clean peak at 5.4 min with no obvious interferences was observed on all runs.
Fig. 1.
LC-MS/MS chromatograms of aldosterone in plasma at 0.07 nmol/L (A) and 0.28 nmol/L (B) and in urine at 9.42 nmol/L (C) and 39.89 nmol/L (D). (m/z 359.2 → 331.1 in blue for aldosterone and 366.2 → 338.2 in red for internal standard d7-aldosterone).
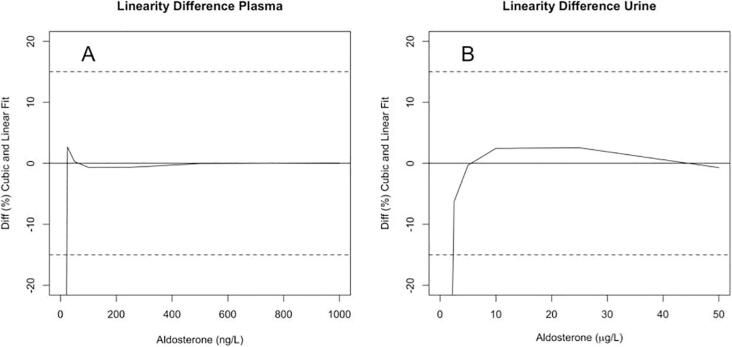
Linearity f the newly developed method was evaluated by cumulative calibration curve data (n = 8 for plasma and n = 6 for urine, separate days), which demonstrated a slightly superior fitting of a cubic rather than quadratic polynomial (RSS = 0.0001 for cubic and 0.0002 for quadratic). Based on non-linearity difference plots (Fig. 2), the plasma method exceeded 15% tolerance in the low aldosterone interval at 0.05 nmol/L for plasma and 5.82 nmol/L for urine. Within the calibration ranges for plasma and urine, non-linearity did not exceed 3%, demonstrating assay linearity up to 2.77 nmol/L for plasma and 138.50 nmol/L for urine.
Fig. 2.
Difference plot between a cubic polynomial fit and ordinary least squares regression fit of mean calibration curve data from 8 plasma-based curves (A) and 6 urine-based curves (B). A pre-defined tolerance limit of 15% linearity is only violated at concentrations below 0.05 nmol/L for plasma and 5.82 nmol/L for urine.
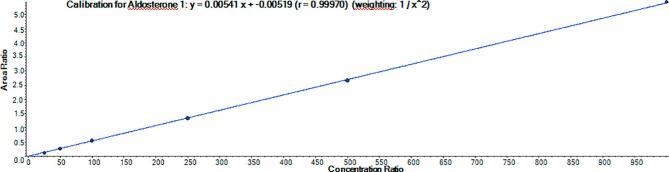
An example calibration curve using plasma is shown in Fig. 3.
Fig. 3.
Example of a routine calibration curve used for the determination of aldosterone in plasma samples.
Precision of the overall analytical procedure was obtained by LC-MS/MS analysis of 5 plasma pools (range: 0.06 to 2.20 nmol/L) and 8 urine pools (range: 3.05 to 311.63 nmol/L), as shown in Table 2.
Table 2.
Imprecision (CV%) and standard deviation (SD) within- and between -run obtained for 5 plasma pools with a range level from 0.06 to 2.20 nmol/L (P1-P5) and 8 urine pools ranging from 2.99 to 311.63 nmol/L (U1-U8). WRCV = within-run CV; WRSD = within-run SD; BRCV = between-run CV; BRSD = between run SD.
| Pool | n | Mean (nmol/L) | WRCV (%) | WRSD (nmol/L) | BRCV (%) | BRSD (nmol/L) |
|---|---|---|---|---|---|---|
| P1 | 9 | 0.06 | 3.6 | 0.00 | 4.8 | 0.00 |
| P2 | 9 | 0.11 | 5.1 | 0.01 | 5.1 | 0.01 |
| P3 | 9 | 0.31 | 3.5 | 0.01 | 3.5 | 0.01 |
| P4 | 9 | 0.71 | 2.9 | 0.02 | 3.1 | 0.02 |
| P5 | 9 | 2.20 | 2.8 | 0.06 | 2.8 | 0.06 |
| U1 | 12 | 2.99 | 15.4 | 0.47 | 15.4 | 0.47 |
| U2 | 12 | 20.25 | 6.4 | 1.30 | 6.4 | 1.30 |
| U3 | 12 | 30.44 | 7.3 | 2.22 | 7.3 | 2.22 |
| U4 | 12 | 90.19 | 2.5 | 2.22 | 4.5 | 4.02 |
| U5 | 12 | 133.60 | 5.0 | 6.62 | 7.0 | 9.34 |
| U6 | 12 | 188.89 | 5.1 | 9.70 | 7.4 | 13.99 |
| U7 | 12 | 311.63 | 5.4 | 16.73 | 5.4 | 16.73 |
| U8 | 12 | 52.74 | 6.0 | 3.19 | 8.6 | 4.54 |
The within run and total CVs calculated across the clinically important intervals were always lower than 5.1% and 7.5% for plasma and urine, respectively, except at a concentration close to the LOD (2.99 nmol/L), where the CV was 15.4% for urine.
