Abstract
Background
Transbronchial lung cryobiopsy (TBLC) is an alternative to surgical lung biopsy for histopathological evaluation of unclassifiable interstitial lung disease (ILD) or ILD diagnosed with low confidence. This meta-analysis synthesised current literature regarding cryobiopsy diagnostic performance and safety, focusing on procedural and sampling techniques.
Methods
Medline and Embase were searched on 11 April 2022. Studies included adults with unclassifiable ILD, reporting diagnostic yield, complications and methodological techniques of TBLC. Meta-analyses were performed for diagnostic yield, pneumothorax and bleeding. Subgroup analyses and meta-regression assessed methodological variables. PROSPERO registration: CRD42022312386.
Results
70 studies were included with 6183 participants. Diagnostic yield of TBLC was 81% (95% CI 79–83%, I2=97%), with better yield being observed with general anaesthesia (p=0.007), ILD multidisciplinary meeting prior to cryobiopsy (p=0.02), 2.4 mm cryoprobe (p=0.04), higher mean forced vital capacity (p=0.046) and higher mean diffusing capacity for carbon monoxide (p=0.023). Pneumothorax rate was 5% (95% CI 4–5%, I2=91%), with higher rates associated with a 2.4 mm cryoprobe (p<0.00001), routine post-procedure imaging (p<0.00001), multiple lobe sampling (p<0.0001), reduced mean diffusing capacity for carbon monoxide (p=0.028) and general anaesthesia (p=0.05). Moderate-to-severe bleeding rate was 12% (11–14%, I2=95%) and higher rates were associated with a 2.4 mm cryoprobe (p=0.001) and bleeding score selection (p=0.04).
Interpretation
Patient characteristics and modifiable factors, including procedural methods and anaesthetic techniques, impacted diagnostic yield and safety outcomes of TBLC in people with unclassifiable ILD and contributed to heterogeneity of clinical outcomes. These variables should be considered for individualised clinical decision making and guideline development and warrant routine reporting in future research.
Shareable abstract
This systematic review identified several variables that contribute to heterogeneity of outcomes following transbronchial lung biopsy in interstitial lung disease, with modifiable procedural factors impacting both diagnostic yield and complications. https://bit.ly/3XqldYm
Introduction
Interstitial lung disease (ILD) comprises a heterogeneous group of pulmonary disorders, manifesting with fibrosis and inflammation in the lung interstitium [1]. Transbronchial lung cryobiopsy (TBLC) is established in clinical practice and within guidelines as an alternative approach to surgical lung biopsy (SLB) in the diagnostic evaluation of people with ILD.
Best practice for ILD diagnosis mandates a multidisciplinary meeting of specialist clinicians, including respiratory physicians, pulmonary radiologists and pathologists [2]. Consensus diagnosis must balance detailed clinical assessment, examination findings, radiological assessment with high-resolution computed tomography of the chest, serological evaluation and, on occasion, bronchoalveolar lavage [2, 3]. A significant minority of people with ILD will receive a diagnosis that is unclassifiable or made with low confidence, in which case lung biopsy may be recommended to increase diagnostic confidence, provide accurate disease prognostication and guide treatment decisions regarding antifibrotic and immunosuppressive pharmacotherapy [3–5]. Many patients will not be eligible to proceed to biopsy due to comorbidities or other reasons, such as the severity of underlying ILD corresponding to unacceptable procedural risk or patient preference.
Decision-making regarding the optimal approach to lung biopsy balances the benefits and risks of available techniques. SLB is the gold standard for diagnostic yield, ranging between 93.5 and 98.0% [6–8]. However, it is an invasive procedure requiring a multi-day hospital stay and placement of an intercostal catheter. The risk of exacerbation of ILD is high (6.1%) [9] and 30-day mortality is approximately 2% for elective procedures, which increases in high-risk groups [10]. TBLC is less invasive than surgical biopsy and, while diagnostic yield is lower than SLB (approximately 80%), risks of morbidity and mortality are lower [6, 11]. Predominant complications are bleeding and pneumothorax, with serious complications of severe bleeding, ILD exacerbation and, rarely, death [11]. The largest study to compare the diagnostic performance of TBLC and SLB found a high level of agreement between techniques [12], although smaller studies have failed to replicate this outcome [13–15]. Consequently, cryobiopsy has been recommended as a first-line method for lung sampling in experienced centres [3–5].
A persistent challenge in TBLC practice has been unexplained heterogeneity reported for the key outcomes of diagnostic yield, bleeding and pneumothorax [6, 11]. A previous expert statement identified that notable variations in procedural and sampling techniques were likely to contribute to disparity in complications [16]. Other authors have reported that heterogeneous outcomes may arise from bias related to low-quality evidence that is frequently nonrandomised or retrospective [11]. A recent meta-analysis attributed some heterogeneity to experience, finding better outcomes in high-volume TBLC centres [6]. Despite these analyses, heterogeneity remains incompletely understood. This study therefore aimed to address this knowledge gap by systematically exploring the presence and impact of current variations in procedural and sampling techniques for TBLC in people with unclassifiable or low diagnostic confidence ILD.
Methods
Search strategy and selection criteria
For this systematic review and meta-analysis, an electronic search for relevant studies from inception to 29 April 2022 was conducted using Medline R (ALL), Embase via OVID and CENTRAL (Cochrane Trials database) via the Cochrane Library hosted by Wiley. Advanced search functions were used in all databases. Two clinical trials registries were searched (International Clinical Trials Registry Platform and ClinicalTrials.gov). Search strategies used a mix of thesaurus and free-text terms (see table S6 for full search strategies). No study filters were used. Reference lists of included studies were hand-searched for qualifying studies.
Inclusion criteria were participants aged ≥18 years old with a diagnosis of ILD of undifferentiated subtype. Included studies consisted of randomised controlled trials, observational studies and case series of three of more patients. Studies were excluded if they were written in languages other than English or nonoriginal research papers, including narrative reviews, editorials, conference abstracts and nonpeer-reviewed papers. Trial registries, grey literature and unpublished studies were not included in the search.
The searches were sequentially undertaken by two authors (J.A. Lachowicz and P. Patel), with conflicts resolved by discussion between authors or adjudication by a third author (D.P. Steinfort) if unable to reach consensus.
