Highlights
-
•
Manifestation of 11β-hydroxylase and 21-hydroxylase deficiencies can be similar.
-
•
Urine steroid metabolome can help differentiate between these two CAH forms.
-
•
A high ratio of THS/(THE + THF + 5α-THF) help to confirm a 11β-hydroxylase deficiency.
-
•
Analysis of the urine steroid metabolome by GC-MS is helpful for CAH diagnosis.
Keywords: 11β-hydroxylase deficiency, Congenital adrenal hyperplasia, Urine steroid metabolome, GC-MS
Abstract
Introduction
11β-hydroxylase deficiency is the second most common form of congenital adrenal hyperplasia (CAH), accounting for 5–8% of all cases. It is an autosomal recessive enzyme defect that impairs the biosynthesis of cortisol and aldosterone. Mutation of the CYP11B1 gene on chromosome 8q22 causes partial or total reduction of enzyme activity. Clinical manifestations of 11β-hydroxylase deficiency include hypertension, and other signs related to overproduction of mineralocorticoids, and virilisation. Here, we report on a case of 11β-hydroxylase deficiency detected by urine steroid metabolome profiling.
Case Subject
The patient, a 3-month-old male, suffered from truncus arteriosus type I (congenital cardiovascular anomaly) and also presented with hyperpigmentation. An endocrinology consultation was sought and biochemical and molecular testing was conducted.
Results
The patient’s urine steroid metabolome, as analysed by GC-MS, showed high excretion of tetrahydrodeoxycortisol (THS) and a THS/(THE + THF + 5αTHF) ratio of 2.3, which was higher than normal. Diagnosis of 11β-hydroxylase deficiency was confirmed by mutation analysis of the CYP11B1 gene.
Conclusion
Analysis of the urine steroid metabolome by GC-MS can be used to assist in diagnosis of 11β-hydroxylase deficiency. We recommend consideration of urine steroid analysis as a first-line test in the diagnosis of CAH.
1. Introduction
Congenital adrenal hyperplasia (CAH) encompasses a group of autosomal recessive disorders that are caused by a deficiency in production of at least one of five enzymes necessary for adrenal cortisol biosynthesis [1]. A defect in any of the enzymes leads to an imbalance in the ratio of hormone precursor and, thus, the final hormone itself. The most common form of the disease is 21-hydroxylase deficiency, which accounts for more than 90% of CAH cases [1], [2]. The second most prevalent form is 11β-hydroxylase deficiency, which accounts for 5–8% of cases [3]. The diagnosis of either of these enzyme deficiencies is typically made by measuring blood levels of adrenal hormones and precursor steroids [4], [5]. A complementary diagnostic method uses gas chromatography-mass spectrometry (GC-MS) for urine steroidomic analysis [6], which allows for the simultaneous assessment and comparison of multiple steroids and is applicable to the diagnosis of many disorders of sex development (DSD) [7], [8].
The Vietnam National Children’s Hospital is a primary children's health care centre in North Vietnam and also a referral centre for the country. Using an Agilent 5975 GC-MS system, our hospital has successfully implemented urinary assays that analyze organic acids to facilitate the screening and diagnosis of inborn errors of metabolism, and quantitate HVA and VMA for use in the diagnosis and monitoring of neuroblastoma [9]. In 2017, our laboratory established a GC-MS method for steroidomic profiling in urine. Previously, in Vietnam, there were no developed methods by which these analytes could be measured; the sole method of diagnosis of CAH with 21-hydoxylase deficiency was analysis of 17-OH-progesterone (17-OHP) from serum by ELISA.
Here, we report a case of a 3-month-old Vietnamese boy with 11β-hydroxylase deficiency who was diagnosed by urine steroid metabolome profiling using GC-MS and confirmed by mutation analysis of the CYP11B1 gene.