Recoveries obtained from plasma and urine samples spiked at different concentrations are presented in Table 3. Mean recoveries were close to 100% in both matrices (i.e., 92–103% for plasma and 87–105% for urine), with the exception of spiked water, which had a mean recovery of 121.2%. This elevated recovery value cannot be explained by endogenous interference since no evidence was observed at the corresponding chromatographic peak for this outlier. Low concentrations of aldosterone were contained in both matrices. CVs (n = 9) were lower than 7% in all cases.
Table 3.
Recovery studies for aldosterone (n = 3) at different concentration levels in spiked, low-aldosterone plasma, urine and water. Uncertainty in the mean is expressed as the 95% confidence interval.
| Sample preparation | Matrix | Concentration (nmol/L) | Mean Recovery (95% CI) |
|---|---|---|---|
| Human plasma | water | 0.07 | 99.4 (86.3–112.5) |
| water | 0.28 | 101.6 (93.1–110.0) | |
| water | 1.39 | 102.5 (96.0–109.0) | |
| plasma | 0.07 | 92.4 (86.3–98.5) | |
| plasma | 0.28 | 103.4 (94.0–112.8) | |
| plasma | 1.39 | 101.6 (95.1–108.1) | |
| Human urine | water | 1.39 | 109.2 (100.2–118.2) |
| water | 27.70 | 104.7 (99.8–109.6) | |
| water | 138.50 | 121.2 (105.1–137.3) | |
| urine | 1.39 | 104.6 (95.2–114.0) | |
| urine | 27.70 | 86.7 (76.1–97.3) | |
| urine | 138.50 | 98.2 (84.1–112.3) | |
Method LOD and LOQ were estimated for plasma to be 0.02 nmol/L (n = 5, average S/N ratio of 5.7) and 0.04 nmol/L (n = 5, average S/N ratio 13.0), respectively. The same calculations were performed using urine and the LOD and LOQ were found to be 3.05 nmol/L (n = 5, average S/N ratio of 6.7) and 6.65 nmol/L (n = 5, average S/N ratio of 13.2), respectively.
Method linearity was evaluated by analyzing high and low patient pools, mixed in proportions of 1:3, 1:1, 3:1. The mean recovery (n = 12) was 103.0% (95% CI: 93.6–112.4%).
Mean (±SD) matrix effects assessed in plasma and urine sample spiked with three different concentrations of standard (i.e., 0.07, 0.28 and 1.39 nmol/L for plasma, and 13.85, 27.70, 138.50 nmol/L for urine) ranged from 101.3 to 112.5% and 95.4 to 102.9%, respectively (Table 4). These results demonstrate that there was no significant ion suppression in either plasma or urine samples.
Table 4.
Matrix effect (%) in plasma and urine.
| Matrix Effect (%) in Plasma | ||
|---|---|---|
| Added analytes (nmol/L) | Mean matrix effect (%) | SD (%) |
| 0.07 (n = 3) | 105.9 | 6.9 |
| 0.28 (n = 3) | 105.8 | 5.6 |
| 1.39 (n = 3) | 109.0 | 6.0 |
| Matrix Effect (%) in Urine | ||
| Added analytes (nmol/L) | Mean matrix effect (%) | SD (%) |
| 13.85 (n = 3) | 95.2 | 3.3 |
| 27.70 (n = 3) | 102.4 | 1.9 |
| 138.50 (n = 3) | 100.0 | 1.3 |
A comparison between aldosterone determination using the quantifier MRM transition versus the qualifier MRM transition was performed in 35 plasma samples. Regression relationship between the two MRM transitions was Qualifier = 1.01 × Quantifier − 0.01 nmol/L as shown in Fig. 4.
Fig. 4.
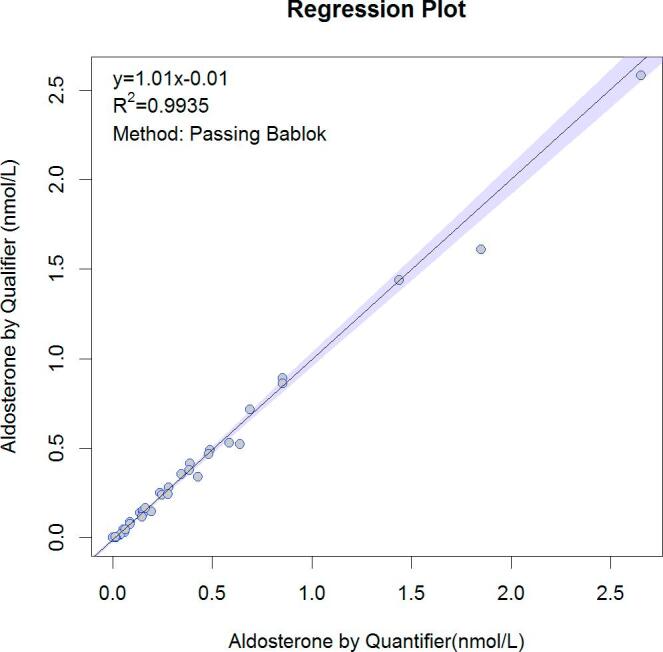
Comparison of the concentration obtained for aldosterone using two MRM transitions on 35 plasma samples. Quantifier transition 359.2 → 331.1 and qualifier transitions 359.2 → 189.