Data analysis
Data extraction was performed by one author (J.A. Lachowicz) using a standardised form with verification by a second author (P. Patel). Data were extracted based on the expert statement from the cryobiopsy working group outlining sources of procedural and sampling variation [16]. These included patient factors, study design, anaesthesia, imaging selection, sampling process, pathological assessment and safety outcomes. When duplicate data were contained in two or more studies, the larger study was included.
Two authors (J.A. Lachowicz and P. Patel) independently assessed studies for risk of bias. Randomised trials were assessed using the revised Cochrane risk-of-bias tool and nonrandomised trials were assessed using a modified Newcastle–Ottawa scale and QUADAS-2 [17–19]. Disagreements between authors were adjudicated by a third author (D.P. Steinfort) or addressed by discussion between authors to reach consensus.
Primary outcomes of diagnostic yield, moderate-to-severe bleeding and pneumothorax were reported as a pooled estimate with 95% confidence intervals. Diagnostic yield was reported as defined by individual studies and, when two definitions of diagnostic yield were reported (relating to histological yield and multidisciplinary discussion yield), histological yield was included. Bleeding was reported as moderate or severe as per the classification of reporting studies. As a reference, the British Thoracic Society (BTS) guidelines define moderate bleeding as requiring the use of cold saline, topical adrenaline or isolation of the bleeding airway segment through intubation or wedging with the bronchoscope to obtain bleeding control [20]. Severe bleeding is defined as requiring an endobronchial balloon blocker or equivalent, use of fibrin sealant, requiring blood transfusion or resuscitation, warranting admission to an intensive care unit, or causing death. Secondary outcomes included measures of procedural and sampling techniques employed during TBLC.
Meta-analyses were performed using a random-effects, generic inverse variance model. Narrative description was undertaken for factors unsuitable for meta-analysis. Variability between studies was assessed using the I2 estimate of study heterogeneity, with values between 75 and 100% representing considerable heterogeneity. Pre-planned subgroup analyses included ILD multidisciplinary meeting assessment, comorbidities, lung function and type of imaging guidance, and were undertaken for categorical variables. Univariate meta-regression was undertaken for continuous variables. Multiple regression was planned for variables of statistical significance but could not be performed due to heterogeneous and incomplete reporting of statistically significant variables, limiting the number of studies that could be included (<10 studies per primary outcome). Several variables found to be statistically significant on univariate meta-regression had negligible R2 values, suggesting they did not contribute to study heterogeneity. Sensitivity analyses were performed for each primary outcome by removing individual studies from the analysis and reassessing the effect size, confidence interval and I2 statistic.
Primary meta-analysis was performed using RevMan 5.4. Meta-regression was performed using Comprehensive Meta-analysis Software version 4. The study was registered at PROSPERO, study number CRD42022312386. There was no funding source for this study and there are no competing interests of the review authors to declare.
Results
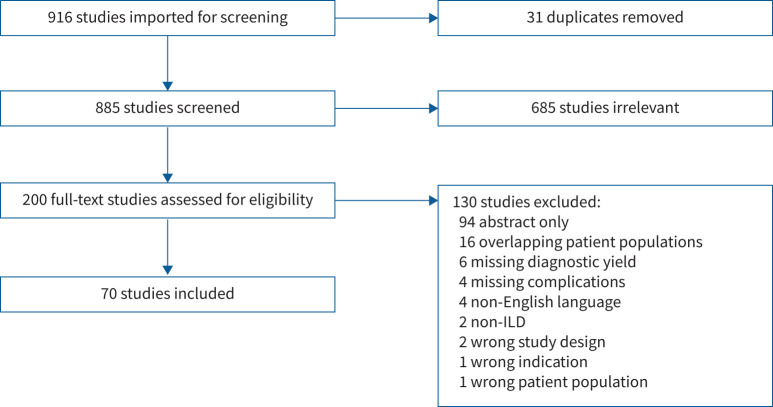
Of 916 studies screened, 70 were included, with 6183 pooled participants (range: 4–699 per study) (figure 1). 56 (80%) studies were single centre and 39 (55.7%) were retrospective. 58 (82.9%) were case series or cohort studies. The remainder comprised single-arm intervention and observational studies; two studies randomised participants to cryobiopsy or transbronchial forceps biopsy [21, 22]. Seven studies evaluated performance of cryobiopsy versus transbronchial forceps biopsy [13, 21–26] and three studies compared cryobiopsy to SLB [12–14]. Study characteristics are summarised in table 1, with details presented in table S1.
FIGURE 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) diagram. ILD: interstitial lung disease
TABLE 1.
Summary of included study characteristics
| Study characteristics | ||
|---|---|---|
| Study type | Number of studies | Number of participants |
| Diagnostic accuracy studies | ||
| Prospective | 3 | 102 |
| Cohort studies and case series | ||
| Prospective | 16 | 1417 |
| Retrospective | 35 | 3360 |
| Randomised control trial | ||
| Prospective | 2 | 51 |
| Single-arm interventional studies using novel imaging guidance | ||
| Prospective | 10 | 777 |
| Retrospective | 4 | 476 |
| Study location | Number of studies | Number of participants |
|---|---|---|
| American continent | 14 | 858 |
| Asia | 13 | 1474 |
| Europe | 35 | 3444 |
| Other | 8 | 407 |
| Study design | Number of studies | Number of participants |
|---|---|---|
| Single centre | 55 | 4775 |
| Multicentre | 13 | 1257 |
| Unclear | 2 | 151 |
| Participant characteristics | Range | |
|---|---|---|
| Male, % | 33–100 | |
| Mean age, years | 46.2–64 | |
| Mean FVC, % pred | 64.6–98.3 | |
| Mean DLCO, % pred | 49.1–76.3 | |
DLCO: diffusing capacity for carbon monoxide; FVC: forced vital capacity.
The overall quality of included studies was low, due to the reliance on nonrandomised, frequently retrospective and nonconsecutive data. Few cohort studies compared the performance of cryobiopsy to a histological reference standard. Risk of bias assessments are summarised in tables S3–S5.
The primary outcome of diagnostic yield was 81% (95% CI 79–83%), with significant heterogeneity (I2=97%) (see figure S1). Definitions of diagnostic yield were presented in only 36 (51.4%) studies and varied considerably. Variations included the presence of any histopathologic yield, final diagnosis at multidisciplinary meeting, histopathologic yield as compared to surgical lung biopsy or whether sufficient histological information was available to contribute to a multidisciplinary diagnosis.