2. Case study
2.1. Patient
The 3-month-old patient was a first child, normal-term delivery with a birth weight of 2.5 kg. His parents were not related and there was no associated positive familial history for CAH. His growth and development were normal for the first month of life. At 35 days, he showed signs of dyspnea and was brought to the Vietnam National Children’s Hospital. He was diagnosed with a truncus arteriosus type I and was treated by operation; this was a coincidental diagnosis in relation to this CAH case presentation. The patient presented to the hospital for a checkup at 3 months, at which time hyperpigmentation was noted. The blood pressure of the patient could not be measured accurately because of the congenital heart defect described above. An endocrinology consultation was sought and biochemical and molecular testing were conducted.
2.2. Serum biochemistry and method
Blood samples were collected from the patient by venepuncture following arrival at the hospital the morning before receiving treatment with hydrocortisone. Blood samples were processed in accordance with the laboratory’s standard operating procedures and in accordance with its ISO15189 accreditation.17-OHP was measured by ELISA (DRG International, Springfield NJ, USA). ACTH, cortisol and testosterone were measured by electrochemiluminescence on a Roche modular Cobas e601 (Roche, Ibaraki, Japan). Plasma electrolytes (i.e., sodium, potassium and chloride) were measured by the indirect ion-selective electrode (ISE) method on a Beckman Coulter AU680 (Beckman Coulter, Mishima, Japan) [10].
2.3. Urine steroid metabolome and method
The urine steroidomic method used here was based on those originally developed by Honour and Greaves [11], [12]. Briefly, a spot urine sample, along with added internal standard (i.e., androstanol), was subjected to enzyme hydrolysis using β-glucuronidase/arylsulfatase followed by solid phase extraction and derivatization with methoxyamine and trimethylsilylimidazole. The prepared sample was loaded onto an Agilent 7890A gas chromatograph coupled to an Agilent 5975C Mass Selective Detector (Agilent Technologies, Santa Clara, CA, USA). The gas chromatograph was equipped with an Agilent HP-5MS fused-silica capillary column (30 m × 0.25 mm id × 0.25 µm film thickness; Agilent Technologies Santa Clara, CA, USA).
The method was calibrated with lyophilised urine calibrator from the Dutch Foundation for Quality Assessment in Medical Laboratories (Stichting Kwaliteitsbewaking Medische Laboratorium Diagnostiek; SKML). Full mass spectra were used for the structural identification of analytes. Specific selected ion monitoring (SIM) groups were assigned for each analyte and included two representative ions (the mass to charge ions selected for SIM are noted in Table 2). Quantitative analysis was based on the analyte’s selected ion response against the response of the internal standard ion. To confirm consistency of results with other laboratories we participate in the University College London (UCL)/SKML urine steroid external quality assurance program [13].
Table 2.
Urine steroid metabolome results for this patient. Results corrected for concentration by measurement of urine creatinine (patient’s creatinine is 1.9 mmol/L).