3.2. Comparison with the DiaSorin RIA
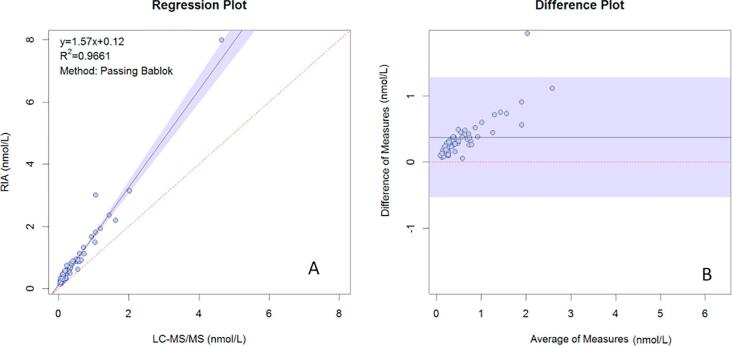
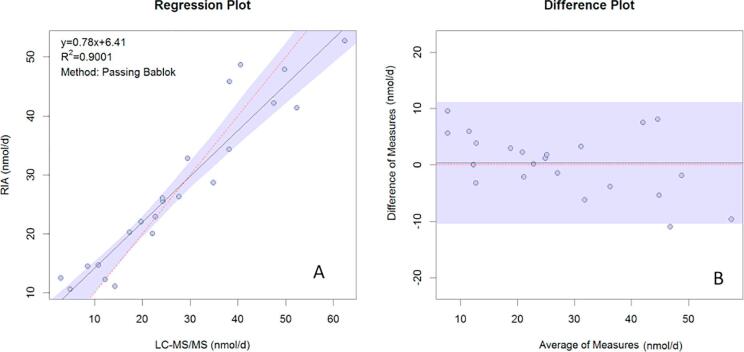
Comparison with the DiaSorin RIA is presented in Fig. 5A and B for plasma samples and Fig. 6A and B shows for urine samples. Passing Bablok regression gave the following equations for plasma: YRIA = 0.12 (CI: 0.09–0.15) + 1.57 (CI: 1.47–1.68) XLC-MS/MS and for urine, YRIA = 6.37 (CI: 1.86–8.31) + 0.78 (CI: 0.70–0.95) XLC-MS/MS.
Fig. 5.
Comparison of aldosterone results obtained in plasma analyzed by LC-MS/MS versus Diasorin RIA. (A) Passing Bablok regression, (B) Difference plot. The horizontal black line represents the mean bias of 0.3757 nmol/L and shaded region represents 95% CI of the mean bias (0.08 and 1.38 nmol/L).
Fig. 6.
Comparison of aldosterone results obtained in urine analyzed by LC-MSMS versus Diasorin RIA. (A) Passing Bablok regression, (B) Difference plot. The horizontal black line represents the mean bias of 0.3435 nmol/L and the shaded region represents 95% CI of the mean bias (−10.3 and 7.8 nmol/d).
The Bland–Altman plot showed a mean bias of 0.38 nmol/L for plasma, with lower and upper 95% limits of agreement of −0.53 nmol/L and 1.28 nmol/L. For urine, after the exclusion of one high sample from the regression, the mean bias was 0.33 nmol/L with lower and upper 95% limits of agreement of −10.67 nmol/L and 11.36 nmol/L.
3.3. Establishment of the reference interval
Eighty-one percent of the subjects who agreed to provide 24 h urine collection presented salt intakes higher than the maximum WHO recommendation of 5 g per day [32]. Salt intakes ranged from 1.0 to 22.1 g/day and were statistically higher (p = 0.02) in men (8.4 g/day (IQR or Interquartile Range: 6.1–9.9) than women (6.8 (IQR: 5.2–7.8) g/day)). There was no correlation between urine or plasma aldosterone concentration and salt intake in men. There was a weak, significant negative correlation observed between plasma aldosterone and salt intakes (rho = −0.34; p = 0.01) for women; although no correlation was noted for urine.