The rate of pneumothorax was 5% (95% CI 4–5%) (see figure S2), I2=91%. Pneumothorax was treated with percutaneous drainage or insertion of an intercostal catheter in 62.5% of cases (3.1% of cryobiopsies). The composite outcome of moderate-to-severe bleeding had an overall incidence of 12% (95% CI 11–14%), I2=95% (see figure S3). Bleeding reporting and definitions were inconsistent. 46 (65.7%) studies utilised a definition that was unvalidated or not previously published. Variations included unit of measure, with categories based on factors such as bleeding volume or duration, need for additional therapeutic drugs, or techniques such as endobronchial blockade, requirement for blood products, haemodynamic instability and need for escalation of care to an intensive care unit or surgery. Seven (10%) studies used the BTS bleeding score for bronchoscopy [27–33]. Interventions for the management of moderate-to-severe bleeding varied with regards to the use of topical adrenaline (routinely or in response to bleeding), cold saline, amchafibrin, bronchial occlusion, rigid bronchoscope ventilation, topical or intravenous tranexamic acid, terlipressin, thrombin, double lumen intubation, a two-bronchoscopy technique, and other procoagulant use.
Procedural and sampling techniques were explored as secondary outcomes. Reporting varied widely, as summarised in table S2. 56 studies (80%) utilised fluorosocopy and 14 studies (20%) employed a novel form of imaging guidance. 11 (15.7%) used radial endobronchial ultrasound [34–44], with a focus on identifying blood vessels in the biopsy zone. Four (5.7%) used cone-beam computed tomography [45–48]. Two (2.9%) employed an integrated navigation system [49, 50] and one (1.4%) used confocal laser endomicroscopy [51]. Target distance to the pleura varied between 0.5 and 2.0 cm.
Relative and absolute physiological parameters as a contraindication to cryobiopsy varied. A minimum forced vital capacity (FVC) of equal to 50% pred was frequently cited [14, 22, 28, 32, 39, 44, 48, 50, 52–57]. Other exclusion criteria included total lung capacity <50% pred [12, 21, 26, 43], FVC <35% pred [11] and diffusing capacity for carbon monoxide (DLCO) <30–50% pred [12, 14, 21, 26, 28, 31, 32, 39, 41, 43, 48, 50, 52, 53, 55–60].
40 (57.1%) studies outlined a target number of biopsies (range: 1–10). Mean±sd number of biopsies was 3.4±0.85. Three (4.3%) studies targeted areas of ground glass opacity over fibrosis or nonfibrotic areas if an alternative radiological pattern was present [13, 56, 61]. Preferences to target lower lobes (18 studies, 25.5%) or the right lung were described [62]. Seven studies included cryobiopsies performed on aspirin [27, 34, 41, 63–66]. Practice otherwise varied regarding antiplatelet and anticoagulant use and was frequently unreported.
Contraindication to cryobiopsy based on respiratory status was inconsistent; the most frequent threshold was alveolar oxygen tension <55–60 mmHg on arterial blood gas sampling and breathing room air [14, 21, 26, 28, 32, 43, 53, 54, 67]. Seven (10%) studies included patients requiring supplemental oxygen or intubated for respiratory failure [23, 41, 55, 56, 64, 68, 69].
Post hoc subgroup analysis and meta-regression of diagnostic yield identified multiple significant factors associated with improved yield (table 2). These included use of general anaesthesia versus sedation (83% versus 75%, p=0.007), ILD multidisciplinary meeting prior to cryobiopsy versus none (82% versus 76%, p=0.02), 2.4 mm cryoprobe size versus 1.9 mm (82% versus 75%, p=0.04), higher mean FVC (regression coefficient 0.0063, p=0.046) and higher mean DLCO (regression coefficient 0.0072, p=0.023) (see figures S4–S9).
TABLE 2.
Impact of procedural and sampling variables on diagnostic yield
| Variable | Diagnostic yield (95% CI) | p-value, R2 analogue# |
|---|---|---|
| GA versus sedation | 0.83 (0.81–0.85) versus 0.75 (0.69–0.80) | p=0.007 |
| ILD MDM prior to cryobiopsy versus no ILD MDM | 0.82 (0.8–0.84) versus 0.76 (0.72–0.81) | p=0.02 |
| 2.4 mm versus 1.9 mm size cryoprobe | 0.82 (0.77–0.87) versus 0.75 (0.71–0.79) | p=0.04 |
| Higher mean FVC (% pred) | Coefficient 0.0063 (0.0001–0.013) | p=0.046, R2=0.24 |
| Higher mean DLCO (% pred) | Coefficient 0.0063 (0.0001–0.013) | p=0.022, R2=0.35 |
| Single lobe versus one or more lobes | 0.74 (0.067–0.82) versus 0.81 (0.78–0.85) | p=0.08 |
| Mean number of biopsies | Coefficient −0.017 (−0.055–0.022) | p=0.40, R2=0.0 |
| Lower lobes targeted for biopsy versus not targeted | 0.81 (0.74–0.88) versus 0.79 (0.77–0.82) | p=0.61 |
| Review by a single pathologist versus >1 pathologist | 0.78 (0.71–0.85) versus 0.80 (0.72–0.89) | p=0.69 |
| DPLD versus non-DPLD (inclusion criteria) | 0.80 (0.75–0.85) versus 0.79 (0.77–0.82) | p=0.79 |
#: Reported for meta-regression analyses. DLCO: diffusing capacity for carbon monoxide; DPLD: diffuse parenchymal lung disease; FVC: forced vital capacity; GA: general anaesthesia; ILD: interstitial lung disease; MDM: multidisciplinary meeting.
Subgroup analysis of pneumothorax rate and meta-regression found multiple significant variables associated with a higher complication rate (table 3). These included a 2.4 mm cryoprobe (11% versus 1%, p<0.00001), routine post-procedure imaging (7% versus 2%, p<0.00001), multiple lobe sampling (9% versus 3%, p<0.0001), reduced mean DLCO (regression coefficient −0.0025, p=0.028) and general anaesthesia (6% versus 5%, p=0.05) (see table 3 and figures S10–S14). Two factors showed a statistically significant association with pneumothorax (mean age and higher mean FVC) but did not significantly contribute to study heterogeneity, as demonstrated by low R2 analogues (figures S15–S16).
TABLE 3.