| Steroids | Abbreviations | Chem Spider ID | Retention time (minute) | Target ion (m/z) | Quantitative Ion (m/z) | Concentration |
||
|---|---|---|---|---|---|---|---|---|
| µmol/L | µmol /mmol creatinine | RI*µmol/mmol | ||||||
| Internal Standard (androstanol) | ISTD | 13.38 | 258 | 243 | ||||
| Androsterone | A | 5668 | 16.96 | 360 | 270 | 0.3 | 0.2 | <0.2 |
| Etiocholanolone | E | 5669 | 17.18 | 360 | 270 | 0.6 | 0.3 | <0.1 |
| Dehydroepiandrosterone | DHEA | 5670 | 18.12 | 358 | 268 | 0.5 | 0.3 | <1.3 |
| 11-Ketoandrosterone | 11-KA | 18.54 | 315 | 300 | 0.1 | 0.1 | <0.2 | |
| 11-OH Androsterone | 11-OH A | 20,171,894 | 19.57 | 358 | 448 | 0.2 | 0.1 | <1.1 |
| 17-hydroxypregnanolone** | 17-OHPN | 19.61 | 296, 386, 476 | Present | ||||
| 11-OH Etiocholanolone | 11-OH E | 92020 | 19.81 | 358 | 448 | 0.1 | 0.1 | <0.2 |
| Pregnanediol | PD | 190585 | 20.51 | 269 | 284 | 4.6 | 2.4 | <0.2 |
| Pregnanetriol | PT | 92121 | 20.88 | 255 | 435 | 2.1 | 1.1 | <0.2 |
| Androstenetriol | A’3 | 21.17 | 432 | 342 | 13.4 | 7.1 | <5.8 | |
| Tetrahydro-deoxycortisol | THS | 29788211 | 21.86 | 474 | 564 | 23.1 | 12.1 | <0.2 |
| Hexahydro-compound S (double peak)** | HHS | 23.16& 23.33 | 255, 345, 435 | Present | ||||
| Tetrahydrocortisone | THE | 29788209 | 23.42 | 488 | 578 | 2.5 | 1.3 | 0.2–5.5 |
| Tetrahydrocortisol | THF | 5665 | 23.93 | 472 | 562 | 1.0 | 0.5 | <0.3 |
| Allo-Tetrahydrocortisol | Allo-THF | 83726 | 24.09 | 472 | 562 | 1.5 | 0.8 | <1.9 |
| Ratios | ||||||||
| Steroids | Result | RI | ||||||
| THS/(THE + THF + Allo-THF) | 2.28 | <0.3 | ||||||
The reference intervals were developed in house from 26 healthy (not in-hospital) Vietnamese children from one-month to 12-months old.
Steroid metabolites were qualitatively assessed based on m/z ions and relative retention time.
Urine creatinine was measured by the Jaffe kinetic method on a Beckman Coulter AU680 chemistry analyser. Patient results were reported relative to creatinine concentration. The urine steroid metabolome results were compared against our in-house interim reference intervals, as well as those from the group of Flück et al. [14].
2.4. Molecular studies and method
Total DNA was extracted from peripheral blood using the QiaAmp DNA blood minikit (Qiagen, Hilden, Germany). The CYP11B1 gene was amplified by PCR. Three pairs of primers were used to amplify exon 1-2, 3-5, and 6-9 of the CYP11B1 gene without amplifying the CYP11B2 gene [15]. All exons and intron/exon boundaries were sequenced on a 3130 Genetic Analyser (Applied Biosystems, Foster City, California, USA) using internal primers, as described previously [15]. Sequence data obtained in this study were compared with the CYP11B1 gene sequence in GeneBank (ENSG00000160882).
2.5. Results
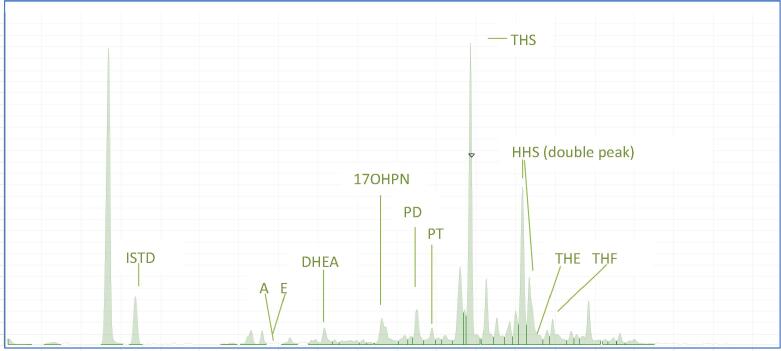
The patient’s testosterone, ACTH and 17-OHP levels were higher than the age-related reference intervals and suggestive of CAH (Table 1). An adrenal ultrasound demonstrated bilateral enlargement of the adrenal glands and a random urine sample was collected for steroid metabolome analysis. The urine steroid metabolome profile showed high excretion of THS compared to that normally expected for an infant. The THS/(THE + THF + Allo-THF) ratio was found to be elevated at 2.3. The two hexahydro-11-deoxycortisol steroid peaks, the result of reduction of the 20-carbonyl group of THS by 20 α- and 20 β-hydroxy steroid dehydrogenase, were present at elevated levels. Steroids from the foetal adrenal zone were found to exist at low concentrations in this profile, indicating involution of this zone in place of the adult adrenal. This abnormal pattern suggested a 11β-hydroxylase deficiency (see Fig. 1 and Table 2).