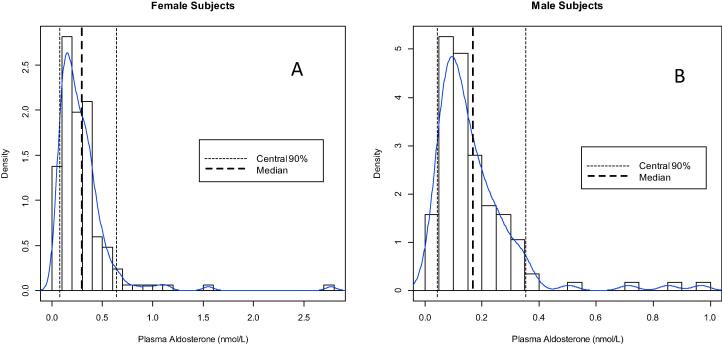
Plasma aldosterone values were not normally distributed (see Fig. 7) and significant differences (p < 0.0001) were found between levels observed in men (0.13 (IQR: 0.08; 0.20) nmol/L) and women (0.22 (IQR: 0.14; 0.35) nmol/L). It was, thus, decided to partition reference levels according to gender, since the z-value of the Harris and Boyd test was at 7.18, much higher than the critical value of 3.19 [33]. We used the robust method according to CLSI C28-A3 after logarithmic back-transformation to establish the plasma aldosterone reference interval. After elimination of 5 outliers in men and 9 outliers in women, according to Tukey [34], the calculated reference interval was 0.07 (90%CI (Confidence Interval): 0.06; 0.08) nmol/L – 0.73 (90%CI: 0.65; 0.83) nmol/L for women and 0.04 (90%CI: 0.04; 0.05) nmol/L – 0.41 (90%CI: 0.36; 0.47) nmol/L for men. We examined the effect of age on the reference interval and noticed no effect (Table 5).
Fig. 7.
Histogram with the non-parametric reference values for aldosterone in plasma for females (A) and males (B) in a healthy, normotensive Belgian cohort.
Table 5.
Reference intervals based on matrix and gender.
| Matrix | Gender | Reference interval |
|---|---|---|
| Plasma | Female | 0.07–0.73 nmol/L |
| Male | 0.04–0.41 nmol/L | |
| Urine | Female | <60.94 nmol/day |
| Male | ||
As with plasma, urine aldosterone levels were not normally distributed. No gender difference was observed and the z-value of the Harris and Boyd test [33] was at 0.94, lower than the critical value of 2.23. Hence, the computed reference interval was <60.94 nmol/day (90%CI: 55.12; 65.54 nmol/day) for both men and women (Table 5).
3.4. Performance of our internal and external controls
Results from internal control tests are presented in Table 6. The CVs obtained from 38 analyses using Lyphochek Levels 1 and 2 (i.e., Biorad QC 40321 and Biorad QC 40312, respectively), were <9%. The CVs obtained from more than 70 analyses for on high and low human urine pools were <11% (Table 6).
Table 6.
Results of the internal controls obtained on Biorad Lyphochek for plasma aldosterone and on urine human pool for urinary aldosterone.
| Lot | N | CV % | Mean (nmol/L) | Standard deviation (nmol/L) |
|---|---|---|---|---|
| Lyphocheck 1 Biorad QC 40321 | 38 | 7.97 | 0.45 | 0.04 |
| Lyphocheck 2 Biorad QC 40312 | 38 | 8.81 | 0.93 | 0.08 |
| Urine Human pool | 75 | 8.37 | 22.71 | 2.22 |
| Urine Human pool | 71 | 10.77 | 55.12 | 6.09 |
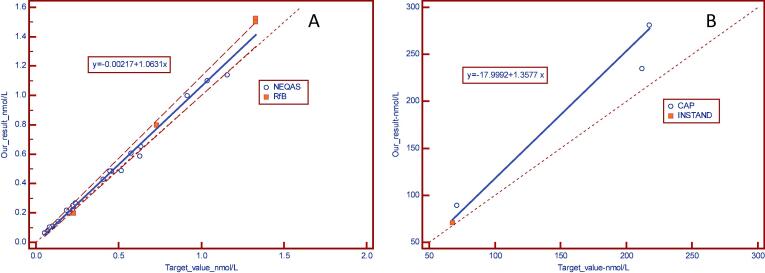
The results of the 2017 external controls are presented as a Passing-Bablok graph, using the target value as the reference as shown in Fig. 8a and b.
Fig. 8.
Passing-Bablok plot presenting: (A): Results of the 2017 external controls (RfB and NEQAS), (B) Results of external controls for urine (Instand and CAP)-results 2017.
Performance of our internal and external controls for 2017 are summarized in the Table 7.
Table 7.