Impact of procedural and sampling variables on pneumothorax
| Variable | Pneumothorax (95% CI) | p-value, R2 analogue# |
|---|---|---|
| 2.4 mm versus 1.9 mm size of cryoprobe | 0.11 (0.08–0.15) versus 0.01 (0.00–0.01) | p<0.00001 |
| Routine post-procedure imaging versus no routine imaging | 0.07 (0.06–0.08) versus 0.02 (0.02–0.03) | p<0.00001 |
| One or more mean lobes versus single lobe | 0.09 (0.07–0.11) versus 0.03 (0.01–0.05) | p<0.0001 |
| Reduced mean DLCO | Coefficient −0.0025 (−0.0047– −0.0003) | p=0.028, R2=0.17 |
| GA versus sedation | 0.06 (0.05–0.07) versus 0.05 (0.04–0.06) | p=0.05 |
| Increasing mean age | Coefficient 0.0015 (0.0003–0.0027) | p=0.013, R2=0.0 |
| Higher mean FVC | Coefficient 0.0036 (0.0012–0.006) | p=0.0031, R2=0.0 |
| No lobes targeted versus lower lobes targeted | 0.05 (0.04–0.06) versus 0.04 (0.03–0.06) | p=0.39 |
| Mean number of biopsies | Coefficient 0.0008 (−0.011–0.013) | p=0.89, R2=0.0 |
| No imaging guidance versus fluoroscopy (sole imaging guidance) | 0.08 (0.04–0.11) versus 0.07 (0.06–0.08) | p=0.89 |
#: Reported for meta-regression analyses. DLCO: diffusing capacity for carbon monoxide; FVC: forced vital capacity; GA: general anaesthesia.
Subgroup analysis and meta-regression of moderate-to-severe bleeding was associated with a 2.4 mm cryoprobe size (12% versus 5%, p=0.001) and bleeding score used (13% versus 7%, p=0.04) (see table 4 and figures S17–S18). Severe bleeding was rare (see figure S19); no deaths occurred due to severe bleeding.
TABLE 4.
Impact of procedural and sampling variables on moderate-to-severe bleeding
| Variable | Moderate-to-severe bleeding (95% CI) | p-value, R2 analogue# |
|---|---|---|
| 2.4 mm versus 1.9 mm size of cryoprobe | 0.12 (0.08–0.16) versus 0.05 (0.03–0.07) | p=0.001 |
| Use of BTS bleeding score versus non-BTS bleeding score | 0.07 (0.02–0.13) versus 0.13 (0.11–0.15) | p=0.04 |
| Mean age | Coefficient 0.0034 (−0.0006–0.0073) | p=0.097, R2=0.0 |
| GA versus sedation | 0.11 (0.08–0.13) versus 0.15 (0.1–0.2) | p=0.13 |
| Lower lobes targeted versus no lobes targeted | 0.15 (0.1–0.2) versus 0.12 (0.1–0.14) | p=0.23 |
| Mean DLCO | Coefficient −0.0038 (−0.01–0.0028) | p=0.26, R2=0.08 |
| Fluoroscopy (sole imaging guidance) versus no imaging guidance | 0.11 (0.09–0.13) versus 0.15 (0.08–0.22) | p=0.26 |
| Single lobe versus one or more lobe sampling | 0.16 (0.01–0.3) versus 0.08 (0.06–0.1) | p=0.29 |
| Mean number of biopsies | Coefficient 0.015 (−0.015–0.044) | p=0.33, R2=0.0 |
| Prophylactic EBB versus no EBB | 0.14 (0.11–0.18) versus 0.13 (0.08–0.18) | p=0.65 |
| Mean FVC | Coefficient −0.0001 (−0.0065–0.0062) | p=0.97, R2=0.0 |
#: Reported for meta-regression analyses. BTS: British Thoracic Society; DLCO: diffusing capacity for carbon monoxide; EBB: endobronchial blocker; FVC: forced vital capacity; GA: general anaesthesia.
Sensitivity analysis was performed by removing each study individually from the forest plots for the three primary outcomes. It did not significantly impact the overall effect sizes, confidence intervals or I2 statistic.
Discussion
This is the largest meta-analysis of cryobiopsy in people with unclassifiable or low diagnostic confidence ILD, with overall estimates of diagnostic yield, pneumothorax and moderate-to-severe bleeding being consistent with previously published estimates, thus demonstrating cryobiopsy safety and efficacy [6, 11]. Critically, this study is the first to explore procedural and sampling variables contributing to heterogeneity in diagnostic and safety outcomes. The identification of modifiable procedural characteristics affecting diagnostic performance and complications, including general anaesthesia, discussion at an ILD multidisciplinary meeting, FVC, DLCO, classification by bleeding score and post-procedure imaging, are novel findings. The impact of multiple lobe sampling and cryoprobe size have not previously been analysed at the level of meta-analysis. These factors must be considered both in routine clinical practice and in research prior to performing cryobiopsy in people with ILD. Importantly, many of our findings regarding modifiable procedural characteristics were described as key points by the expert working group and the discussion of all candidates for cryobiopsy at an ILD multidisciplinary meeting prior to the procedure is in accordance with international guideline recommendations [3, 5, 16]. Therefore, our findings provide evidence to support these recommendations, including cryobiopsy performance via endotracheal tube or rigid bronchoscopy, and validate previously unclear procedural aspects, including sampling from multiple lobes.
Just as crucially, our findings demonstrate that some of the heterogeneity of cryobiopsy outcomes is due to variable reporting, including differing use of definitions of diagnostic yield, bleeding severity and mode of detection of pneumothorax. Notably, many of the included studies were published or performed prior to the publication of the Nashville bleeding scale [70], which was developed with specific reference to cryobiopsy. The Nashville and BTS scores have notable differences in the classification of bleeding, with the use of cold saline or the use of an endobronchial balloon both corresponding to “moderate” bleeding in BTS but categorised as grade 2 or 3 bleeding, respectively, in the Nashville classification [70]. The lack of equivalence may lead to under or over-reporting of true bleeding rates in the meta-analysis. Equally, routine chest radiography is associated with increased rates of pneumothorax, presumably to due greater detection of asymptomatic, clinically insignificant pneumothorax. Reporting of intercostal catheter insertion rates may be a more important measure than the presence of incidental pneumothorax.
Larger cryoprobe sizes affected all major outcomes, with higher diagnostic yield and increased complication rates of pneumothorax and bleeding identified. Improvement in yield may relate to larger biopsy specimens reported with larger probes [48]. The balance of risk to benefit when optimising yield relative to traumatic complications through probe selection may vary with the clinical status of patients and their physiological reserve to tolerate bleeding or pneumothorax. General anaesthesia was associated with higher diagnostic yield but higher pneumothorax risk. Lentz et al. [71] hypothesised that general anaesthesia increases pneumothorax due to positive pressure ventilation. The mechanism by which general anaesthesia improves diagnostic yield relative to sedation is unknown but may relate to greater control of cough and patient movement.