Table 1.
Routine laboratory test results.
| Analyte | Result | Reference Intervals | Comment |
|---|---|---|---|
| Testosterone | 4.25 nmol/L | 1–4 | High |
| ACTH | 51.3 pmol/L | 1.6–13.9 | High |
| Cortisol at 8 a.m. | 953 nmol/L | 69–630 | High |
| 17-OHP | 83.8 nmol/L | 5.1–12.1 | High |
| Sodium | 132 mmol/L | 134–142 | Slight low |
| Potassium | 4.5 mmol/L | 3.5–5.6 | Normal |
| Chloride | 99 mmol/L | 98–105 | Normal |
Fig. 1.
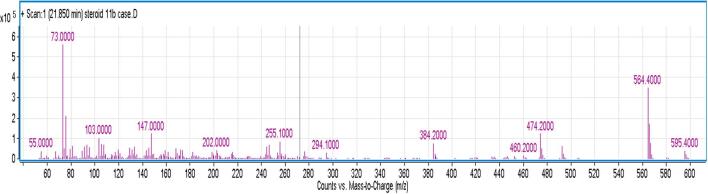
(a): The total ion chromatography of the urine steroid metabolome of this patient. The main peaks in this profile are the internal standard (androstanol, ISTD at 13.38 min), androsterone (A, 16.96 min), etiocholanolone (E, 17.18 min), 17-hydroxyprenanolone (17-OHPN, 19.61 min), pregnanetriol (PT, 20.88 min), tetrahydro 11-deoxycortisol (THS, markedly elevated at 21.85 min), hexahydro11-deoxycortisol (HHS at 23.02 and 23.16 min), tetrahydro cortisone (THE, 23.42 min) and tetrahydro cortisol (THF, supressed levels at 23.93 min). (b): Ion profile inside the TIC peak at 21.86 min. This EI fragmentation pattern demonstrates the presence of THS (based on the retention time and library match). THS chemical name is 5b-P-3a, 17a,21-TRIOL-20-ONE and molecular weight is 350.492 Da (g/mol). The major ions of THS are 246, 294, 384, 474 and 564.
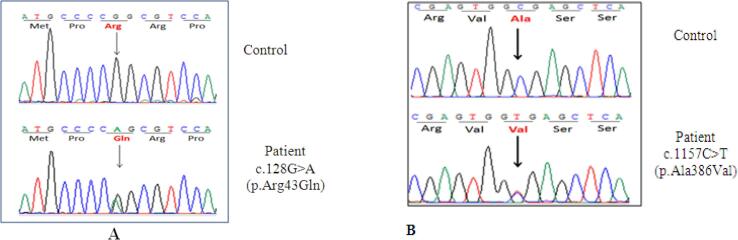
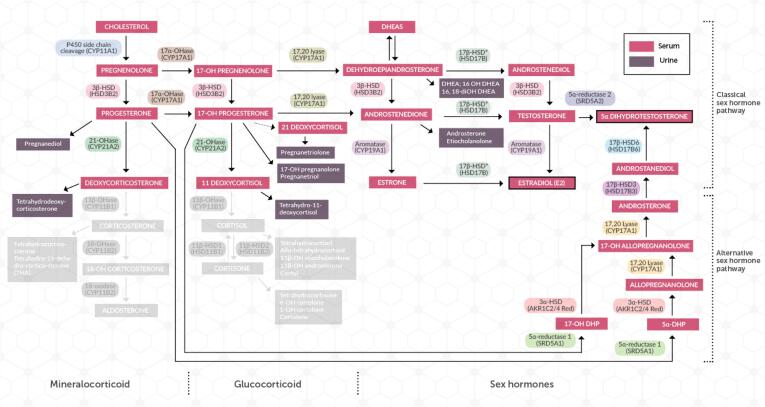
As a result of these findings, molecular genetic analysis of the CYP11B1 gene was performed and revealed the patient had two compound heterozygous mutations c.128G > A (p.Arg43Gln) and c.1157C > T (p.Ala386Val) (Fig. 2). These are commonly found in 11β-hydroxylase deficiency patients [16], [17]. The alteration in the steroid pathway due to this enzyme deficiency is shown in Fig. 3.