Results obtained (bias ± SD) by comparing our results with the target values given by each EQA (n = number of participating laboratories).
| Plasma |
Urine |
||||
|---|---|---|---|---|---|
| Rfb |
UKNEQAS | CAP | Instand | ||
| GC-MS Ref method | Mean of LCMS users | Mean of LCMS users | Mean of extracted other RIA users | Mean non Diasorin users | |
| Bias ± SD | 11.9% ± 12.5% | 7.7% ± 12.2% | 9.5% ± 11.0% | 21.6 ± 9.6% | 2% ± 3.5% |
| n | 12 | 14 | 21 | 23 | |
4. Discussion
The measurement of aldosterone is not easy given its relatively low concentration in plasma and the presence of numerous potential interferences, as well as cross-reactivity that is observed with immunoassays. Differences between commercial immunoassays and LC-MS/MS results [20] highlight the need for harmonization and standardization; a need reiterated by the Endocrine Society Practice Guideline for PA [35]. Although aldosterone is a well-defined molecule with known molecular weight and structure, reference material has only been available since October 2017, and then only from the National Metrology Institute of Japan (NMIJ CRM6402). A reference method does exist to provide a target value for RfB external controls, but it uses GC-MS, not LC-MS/MS.
Here, our focus was to validate an LC-MS/MS method adapted from Holmes et al. [23], and to provide well-characterized reference intervals for plasma and urine aldosterone. Results of our validation show that the within run and total CVs calculated across the clinically important intervals were consistently lower than 5.1% for plasma and 7.5% for urine (except, in one case for urine where the CV was 15.4% at a concentration close to the LOD). To understand the value, these results should be interpreted within the context of the biological variation of aldosterone. Unfortunately, no data is available on the biological variation of urine aldosterone, but according to the biological variation of plasma aldosterone [36], [37], the total uncertainty budget for the allowable coefficient of variation is 14.7% and the budget for the bias is 12.4%. In our method, the highest CV observed for measurement of aldosterone in serum was 5.1% and the highest bias was 6%, compared to the reference GC-MS method used to set the target of the RfB external quality control. We interpret this to mean that the performance of our method for plasma aldosterone measurement is in accordance with expected ranges for biological variation. The robustness of our LC-MS/MS method was further confirmed by a retrospective analysis of the calibration curve slopes constructed over the past years since we have been using this assay methodology. Eighteen calibration curves out of a pool of 156 that had been performed weekly over multiple years were randomly selected (i.e., 7 from 2015, 6 from 2016, 5 from 2017 and 1 from 2018) and it was confirmed that the coefficient of variation of the slopes of these curves was 13.1% on average, which is below the 15% allowable CV according to biological variation. The range of the slopes was between 0.00438 and 0.00681.
The LOQ obtained with our LC-MS/MS method (0.04 nmol/L for plasma and 6.65 nmol/L for urine) was much lower than what has been reported for RIA methods (i.e., 0.21 nmol/L for plasma and 18.56 nmol/L for urine) [38]. Other groups investigating aldosterone measurement using LC-MS/MS have reported LOQs similar to those we observed with our method: 0.03 nmol/L [25] or 0.04 nmol/L [31] for plasma, and 5.54 nmol/L for urine [39]. From a practical standpoint, the LOQ of our method is well below the diagnostic threshold used in routine clinical practice.
Regarding interferences, we observed no evidence of coelution with the aldosterone peak at 5.31 min, and we were able to chromatographically resolve the known interference from 18-hydroxycorticosterone [24].
Compared with LC-MS/MS, RIA showed a strong positive bias in plasma and moderate positive bias in urine. The disparity between LC-MS/MS and RIA widened at increasing plasma concentration. Antibodies by their nature detect a family of structurally similar molecules, meaning falsely elevated results due to cross-reacting compounds become more of a problem in modern homogenous immunoassays (both automated and manual), as compared to older methods, which required an initial extraction step. The sample preparation procedure used for LC-MS/MS was expected to remove water-soluble aldosterone glucuronides, often present in chronic kidney disease (CKD) patients, which have been shown to cause false positives in this patient group when analyzed by homogeneous immunoassay because they are recognized as alodosterone [40].
We have revised our reference values for plasma and urinary aldosterone measurement by LC-MS/MS in a population of healthy Belgian adults. Since sodium intake can dramatically impact aldosterone concentrations [41], we quantified the salt intake in a subset of subjects representing 20% of the Belgian population demographic. Our results show that salt consumption is important in our sample group, which is consistent with previously published epidemiological studies in Wallonia [42].
We found that the upper limit of the reference interval for plasma was 1.8-times higher in women (0.75 nmol/L) versus men (0.42 nmol/L). This raised the question of whether there should be gender-specific reference intervals for aldosterone. The available literature is not clear on this point. Although a number of groups have evaluated reference intervals for plasma aldosterone, several were using RIA methods [26], [28], [43], [44], [45] which potentially renders the transposition of their results to those garnered from LCMS methods meaningless. Only, a few have reported reference intervals obtained by LC-MS/MS. In France, Meunier et al., on a relatively small population of normotensive men (n = 16) and women (n = 37), proposed an aldosterone reference range of 0.08–0.92 nmol/L [31]. There was no mention of any gender differential in this study, nor any drug exclusion. In the UK, Taylor et al., evaluated a cohort of upright normotensive men (n = 54) and women (n = 43) and proposed a reference interval of <0.10 to 0.95 nmol/L; again, it was not specified whether there were any differences between males and females [26]. Finally, in Germany, Eisenhofer et al. studied the impact of gender, age, oral contraception, BMI and blood pressure status on various steroids measured by LC-MS/MS in 225 women and 232 men [46]. They did not detect any difference in median aldosterone levels between genders (i.e., 0.13 (men) versus 0.14 (women) nmol/L, p = 0.1462), but the gender-specific reference intervals they proposed (i.e., 0.03–0.67 for women versus 0.01–0.45 nmol/L for men) are similar to those observed in our study.