Lung function parameters were the key patient characteristic to impact outcomes, suggesting that cryobiopsy performed earlier when lung function is higher may confer benefits for safety and diagnostic performance compared to a procedure delayed until lung function declines with ILD progression. Higher mean DLCO and FVC were associated with higher diagnostic yield and a lower mean DLCO was associated with increased pneumothorax risk. The association between reduced total lung capacity, DLCO, FVC and increased pneumothorax has been previously observed [64, 72]. Dense fibrosis, often associated with advanced ILD, is less favourable for histological assessment than milder fibrosis [51] and may contribute to the mechanism by which reduced lung function is associated with lower yield and pneumothorax. While undertaking an ILD multidisciplinary meeting after lung biopsy is known to increase diagnostic confidence [2], the positive impact on diagnostic yield of a meeting prior to cryobiopsy is novel. ILD discussion may promote selection of appropriate patients and exclusion of inappropriate candidates. It may also promote consensus discussion by relevant experts regarding the ideal target zone for cryobiopsy, balancing issues such as proximity to pleura with the need to target radiological abnormalities [50, 73].
Due to incomplete reporting, participants could not be conclusively divided into single versus multiple lobe sampling groups, rather, single lobe studies which allowed single or multiple lobe sampling dependent on clinical judgement. This likely underestimated the impact of multiple lobe sampling, although a trend to increased diagnosed yield was still observed in the “one or more lobes” group, as well as the novel finding of increased pneumothorax. Multiple studies have reported an increased diagnostic yield by sampling at least two separate pulmonary segments (especially in fibrotic disease) [57, 72] or through multiple lobe sampling [33, 55]. The mean number of biopsies did not influence diagnostic yield, contrasting with previous studies. An increase in yield has been reported between one and two but not two and three biopsies [30, 57, 72]. As the mean number of biopsies per study exceeded three, this may have led to the lack of observed effect. Increased pneumothorax with higher mean number of biopsies has been described but was not replicated in our systematic review [31, 72].
Other factors previously reported as altering cryobiopsy outcomes could not be evaluated due to insufficient data, including proceduralist learning curve and patient periprocedural risk. Routine use of endobronchial balloon blockers is recommended [16] and beneficial for bleeding management [54, 72, 74], but did not impact moderate-to-severe bleeding in this systematic review. It is noted that severe bleeding remained low.
A limitation of this meta-analysis, in keeping with previous assessments of the cryobiopsy literature, is the reliance on studies of low quality and containing high levels of bias. Data were largely nonrandomised and nonconsecutive, with inconsistent definitions and classifications were employed between studies in relation to the key outcomes of diagnostic yield and bleeding severity.
In conclusion, this meta-analysis informs how clinicians and institutions can adopt procedural and sampling decisions to optimise diagnostic performance and minimise complications for cryobiopsy in people with unclassifiable ILD or with low diagnostic confidence. Patient selection at an earlier stage of disease severity in relation to lung function is favourable and the procedure should be discussed at an ILD multidisciplinary meeting prior to cryobiopsy. Selection of a 2.4 mm cryoprobe size and the decision for multiple lobe sampling will augment the diagnostic yield, but also increases the complication risk for patients. Delivery of general anaesthesia and routine screening for pneumothorax post-procedure should be encouraged at an institutional level. Use of a validated bleeding score (such as the BTS scale) supports accurate assessment of bleeding. The identification of these factors will inform future cryobiopsy clinical practice guidelines and highlights key variables that must be reported in future cryobiopsy research.
Points for clinical practice
Evidence before this study
TBLC is recommended as an alternative to SLB in the evaluation of unclassifiable ILD or ILD diagnosed with low confidence. Previous studies identified heterogeneous outcomes in relation to diagnostic yield and complication rate using low quality evidence, though factors contributing to this remain largely unknown.
Added value of this study
This systematic review has identified several variables that contribute to heterogeneity of outcomes following TBLC in ILD, with multiple modifiable procedural factors noted to impact both diagnostic yield and complication rates. Diagnostic yield is influenced by cryoprobe size, mode of anaesthesia, respiratory function parameters and use of an ILD multidisciplinary meeting assessment prior to TBLC. Pneumothorax rates are increased with a 2.4 mm cryoprobe, routine post-procedure imaging, multiple lobe sampling, reduced mean DLCO and general anaesthesia. Moderate-to-severe bleeding in TBLC increased with a 2.4 mm cryoprobe and bleeding score selection.
Implications of all the available evidence
TBLC procedural factors can be modified to maximise diagnostic yield and safety. These factors should be considered for individualised clinical decision-making and guideline development. Guidelines for optimal TBLC practice should be updated to reflect these findings in relation to patient pre-procedure assessment, anaesthetic modality and sampling techniques. More consistent description of cryobiopsy techniques is required in future studies to more accurately characterise patient outcomes.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures S1-S19 ERR-0035-2024.SUPPLEMENT (10.8MB, pdf)
Supplementary tables S1 and S2 ERR-0035-2024.SUPPLEMENT2 (192.1KB, pdf)
Supplementary tables S3-S5 (Quality assessments) ERR-0035-2024.SUPPLEMENT3 (211.5KB, pdf)
Supplementary table S6 (Search strategy) ERR-0035-2024.SUPPLEMENT4 (103.7KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: J.A. Lachowicz reports grants from University of Melbourne, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca. N.E. Smallwood reports grants from NHMRC, MRFF, Cancer Council Australia, Fisher & Paykel Healthcare (FPH), Windermere Foundation, Lung Foundation Australia and Lord Mayor's Foundation Melbourne, consultancy fees from The Limbic and Orchard Consulting, payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, FPH and Health Ed, support for attending meetings from Chiesi and Boehringer Ingelheim, leadership roles with the Thoracic Society of Australia and New Zealand (Board Director and past state president), Victorian Doctors’ Program (Board Director), European Respiratory Society (Co-chair guidelines committee), and receipt of equipment, materials, drugs, medical writing, gifts or other services from FPH. J.D. Prasad, P. Patel and C. Voutier have nothing to disclose. Y.H. Khor reports grants from NHMRC, MRFF, Air Liquide Healthcare, Austin Medical Research Foundation, Lung Foundation Australia/Thoracic Society of Australia and New Zealand and RACP, and leadership roles with the Thoracic Society of Australia and New Zealand (Board director, Special Interest Group Convenor), American Thoracic Society (Clinical Problems Assembly Program Committee; guideline methodologist) and European Respiratory Society (Associate editor for European Respiratory Journal). D.P. Steinfort reports grants from the Australian National Health and Medical Research Council (Leadership Investigator Grant (#2008317)).