Fig. 2.
Mutations detected on the CYP11B1 gene. (A): Mutation c.128 G > A (p.Arg43Gln). (B): Mutation c1157C > T (p.Ala 386 Val).
Fig. 3.
Steroid pathway demonstrating block associated with 11β-hydroxylase deficiency. This block results in decreased mineralocorticoids and glucocorticoids, as demonstrated by the greyed out section of the pathway. An increase in steroids proximal to the block, particularly THS and PT, and shunting of the pathway towards DHEA production results.
3. Discussion
Currently, in Vietnam, CAH that results from 21-hydroxylase deficiency is diagnosed by analysis of 17-OHP from blood using ELISA. However, the issue of immunoassay cross-reactivity remains, and the complex steroid milieu of the newborn human gives rise to considerable variation in their performance. A definitive neonatal diagnosis is complicated by non-elevated 17-OHP levels in some infants with 21-hydroxylase deficiency, and by the presence of residual maternal- placental and foetal steroid products, which can lead to misdiagnoses. Moreover, CAH due to 11β-hydroxylase, 17-hydroxylase, 20–22 desmolase, 3β-hydroxysteroid dehydrogenase or 5α-reductase deficiency cannot be diagnosed by blood 17-OHP measurement. By May 2014, there were 715 children with CAH at our hospital (703 with 21-hydroxylase deficiency, nine with11β-hydroxylase deficiency and three with 3β-hydroxysteroid dehydrogenase deficiency) who had been diagnosed and were having their treatment managed with the inclusion of steroid hormone quantification by immunoassay [18]. While generally successful, more complicated cases could not be diagnosed in this manner. In such situations, the confirmed diagnosis was obtained by sending samples overseas, which is time-intensive, costly, and results in significant delays. Here, we demonstrate the value of urine steroid analysis by GC-MS in the differential diagnosis of CAH. The implementation of this method in Vietnam will improve the quality of diagnosis and treatment monitoring of inborn errors of steroid synthesis, as well as other adrenal disorders in Vietnam.
Clinical manifestations of 11β-hydroxylase deficiency include hypertension (which occurs in 75% of patients, but is not always present during childhood), and other signs related to overproduction of mineralocorticoids, such as hypokalemia, muscle weakness and virilisation [16], [19]. Signs of androgen-excessive secretion include early closure of the epiphysis (short stature) [16], [19], [20]. The diagnosis of 11β-hydroxylase deficiency is indicated by high serum basal levels of deoxycorticosterone, 11-deoxycortisol and/or urine tetrahydro-metabolites. This disorder should be considered in patients with ACTH that is three times higher than the 95th percentile, as predicted for the patient’s age [21]. However, for our patient, the clinical findings and routine laboratory tests showed none of the specific features for 11β-hydroxylase deficiency: the blood pressure of this patient could not be measured accurately because of his congenital heart defect; the serum testosterone was high; and plasma ACTH and 17-OHP levels were unusually high, which may have led to the misdiagnosis of 21-hydroxylase deficiency. Our laboratory did not perform deoxycorticosterone and/or 11-deoxycortisol serum measurement. Additionally, the patient’s blood cortisol level was also high.