Based on a combination of our data and these reports, it is not clear whether gender-specific reference intervals for aldosterone should be employed. However, this issue has important clinical consequences since an ARR cut-off of 30 is generally used to screen for primary hyperaldosteronism. This cut-off was obtained with older RIA methods and is still applied irrespective of the assay used or patient gender. By way of example of the impact this cut-off could have, a hypertensive man with an aldosterone concentration at 0.69 nmol/L, obtained by LC-MS/MS, and plasma renin activity value of 1 ng/mL/h will have an ARR at 25 ng/dL:ng/mL/h, which is considered normal. However, 0.69 nmol/L corresponds to 1.7 times the upper limit of normal for our LC-MS/MS method. By regression-transformation, the RIA equivalent of 0.69 nmol/L would be approximately 1.19 nmol/L and the ratio would be 43 ng/dL:ng/mL/h, which would be positive.
The methodological biases we observed raise questions about the applicability of previously identified ARR screening cutoffs and thresholds for aldosterone suppression used in diagnostic tests, such as saline suppression and oral salt loading. Evidently, until aldosterone methods are standardized, it would seem prudent to identify cutoffs specific to the aldosterone and renin methods in use, whether that be RIA or LC-MS/MS.
Finally, the availability of an internationally recognized standardized reference material for aldosterone would facilitate the standardization of LC-MS/MS methods between labs, and similar efforts could be made for renin activity by standardizing the angiotensin I generation protocols and analysis.
5. Conclusions
Our LC-MS/MS method has reduced the total sample preparation time from 22 h to 2 h and from 45 h to 24 h, in comparison with our former RIA method, for serum and urine, respectively. This facilitates the applicability of this methodology for routine clinical analysis. We have provided reference intervals for plasma and urine aldosterone on a well-characterized population of normotensive, healthy young subjects free of interfering medications. Finally, we anticipate that the recently developed international standard reference material for aldosterone, by NMIJ, will contribute to harmonization of aldosterone results and that a candidate reference method for LC-MS/MS will soon be available. This would support the establishment of new reference values and ARR cut-offs in order to more effectively screen patients at risk for PA.
Acknowledgments
Competing interests: The authors declare no conflict of interest for this study.
References
- 1.Conn J. Primary aldosteronism, a new clinical syndrome. J. Lab. Clin. Med. 1955;45:3–17. [PubMed] [Google Scholar]
- 2.Quinkler M., Born-Frontsberg E.F.V. Comorbidities in primary aldosteronism. Horm. Metab. Res. 2010;42:429–434. doi: 10.1055/s-0029-1243257. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.-G., Lee W., Kwon O.H., Kim J.-H. Association of urinary sodium/creatinine ratio and urinary sodium/specific gravity unit ratio with blood pressure and hypertension: KNHANES 2009–2010. Clin. Chim. Acta. Elsevier B.V. 2013;424:168–173. doi: 10.1016/j.cca.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. A global brief on Hypertension - World Health Day 201World Heal Organ. 2013;1–40.
- 5.Chockalingam A. Impact of World Hypertension Day. Can. J. Cardiol. 2007;23(7):517–519. doi: 10.1016/s0828-282x(07)70795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horton R. Aldosterone and aldosteronism. Steroids. 2003;68(14):1135–1138. doi: 10.1016/j.steroids.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Galati Sandi-Jo. Primary aldosteronism: challenges in diagnosis and management. Endocrinol. Metab. Clin. North Am. 2015;44:355–369. doi: 10.1016/j.ecl.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Ceral J., Malirova E., Ballon M.S.M. The role of urinary aldosterone for the diagnosis of primary aldosteronism. Horm. Metab. Res. 2014;46(9):663–667. doi: 10.1055/s-0034-1374638. [DOI] [PubMed] [Google Scholar]
- 9.Hannemann A., Friedrich N., Lüdemann J., Völzke H., Rettig R., Peters J., et al. Reference intervals for aldosterone, renin, and the aldosterone-to-renin ratio in the population-based Study of Health in Pomerania (SHIP-1) Horm. Metab. Res. 2010;42(6):392–399. doi: 10.1055/s-0030-1247545. [DOI] [PubMed] [Google Scholar]
- 10.Stowasser M., Taylor P.J., Pimenta E., Ahmed A.H.A.-A., Gordon R.D. Laboratory investigation of primary aldosteronism. Clin. Biochem. Rev. 2010;31(2):39–56. [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzolo F., Rafaeli R., Chiecchi L., Pavan C.G.P., et al. Effects of female sex hormones and contraceptives pill on the diagnostic work-up for primary aldosteronism. J. Clin. Endocrinol. Metab. Clin. Endocrinol. Metab. 2010;28:135–142. doi: 10.1097/HJH.0b013e32833266e3. [DOI] [PubMed] [Google Scholar]