References
- 1.Cottin V, Hirani NA, Hotchkin DL, et al. . Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27: 180076. doi: 10.1183/16000617.0076-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad JD, Mahar A, Bleasel J, et al. . The interstitial lung disease multidisciplinary meeting: a position statement from the Thoracic Society of Australia and New Zealand and the Lung Foundation Australia. Respirology 2017; 22: 1459–1472. doi: 10.1111/resp.13163 [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Remy-Jardin M, Richeldi L, et al. . Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korevaar DA, Colella S, Fally M, et al. . European Respiratory Society guidelines on transbronchial lung cryobiopsy in the diagnosis of interstitial lung diseases. Eur Respir J 2022; 60: 2200425. doi: 10.1183/13993003.00425-2022 [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Remy-Jardin M, Ryerson CJ, et al. . Diagnosis of hypersensitivity pneumonitis in adults. An Official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e36–e69. doi: 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues I, Estevao Gomes R, Coutinho LM, et al. . Diagnostic yield and safety of transbronchial lung cryobiopsy and surgical lung biopsy in interstitial lung diseases: a systematic review and meta-analysis. Eur Respir Rev 2022; 31: 210280. doi: 10.1183/16000617.0280-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravaglia C, Bonifazi M, Wells AU, et al. . Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration 2016; 91: 215–227. doi: 10.1159/000444089 [DOI] [PubMed] [Google Scholar]
- 8.Han Q, Luo Q, Xie JX, et al. . Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015; 149: 1394–1401. doi: 10.1016/j.jtcvs.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson JP, Fogarty AW, McKeever TM, et al. . In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016; 193: 1161–1167. doi: 10.1164/rccm.201508-1632OC [DOI] [PubMed] [Google Scholar]
- 10.Hariri LP, Roden AC, Chung JH, et al. . The role of surgical lung biopsy in the diagnosis of fibrotic interstitial lung disease: perspective from the pulmonary fibrosis foundation. Ann Am Thorac Soc 2021; 18: 1601–1609. doi: 10.1513/AnnalsATS.202009-1179FR [DOI] [PubMed] [Google Scholar]
- 11.Kheir F, Uribe Becerra JP, Bissell B, et al. . Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc 2022; 19: 1193–1202. doi: 10.1513/AnnalsATS.202102-198OC [DOI] [PubMed] [Google Scholar]
- 12.Troy LK, Grainge C, Corte TJ, et al. . Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020; 8: 171–181. doi: 10.1016/S2213-2600(19)30342-X [DOI] [PubMed] [Google Scholar]
- 13.Wahidi MM, Argento AC, Mahmood K, et al. . Comparison of forceps, cryoprobe, and thoracoscopic lung biopsy for the diagnosis of interstitial lung disease – the CHILL study. Respiration 2022; 101: 394–400. doi: 10.1159/000519674 [DOI] [PubMed] [Google Scholar]
- 14.Romagnoli M, Colby TV, Berthet JP, et al. . Poor concordance between sequential transbronchial lung cryobiopsy and surgical lung biopsy in the diagnosis of diffuse interstitial lung diseases. Am J Respir Crit Care Med 2019; 199: 1249–1256. doi: 10.1164/rccm.201810-1947OC [DOI] [PubMed] [Google Scholar]
- 15.Fortin M, Liberman M, Delage A, et al. . Transbronchial lung cryobiopsy and surgical lung biopsy: a prospective multi-centre agreement study (CAN-ICE). Am J Respir Crit Care Med 2023; 207: 1612–1619. doi: 10.1164/rccm.202209-1812OC [DOI] [PubMed] [Google Scholar]
- 16.Hetzel J, Maldonado F, Ravaglia C, et al. . Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration 2018; 95: 188–200. doi: 10.1159/000484055 [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savovic J, Page MJ, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 18.Whiting PF, Rutjes AW, Westwood ME, et al. . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O'Connell D, et al. . The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Date last accessed: 1 October 2023. Date last updated: 3 May 2021. www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 20.Du Rand IA, Blaikley J, Booton R, et al. . British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013; 68: Suppl. 1, i1–i44. doi: 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 21.Pajares V, Puzo C, Castillo D, et al. . Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology 2014; 19: 900–906. doi: 10.1111/resp.12322 [DOI] [PubMed] [Google Scholar]
- 22.Shafiek H, Elbialy S, El Achy SN, et al. . Transbronchial cryobiopsy validity in diagnosing diffuse parenchymal lung diseases in Egyptian population. J Multidiscip Healthc 2019; 12: 719–726. doi: 10.2147/JMDH.S208824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babiak A, Hetzel J, Krishna G, et al. . Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009; 78: 203–208. doi: 10.1159/000203987 [DOI] [PubMed] [Google Scholar]
- 24.Cirak AK, Katgi N, Erer OF, et al. . Diagnostic approach in parenchymal lung diseases: transbronchial lung biopsy or cryobiopsy? Turk J Med Sci 2020; 50: 1535–1539. doi: 10.3906/sag-1910-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koslow M, Edell ES, Midthun DE, et al. . Bronchoscopic cryobiopsy and forceps biopsy for the diagnostic evaluation of diffuse parenchymal lung disease in clinical practice. Mayo Clin Proc Innov Qual Outcomes 2020; 4: 565–574. doi: 10.1016/j.mayocpiqo.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pajares V, Nunez-Delgado M, Bonet G, et al. . Transbronchial biopsy results according to diffuse interstitial lung disease classification. Cryobiopsy versus forceps: MULTICRIO study. PLoS One 2020; 15: e0239114. doi: 10.1371/journal.pone.0239114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aburto M, Perez-Izquierdo J, Agirre U, et al. . Complications and hospital admission in the following 90 days after lung cryobiopsy performed in interstitial lung disease. Respir Med 2020; 165: 105934. doi: 10.1016/j.rmed.2020.105934 [DOI] [PubMed] [Google Scholar]