The high serum cortisol level found in this patient is counterintuitive given the clinical scenario, as cortisol is produced downstream of the enzyme block. The urine steroid metabolome demonstrated low cortisol metabolite output (i.e., THF and the cortols). The difference is that the serum cortisol was performed by immunoassay on the Roche Cobas e601 analyser using the Cortisol II kit, compared to the urine being measured using mass spectrometry [22]. The difference here is in method specificity with mass spectrometry relying on the chemical structure, molecular weight and retention time, while immunoassay analysis is dependent on the specificity of the antibody. 11-deoxycortisol is a significant cross reactant in the Roche Cobas e601 Cortisol II assay, which is a likely contributor to the “high” serum cortisol seen in our patient. Hence, this can be misleading in the differential diagnosis of CAH, and, as such, immunoassay cortisol results should not be used to rule out CAH [23], [24], [25].
The patient's urine steroid analysis by GC-MS showed a slight increase in metabolites of 17-OHP, including17-OH-pregnanolone and pregnanetriol. However, the increase of these metabolites is also seen in 21-hydroxylase deficiency. The metabolite with the highest specificity for 11β-hydroxylase deficiency is tetrahydro–deoxycortisol (THS), a metabolite of 11-deoxycortisol. In 21-hydroxylase deficiency, this metabolite is in the normal range. High excretion of THS is a specific characteristic of 11β-hydroxylase deficiency. This pattern is specific for 11β-hydroxylase deficiency and is a criterion for definitive diagnosis of this CAH form. In a study by Chan and Shek, the urine THS level was five times higher than normal in an 11β-hydroxylase deficient patient [8]. Caulfield et al. also found that two patients with 11β-hydroxylase deficiency had high levels of THS in their urine [7].
CAH, caused by 11β-hydroxylase deficiency, is rare, with an estimated frequency of 1 in 100,000 live births [3]. The robust serum marker for 11β-hydroxylase deficiency is the accumulated substrate 11-deoxycortisol. Additionally, elevated levels of serum markers 11-deoxycorticosterone and androstenedione have been observed [16]; although, these steroids are not routinely measured. Blood sampling reflects a single time point rather than an integrated picture of the steroid metabolome over a longer time span, as obtained by GC-MS urine steroid analysis [26]. As such, there are several advantages to urine steroid analysis for CAH diagnosis: sample collection is non-invasive; a random urine sample is suitable; and a full spectrum of steroid hormone metabolites and precursor/product ratio assessment can be measured simultaneously, increasing accuracy and confidence in the diagnosis [7], [8].
4. Conclusions
11β-hydroxylase deficiency is a rare form of CAH whose clinical and laboratory findings can be similar to those for 21-hydroxylase deficiency. Accurate diagnosis of the form of CAH not only facilitates proper treatment, but also guides genetic counselling of the parents of affected children. The urine steroid metabolome with high excretion of THS and a high THS/(THE + THF + 5α-THF) ratio helps to confirm 11β-hydroxylase deficiency. Analysis of the urine steroid metabolome by GC-MS provides comprehensive diagnostic information and should be considered for further development as a first-line test for CAH patients due to 11β-hydroxylase deficiency. Although further research will be necessary to establish a robust cut-off value for a THS/(THE + THF + 5α-THF) ratio that can be used to definitively identify patients with 11β-hydroxylase deficiency.
Funding
Asia Pacific Federation of Clinical Chemistry and Laboratory Medicine project grant.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
Nothing to declare.
Contributors Statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of this manuscript. The manuscript consists of original material and has not been published elsewhere. All authors listed contributed to this work. Dr. CD Vu and MD Tran performed clinical examination. Dr. NAT Tran performed the urine steroid analysis. Dr. PM Nguyen, DN Ngo and Dr. HH Nguyen performed CYP11B1 gene mutation analysis. Dr. NAT Tran and MTC Tran wrote the the first draft of this manuscript and together Dr. TCM Tran and Dr. RF Greaves reviewed and edited the manuscript. All authors approved the final manuscript. All the authors have accepted responsibility for the entire content of this submission.