- 12.Yalow R.S., Berson S.A. Immunoassay of endogenous plasma insulin in man. J. Clin. Invest. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorrian C.A., Toole B.J., Alvarez-Madrazo S., Kelly A., Connell J.M.W.A. A screening procedure for primary aldosteronism based on the Diasorin Laision automated chemilumuniscent immunoassay for direct renin. Ann. Clin. Biochem. 2010;47:195–200. doi: 10.1258/acb.2010.009230. [DOI] [PubMed] [Google Scholar]
- 14.Stowasser M.G.R. Aldosterone assays: an urgent need for improvement. Clin. Chem. 2006;9(9):1640–1642. doi: 10.1373/clinchem.2006.073460. [DOI] [PubMed] [Google Scholar]
- 15.Belaidi N., Georges A., Brossaud J., Corcuff J.-B. Aldosterone determination: Comparison of a RIA assay and a CLIA assay. Clin. Biochem. The Canadian Soc. Clin. Chem. 2015;48(1–2):89–92. doi: 10.1016/j.clinbiochem.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Al-Dujaili E.A., Edwards C.R. Development and application of a simple radioimmunoassay for urinary aldosterone. Clin. Chim. Acta; Int. J. Clin. Chem. 1981:277–287. doi: 10.1016/0009-8981(81)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Fredline V.F., Taylor P.J., Dodds H.M., Johnson G.A. A reference method for the analysis of aldosterone in blood by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal. Biochem. 1997;252(252):308–313. doi: 10.1006/abio.1997.2340. [DOI] [PubMed] [Google Scholar]
- 18.S. Becker, L. Kortz, C. Helmschrodt, J. Thiery, U. Ceglarek, R.M. Büttler, et al., Clinica Chimica Acta Measurement of dehydroepiandrosterone sulphate (DHEAS): A comparison of Isotope-Dilution Liquid Chromatography Tandem Mass Spectrometry (ID-LC-MS/MS) and seven currently available immunoassays, Clin. Chim. Acta. Elsevier B.V.; 424(May 2012) (2013) 68–75. Available from. [DOI] [PubMed]
- 19.Allen K.R., Azad R., Field H.P.B.D. Replacement of immunoassay by LC tandem mass spectrometry for the routine measurement of drugs of abuse in oral fluid. Ann. Clin. Biochem. 2005;42:277–284. doi: 10.1258/0004563054255632. [DOI] [PubMed] [Google Scholar]
- 20.Ray J.A., Kushnir M.M., Palmer J., Sadjadi S., Rockwood A.L., Meikle A.W. Enhancement of specificity of aldosterone measurement in human serum and plasma using 2D-LC-MS/MS and comparison with commercial immunoassays. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. Elsevier B.V. 2014;970:102–107. doi: 10.1016/j.jchromb.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Vogeser M., Kirchhoff F. Progress in automation of LC-MS in laboratory medicine. Clin. Biochem. The Canadian Soc. Clin. Chem. 2011;44(1):4–13. doi: 10.1016/j.clinbiochem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Grebe S.K.G., Singh R.J. LC-MS/MS in the clinical laboratory - Where to from here? Clin. Biochem. Rev. 2011;32(1):5–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Becker S., Kortz L., Helmschrodt C., Thiery J., Ceglarek U. LC–MS-based metabolomics in the clinical laboratory. J. Chromatogr. B. Elsevier B.V. 2012;883–884:68–75. doi: 10.1016/j.jchromb.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Gugten J.G., Dubland J., Liu H.-F., Wang A., Joseph C., Holmes D.T. Determination of serum aldosterone by liquid chromatography and tandem mass spectrometry: a liquid-liquid extraction method for the ABSCIEX API-5000 mass spectrometry system. J. Clin. Pathol. 2012;65(5):457–462. doi: 10.1136/jclinpath-2011-200564. [DOI] [PubMed] [Google Scholar]
- 25.Hinchliffe E., Carter S., Owen L.J., Keevil B.G. Quantitation of aldosterone in human plasma by ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. Elsevier B.V. 2013;913–914:19–23. doi: 10.1016/j.jchromb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Taylor P.J., Cooper D.P., Gordon R.D., Stowasser M. Measurement of aldosterone in human plasma by semiautomated HPLC-tandem mass spectrometry. Clin. Chem. 2009;55(6):1155–1162. doi: 10.1373/clinchem.2008.116004. [DOI] [PubMed] [Google Scholar]
- 27.Guo T., Taylor R.L., Singh R.J., Soldin S.J. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin. Chim. Acta. 2006;372(1–2):76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Meunier C., Blondelle D., Faure P., Baguet J.-P., Le Goff C., Chabre O., et al. Development and validation of a method using supported liquid extraction for aldosterone determination in human plasma by LC-MS/MS. Clin. Chim. Acta. 2015;447 doi: 10.1016/j.cca.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation, US Dep Heal Hum Serv. (May) (2001) 4–10.