- 28.Almeida LM, Lima B, Mota PC, et al. . Learning curve for transbronchial lung cryobiopsy in diffuse lung disease. Rev Port Pneumol 2018; 24: 23–31. doi: 10.1016/j.rppnen.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 29.Barata M, Caetano Mota P, Melo N, et al. . Transbronchial lung cryobiopsy in smoking-related interstitial lung diseases. Sarcoidosis Vasc Diffuse Lung Dis 2020; 37: e2020013. doi: 10.36141/svdld.v37i4.9934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cascante JA, Cebollero P, Herrero S, et al. . Transbronchial cryobiopsy in interstitial lung disease: are we on the right path? J Bronchology Interv Pulmonol 2016; 23: 204–209. doi: 10.1097/LBR.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 31.Hagmeyer L, Theegarten D, Wohlschlager J, et al. . Transbronchial cryobiopsy in fibrosing interstitial lung disease: modifications of the procedure lead to risk reduction. Thorax 2019; 74: 711–714. doi: 10.1136/thoraxjnl-2018-212095 [DOI] [PubMed] [Google Scholar]
- 32.Jacob M, Bastos HN, Mota PC, et al. . Diagnostic yield and safety of transbronchial cryobiopsy in sarcoidosis. ERJ Open Res 2019; 5: 00203-2019. doi: 10.1183/23120541.00203-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Mahony AM, Burke L, Cavazza A, et al. . Transbronchial lung cryobiopsy (TBLC) in the diagnosis of interstitial lung disease: experience of first 100 cases performed under conscious sedation with flexible bronchoscope. Ir J Med Sci 2021; 190: 1509–1517. doi: 10.1007/s11845-020-02453-7 [DOI] [PubMed] [Google Scholar]
- 34.Abdelghani R, Thakore S, Kaphle U, et al. . Radial endobronchial ultrasound-guided transbronchial cryobiopsy. J Bronchology Interv Pulmonol 2019; 26: 245–249. doi: 10.1097/LBR.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 35.Berim IG, Saeed AI, Awab A, et al. . Radial probe ultrasound-guided cryobiopsy. J Bronchology Interv Pulmonol 2017; 24: 170–173. doi: 10.1097/LBR.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Li J, Luo F, et al. . The application of transbronchial cryobiopsy in interstitial lung disease: a prospective, multicenter, real-world study. Ann Transl Med 2021; 9: 1645. doi: 10.21037/atm-21-3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho R, Zamora F, Gibson H, et al. . Transbronchial lung cryobiopsy in the diagnosis of interstitial lung disease: a retrospective single-center experience. J Bronchology Interv Pulmonol 2019; 26: 15–21. doi: 10.1097/LBR.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 38.Gnass M, Filarecka A, Bartczak A, et al. . Transbronchial lung cryobiopsy guided by radial mini-probe endobronchial ultrasound in interstitial lung diseases – a multicenter prospective study. Adv Respir Med 2020; 88: 123–128. doi: 10.5603/ARM.2020.0086 [DOI] [PubMed] [Google Scholar]
- 39.Goel MK, Kumar A, Maitra G, et al. . Safety and diagnostic yield of transbronchial lung cryobiopsy by flexible bronchoscopy using laryngeal mask airway in diffuse and localized peripheral lung diseases: a single-center retrospective analysis of 326 cases. Lung India 2021; 38: 109–116. doi: 10.4103/lungindia.lungindia_220_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inomata M, Kuse N, Awano N, et al. . Prospective multicentre study on the safety and utility of transbronchial lung cryobiopsy with endobronchial balloon. ERJ Open Res 2020; 6: 00008-2020. doi: 10.1183/23120541.00008-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kheir F, Alkhatib A, Berry GJ, et al. . Using bronchoscopic lung cryobiopsy and a genomic classifier in the multidisciplinary diagnosis of diffuse interstitial lung diseases. Chest 2020; 158: 2015–2025. doi: 10.1016/j.chest.2020.05.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuse N, Inomata M, Awano N, et al. . Management and utility of transbronchial lung cryobiopsy in Japan. Respir Investig 2019; 57: 245–251. doi: 10.1016/j.resinv.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 43.Li X, Pan J, Ma Y, et al. . Diagnosis of diffuse parenchymal lung diseases using transbronchial cryobiopsy guided by endobronchial ultrasound compared to clinicoradiological diagnosis. Quant Imaging Med Surg 2022; 12: 1139–1148. doi: 10.21037/qims-21-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Xu J, Liu C, et al. . The significance of multidisciplinary classifications based on transbronchial pathology in possible idiopathic interstitial pneumonias. Medicine 2020; 99: e20930. doi: 10.1097/MD.0000000000020930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castellani C, Castellani H, Benn BS. Transbronchial lung cryobiopsy is safe and effective for diagnosing acutely ill hospitalized patients with new diffuse parenchymal lung disease. Lung 2022; 200: 153–159. doi: 10.1007/s00408-022-00513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinfort DP, D'Agostino RD, Vrjlic I, et al. . CT-fluoroscopic guidance for performance of targeted transbronchial cryobiopsy: a preliminary report. Respiration 2018; 96: 472–479. doi: 10.1159/000490547 [DOI] [PubMed] [Google Scholar]
- 47.Zhao JG, Zhou GW, Zhao L, et al. . Safety and accuracy of transbronchial lung cryobiopsy in diagnosing desquamative interstitial pneumonia. Clin Respir J 2022; 16: 309–316. doi: 10.1111/crj.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou G, Ren Y, Li J, et al. . The effect of 1.9-mm versus 2.4-mm probes in transbronchial cryobiopsies for interstitial lung diseases: a prospective analysis. Ann Transl Med 2021; 9: 20. doi: 10.21037/atm-20-4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Li H, An Y, et al. . Combination of the Archimedes Navigation System and cryobiopsy in diagnosis of diffuse lung disease. J Int Med Res 2021; 49: 3000605211016665. doi: 10.1177/03000605211016665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronborg-White S, Sritharan SS, Madsen LB, et al. . Integration of cryobiopsies for interstitial lung disease diagnosis is a valid and safe diagnostic strategy-experiences based on 250 biopsy procedures. J Thorac Dis 2021; 13: 1455–1465. doi: 10.21037/jtd-20-2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijmans L, Bonta PI, Rocha-Pinto R, et al. . Confocal laser endomicroscopy as a guidance tool for transbronchial lung cryobiopsies in interstitial lung disorder. Respiration 2019; 97: 259–263. doi: 10.1159/000493271 [DOI] [PubMed] [Google Scholar]