Acknowledgements
We acknowledge the Asia-Pacific Federation of Clinical Biochemistry and Laboratory Medicine for funding of the urine steroid metabolome method development.
References
- 1.Specier P.W., White P.C. Congenital adrenal hyperplasia. N. Engl. J. Med. 2003;349:776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.M.I. New, O. Lekarev, E. Mancenido, A. Parsa, T. Yuen, Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency, Genetics Steroid Disorders, ed MI New et al., 2014, pp. 29–51.
- 3.P.C. White, Steroid 11-beta-hydroxylase deficiency and related disorders. Genetic Steroid Disorders. ed MI New et al., 2014, pp. 71–85.
- 4.Biason-Lauber A., Zachmann M. Physician’s Guide to the laboratory diagnosis of metabolic diseases. Second edition. Springer; 1996. Disorders of Steroid synthesis and metabolism; pp. 551–572. [Google Scholar]
- 5.Kulle A., Krone N., Holterhus P.M., Schuler G., Juul A., Greaves R.F., deRijke Y.B., Hartmann M.F., Saba A., Hiort O., Wudy S.A. Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST action BM 1303 DSDnet. Eur. J. Endocrinol. 2017;176(5):1–9. doi: 10.1530/EJE-16-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wudy S.A., Schulerb G., Sánchez-Guijoa A., Hartmann M.F. The art of measuring steroids - principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2017 doi: 10.1016/j.jsbmb.2017.09.003. in press. [DOI] [PubMed] [Google Scholar]
- 7.Caulfield M.P., Lynn T., Gottschalk M.E., et al. The diagnosis of congenital adrenal hyperplasia in the newborn by Gas Chromatography/Mass Spectrometry analysis of random urine specimens. J. Clin. Endocrinol. Metabol. 2002;87:3682–3690. doi: 10.1210/jcem.87.8.8712. [DOI] [PubMed] [Google Scholar]
- 8.Chan A.O., Shek C.C. Urinary steroid profiling in the diagnosis of congenital adrenal hyperplasia and disorders of sex development: experience of a urinary steroid referral centre in Hong Kong. Clin. Biochem. 2012;46:327–334. doi: 10.1016/j.clinbiochem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Tran M.T.C., Baglin J., Tran T.T.T., Hoang K.T., Phung L.T., Read A., Greaves R.F. Development of a new biochemical test to diagnose and monitor neuroblastoma in Vietnam: homovanillic and vanillylmandelic acid by gas chromatography –mass spectrometry. Clin. Biochem. 2014;47:206–215. doi: 10.1016/j.clinbiochem.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Tran M.T.C., Hoang K.T., Greaves R.F. Practical application of biological variation and SigmaMetrics quality models to evaluate 20 chemistry analytes on the Beckman AU680. Clin. Biochem. 2016;49:1259–1266. doi: 10.1016/j.clinbiochem.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Honour J.W. Steroid profiling. Ann. Clin. Biochem. 1997;34:32–44. doi: 10.1177/000456329703400106. [DOI] [PubMed] [Google Scholar]
- 12.Greaves R.F., Poomthavorn P., Zacharin M. 11β-hydroxylase deficiency masked by alternative medicines. J. Paediatric Child Health. 2006;42:652–654. doi: 10.1111/j.1440-1754.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 13.UCL/SKML - Clinical Biochemistry - HSL Analytics LLP.Floor 2,1 Mabledon Place, London WC1H 9AX(Dr Francis Lam), steroidprofile.eqa@hslpathology.com.