- 30.P.A. Wayne, EP06-A: Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline, Approv Guidel CLSI Doc EP6-A (April) (2003) 23.
- 31.Meunier C., Blondelle D., Faure P., Baguet J.-P., Le Goff C., Chabre O., et al. Development and validation of a method using supported liquid extraction for aldosterone determination in human plasma by LC-MS/MS. Clin. Chim. Acta. Elsevier B.V. 2015;447:8–15. doi: 10.1016/j.cca.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Organization World Health. WHO | Sodium intake for adults and children. World Heal Organ [Internet]. 2012;(ISBN 978 92 4 150483 6):56. Available from: http://www.ncbi.nlm.nih.gov/pubmed?term=Sodium[Title] AND intake[Title] AND adults[Title] AND children[Title] AND WHO[Title].
- 33.Harris E.K., Boyd J.C. On dividing reference data into subgroups to produce separate reference ranges. Clin. Chem. 1990;36(2):265–270. [PubMed] [Google Scholar]
- 34.Tukey J.W. Addison-Wesely; 1977. Exploratory Data Analysis. [Google Scholar]
- 35.Funder J.W., Carey R.M., Fardella C., Gomez-Sanchez C.E., Mantero F., Stowasser M., et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008;93(9):3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 36.Ricós C., Iglesias N., García-Lario J.-V., Simón M., Cava F., Hernández A., et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann. Clin. Biochem. 2007;44:343–352. doi: 10.1258/000456307780945633. [DOI] [PubMed] [Google Scholar]
- 37.Ahokoski O., Virtanen A., Kairisto V., Scheinin H., Huupponen R., Irjala K. Biological day-to-day variation and reference change limits of serum cortisol and aldosterone in healthy young men on unrestricted diets. Clin. Chem. 1999;45(7):1097–1099. [PubMed] [Google Scholar]
- 38.Fortunato A., Prontera C., Masotti S., Franzini M., Marchetti C., Giovannini S., et al. State of the art of aldosterone immunoassays. A multicenter collaborative study on the behalf of the Cardiovascular Biomarkers Study Group of the Italian Section of European Society of Ligand Assay (ELAS) and Società Italiana di Biochimica Clinica (SIBIOC. Clin Chim Acta. Elsevier B.V. 2015;444:106–112. doi: 10.1016/j.cca.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Cho H.-J., Kim J.D., Lee W.-Y., Chung B.C., Choi M.H. Quantitative metabolic profiling of 21 endogenous corticosteroids in urine by liquid chromatography-triple quadrupole-mass spectrometry. Anal. Chim. Acta. 2009;632(1):101–108. doi: 10.1016/j.aca.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 40.Rehan M., Raizman J.E., Cavalier E., Don-Wauchope A.C., Holmes D.T. Laboratory challenges in primary aldosteronism screening and diagnosis. Clin. Biochem. 2015;48(6):377–387. doi: 10.1016/j.clinbiochem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Drenjacnevic-Peric I., Jelaković B., Lombard J.H., Kunert M.P., Kibel A., Gros M. High-salt diet and hypertension: Focus on the renin-angiotensin system. Kidney Blood Press Res. 2011;34(1):1–11. doi: 10.1159/000320387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandevijvere S., De Keyzer W., Chapelle J.-P., Jeanne D., Mouillet G., Huybrechts I., et al. Estimate of total salt intake in two regions of Belgium through analysis of sodium in 24-h urine samples. Eur. J. Clin. Nutr. Nature Publishing Group. 2010;64(11):1260–1265. doi: 10.1038/ejcn.2010.148. [DOI] [PubMed] [Google Scholar]
- 43.Eisenhofer G., Peitzsch M., Kaden D., Langton K., Pamporaki C., Masjkur J., et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin. Chim. Acta. 2017;470 doi: 10.1016/j.cca.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Shea P., Brady J.J., Gallagher N., Dennedy M.C., Fitzgibbon M. Establishment of reference intervals for aldosterone and renin in a Caucasian population using the newly developed Immunodiagnostic Systems specialty immunoassay automated system. Ann. Clin. Biochem. An Int. J. Biochem. Lab. Med. 2015;(August):1–9. doi: 10.1177/0004563215603401. [DOI] [PubMed] [Google Scholar]
- 45.Y. Bekkach, A.C. Heijboer, E. Endert, Determination of urinary aldosterone using a plasma aldosterone 2D ID LC–MS/MS method. 8 (2016) 1765–1775. [DOI] [PubMed]
- 46.Eisenhofer G., Peitzsch M., Kaden D., Langton K., Pamporaki C., Masjkur J., et al. Clinica Chimica Acta Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin. Chim. Acta. Elsevier. 2017;470(June 2016):115–124. doi: 10.1016/j.cca.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]