- 52.Bango-Alvarez A, Ariza-Prota M, Torres-Rivas H, et al. . Transbronchial cryobiopsy in interstitial lung disease: experience in 106 cases – how to do it. ERJ Open Res 2017; 3: 00148-2016. doi: 10.1183/23120541.00148-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casoni GL, Tomassetti S, Cavazza A, et al. . Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One 2014; 9: e86716. doi: 10.1371/journal.pone.0086716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhooria S, Mehta RM, Srinivasan A, et al. . The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J 2018; 12: 1711–1720. doi: 10.1111/crj.12734 [DOI] [PubMed] [Google Scholar]
- 55.Marcoa R, Linhas R, Apolinario D, et al. . Diagnostic yield of transbronchial lung cryobiopsy in interstitial lung diseases. Rev Port Pneumol 2017; 23: 296–298. [DOI] [PubMed] [Google Scholar]
- 56.Samitas K, Kolilekas L, Vamvakaris I, et al. . Introducing transbronchial cryobiopsies in diagnosing diffuse parenchymal lung diseases in Greece: implementing training into clinical practice. PLoS One 2019; 14: e0217554. doi: 10.1371/journal.pone.0217554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turan D, Ugur Chousein EG, Koc AS, et al. . Transbronchial cryobiopsy for diagnosing parenchymal lung diseases: real-life experience from a tertiary referral center. Sarcoidosis Vasc Diffuse Lung Dis 2021; 38: e2021004. doi: 10.36141/svdld.v38i1.11029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davidsen JR, Skov IR, Louw IG, et al. . Implementation of transbronchial lung cryobiopsy in a tertiary referral center for interstitial lung diseases: a cohort study on diagnostic yield, complications, and learning curves. BMC Pulm Med 2021; 21: 67. doi: 10.1186/s12890-021-01438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han Q, Chen X, Xu X, et al. . The application of transbronchial lung cryobiopsy and uniportal and tubeless video-assisted thoracic surgery in the multidisciplinary diagnosis of interstitial lung disease–a real-world prospective study. Front Mol Biosci 2021; 8: 681669. doi: 10.3389/fmolb.2021.681669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riviere F, Cazes A, Bylicki O, et al. . Single-centre experience of transbronchial cryobiopsy as a first choice method for the diagnosis of interstitial lung disease. Indian J Thorac Cardiovasc Surg 2022; 38: 142–148. doi: 10.1007/s12055-021-01299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ussavarungsi K, Kern RM, Roden AC, et al. . Transbronchial cryobiopsy in diffuse parenchymal lung disease: retrospective analysis of 74 cases. Chest 2017; 151: 400–408. doi: 10.1016/j.chest.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 62.Shkeiri R, Schneer S, Avarmovich A, et al. . Transbronchial cryobiopsy in diffuse parenchymal lung diseases in a community medical center. Isr Med Assoc J 2020; 22: 781–783. [PubMed] [Google Scholar]
- 63.Cooley J, Balestra R, Aragaki-Nakahodo AA, et al. . Safety of performing transbronchial lung cryobiopsy on hospitalized patients with interstitial lung disease. Respir Med 2018; 140: 71–76. doi: 10.1016/j.rmed.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 64.Hackner K, Stadler A, Schragel F, et al. . Transbronchial lung cryobiopsy: prospective safety evaluation and 90-day mortality after a standardized examination protocol. Ther Adv Respir Dis 2022; 16: 17534666221077562. doi: 10.1177/17534666221077562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hostettler KE, Tamm M, Bubendorf L, et al. . Integration of transbronchial cryobiopsy into multidisciplinary board decision: a single center analysis of one hundred consecutive patients with interstitial lung disease. Respir Res 2021; 22: 228. doi: 10.1186/s12931-021-01821-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kropski JA, Pritchett JM, Mason WR, et al. . Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One 2013; 8: e78674. doi: 10.1371/journal.pone.0078674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bondue B, Schlossmacher P, Allou N, et al. . Trans-bronchial lung cryobiopsy in patients at high-risk of complications. BMC Pulm Med 2021; 21: 135. doi: 10.1186/s12890-021-01503-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernandez-Gonzalez F, Lucena CM, Ramirez J, et al. . Cryobiopsy in the diagnosis of diffuse interstitial lung disease: yield and cost-effectiveness analysis. Arch Bronconeumol 2015; 51: 261–267. doi: 10.1016/j.arbres.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 69.Matta A, Gupta E, Swank Z, et al. . The use of transbronchial cryobiopsy for diffuse parenchymal lung disease in critically ill patients with acute hypoxemic respiratory failure–a case series. Clin Respir J 2021; 15: 788–793. doi: 10.1111/crj.13362 [DOI] [PubMed] [Google Scholar]
- 70.Folch EE, Mahajan AK, Oberg CL, et al. . Standardized definitions of bleeding after transbronchial lung biopsy: a Delphi consensus statement from the Nashville Working Group. Chest 2020; 158: 393–400. doi: 10.1016/j.chest.2020.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lentz RJ, Taylor TM, Kropski JA, et al. . Utility of flexible bronchoscopic cryobiopsy for diagnosis of diffuse parenchymal lung diseases. J Bronchology Interv Pulmonol 2018; 25: 88–96. doi: 10.1097/LBR.0000000000000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravaglia C, Wells AU, Tomassetti S, et al. . Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med 2019; 19: 16. doi: 10.1186/s12890-019-0780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kronborg-White S, Bendstrup E, Gori L, et al. . A pilot study on the use of the super dimension navigation system for optimal cryobiopsy location in interstitial lung disease diagnostics. Pulmonology 2021; 12: 119–123. doi: 10.1016/j.pulmoe.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 74.Deasy KF, Walsh LJ, Kennedy MP, et al. . Endobronchial balloon blockers: a retrospective analysis of their implementation for use in transbronchial cryobiopsy under conscious sedation. Lung 2021; 199: 187–193. doi: 10.1007/s00408-021-00424-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures S1-S19 ERR-0035-2024.SUPPLEMENT (10.8MB, pdf)
Supplementary tables S1 and S2 ERR-0035-2024.SUPPLEMENT2 (192.1KB, pdf)
Supplementary tables S3-S5 (Quality assessments) ERR-0035-2024.SUPPLEMENT3 (211.5KB, pdf)
Supplementary table S6 (Search strategy) ERR-0035-2024.SUPPLEMENT4 (103.7KB, pdf)