- 14.Dhayat N.A., Frey A.C., Frey B.M., d’Uscio C.H., Vogt B., Rousson V., Dick B., Flück C.E. Estimation of reference curves for the urinary steroid metabolome in the first year of life in healthy children: tracing the complexity of human postnatal steroidogenesis. J. Steroid Biochem. Mol. Biol. 2015;154:226–236. doi: 10.1016/j.jsbmb.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen H.H., Nguyen T.H., Vu C.D., Nguyen K.T., Le B.V., Nguyen T.L., Nong V.H. Novel homozygous p. Y395X mutation in the CYP11B1 gene found in a Vietnamese patient with 11beta-hydroxylase deficiency. Gene. 2012;509:295–297. doi: 10.1016/j.gene.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Khattab A., Haider S., Kumar A., Dhawan S., Alam D., Romero R., Burns J., Li D., Estatico J., Rahi S., Fatima S., Alzahrani A., Hafez M., Musa N., RazzghyAzar M., Khaloul N., Gribaa M., Saad A., Charfeddine I.B., Bilharinho de Mendonça B., Belgorosky A., Dumic K., Dumic M., Aisenberg J., Kandemir N., Alikasifoglu A., Ozon A., Gonc N., Cheng T., Kuhnle-Krahl U., Cappa M., Holterhus P.M., Nour M.A., Pacaud D., Holtzman A., Li S., Zaidi M., Yuen T., New M.I. Clinical, genetic and structural basis of congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. Proc. Natl. Acad. Sci. U.S.A. 2017;114(10):E1933–E1940. doi: 10.1073/pnas.1621082114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krone N., Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract. Res. Clin. Endocrinol. Meta. 2009;23:181–192. doi: 10.1016/j.beem.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dung Vu Chi, Thao Bui Phuong, Ngoc Can ThiBich, Khanh Nguyen Ngoc, PhuDat Nguyen, ThiHoan Nguyen, Mai Do Thanh, ThiHuong Bui. Registry of congenital adrenal hyperplasia at the north pediatric referral centre of Vietnam during 15 years. Ann. Transl. Med. 2015;3(S2):S48. [Google Scholar]
- 19.Efat K., Rahim V. Congenital adrenal hyperplasia and SchmidmetaphysealChondrodysplasia in a child. Iran J. Med. Sci. 2016;41(1):64–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Ramires T.S., Haroldo S.S., Alexandre T. Congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. Arquivos Brasilairos de Cardiologia. 2005;85(6):1–4. doi: 10.1590/s0066-782x2005001900008. [DOI] [PubMed] [Google Scholar]
- 21.Sathya A., Ganesan R., Kumar A. Congenital adrenal hyperplasia masquerading as periodic paralysis in an aldolescent girl. Singapore Med. J. 2012;53:48–149. [PubMed] [Google Scholar]
- 22.Roche cortisol Gen 2, Available at: https://www.accessdata.fda.gov/cdrh_docs/reviews/K152227.pdf. Accessed 6th September 2017.
- 23.Ratcliffe W.A., McClure J.P., Auld W.H.R., Honour J.W., Fraser R., Ratcliffe J.G. Precociouspseudopuberty due to a rare form of congenital adrenal hyperplasia. Ann. Clin. Biochem. 1982;19:145–150. doi: 10.1177/000456328201900303. [DOI] [PubMed] [Google Scholar]
- 24.El-Farhan N., Rees D.A., Evans C. Measuring cortisol in serum, urine andsaliva – are our assays good enough? Ann. Clin. Biochem. 2017;54(3):308–322. doi: 10.1177/0004563216687335. [DOI] [PubMed] [Google Scholar]
- 25.Reisch N., Högler W., Parajes S., Rose I.T., Dhir V., Götzinger J., Arlt W., Krone N. A diagnosis not to be missed: nonclassic steroid 11β-hydroxylase deficiency presenting with premature adrenarche and hirsutism. J. Clin. Endocrinol. Metab. 2013;98(10):E1620–E1625. doi: 10.1210/jc.2013-1306. [DOI] [PubMed] [Google Scholar]
- 26.Krone N., Hughes B.A., Lavary G.G., Stewart P.M., Arlt W., Shackleton C.H.L. Gas chromatography/mass spectrometry (GC-MS) remains a preeminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J. Steroid Biochem. Mol. Biol. 2010;121(3-5):496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]