Abstract
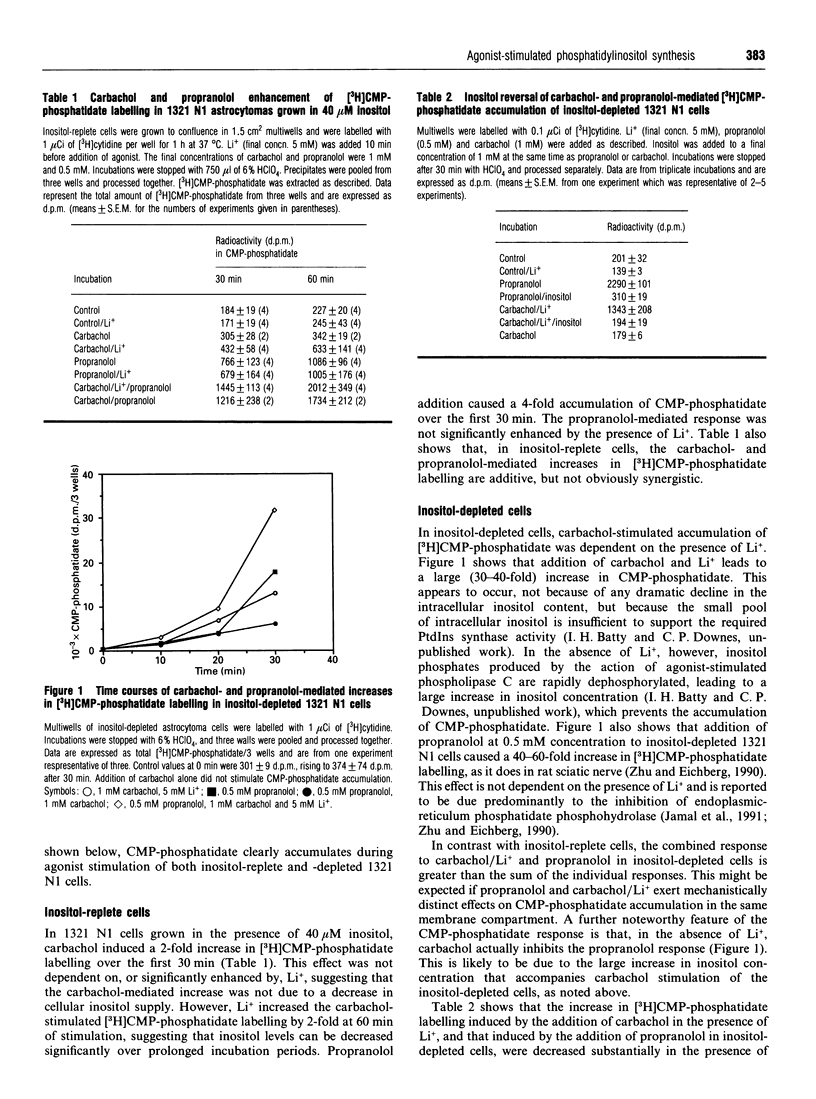
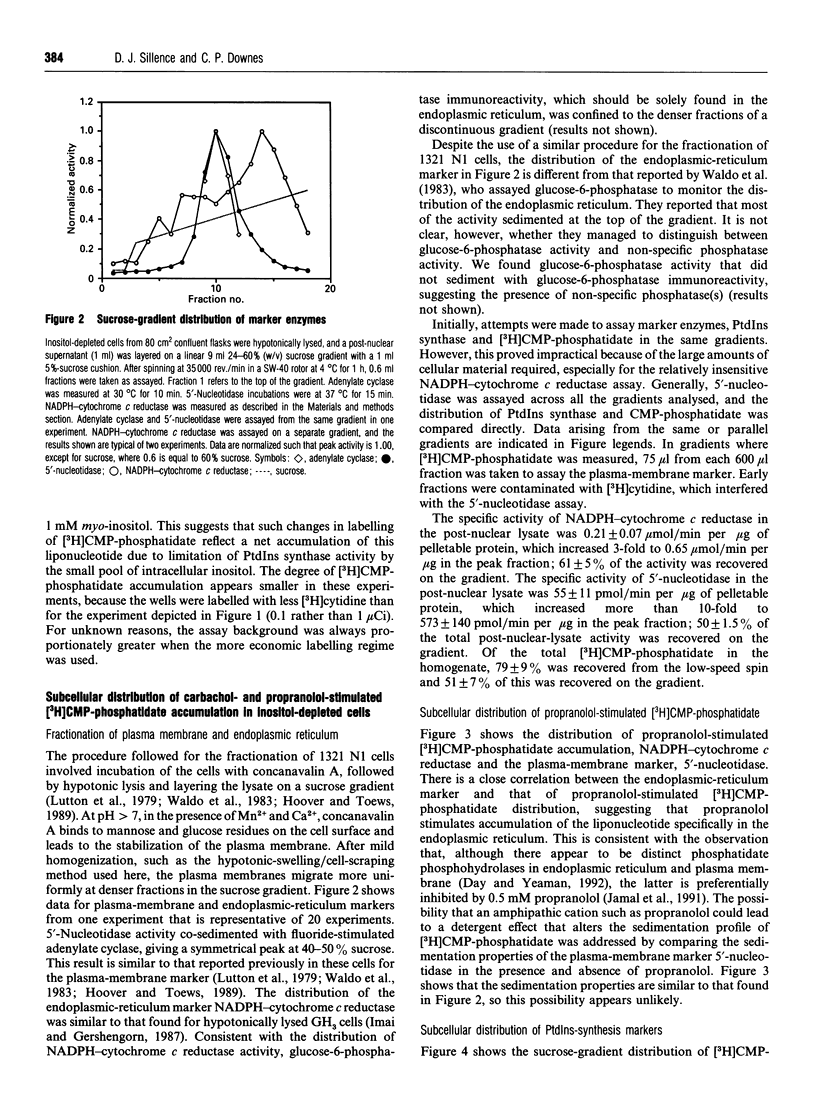
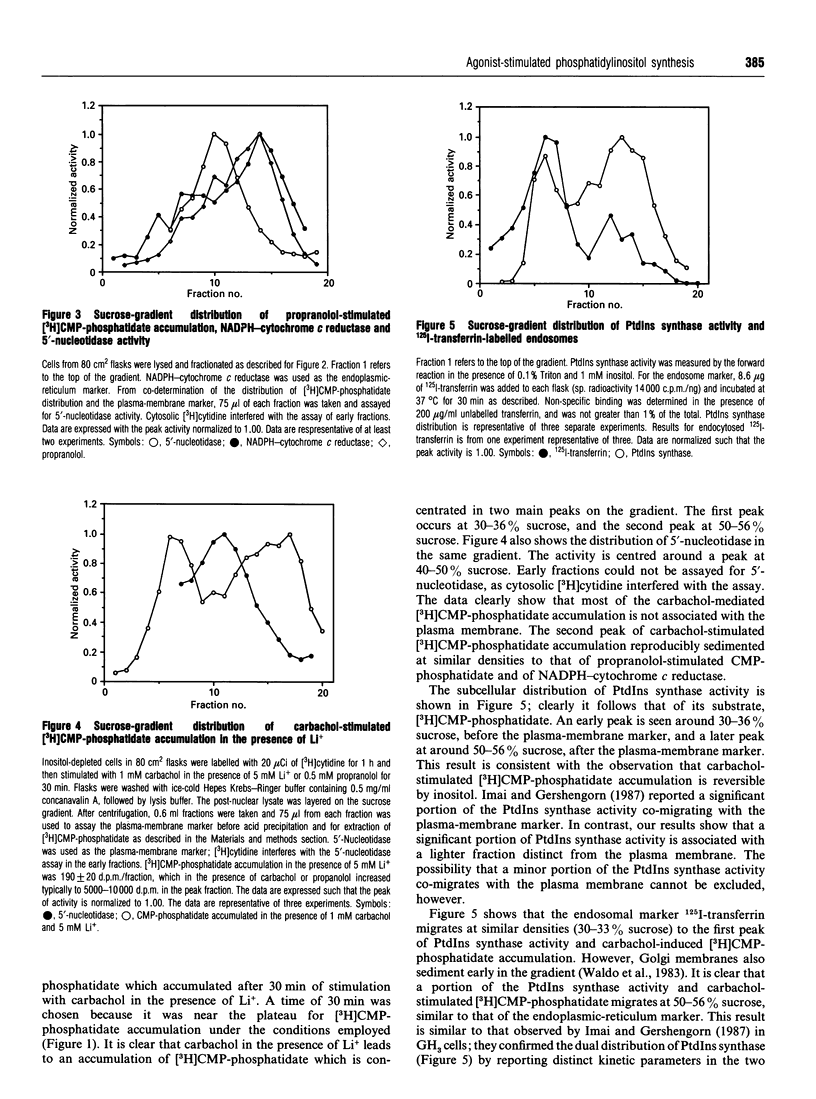
In an inositol-depleted 1321 N1 astrocytoma cell line, propranolol at 0.5 mM concentration and carbachol in the presence of Li+ induce a large increase (30-60-fold) in the amount of CMP-phosphatidate, the lipid substrate of PtdIns synthase. The actions of both agents on CMP-phosphatidate accumulation were reversed by co-incubation with 1 mM inositol. In cells grown in the presence of 40 microM inositol the propranolol- and carbachol-mediated CMP-phosphatidate accumulation was much smaller (2-4-fold). Propranolol- and carbachol-mediated increases in CMP-phosphatidate accumulation were at least additive in both inositol-replete and -depleted cells. The subcellular distribution of accumulated CMP-phosphatidate was investigated by sucrose-density-gradient centrifugation of a lysate of inositol-depleted cells. There were two coincident peaks of carbachol-stimulated [3H]CMP-phosphatidate and PtdIns synthase activity, respectively. The first peak of accumulated [3H]CMP-phosphatidate and PtdIns synthase activity is characteristic of a 'light vesicle' fraction, since it sediments at sucrose densities similar to that of endocytosed 125I-transferrin. The later peak, containing both carbachol-stimulated [3H]CMP-phosphatidate and PtdIns synthase activity, has a distribution in the gradient that is similar to NADPH-cytochrome c reductase activity, an endoplasmic-reticulum marker. By contrast, propranolol-stimulated [3H]CMP-phosphatidate accumulates in membranes which sediment as a single peak corresponding to the endoplasmic-reticulum marker. These observations suggest that agonist-stimulated PtdIns synthesis occurs in the endoplasmic reticulum and in at least one additional membrane compartment which is insensitive to propranolol, an inhibitor of endoplasmic-reticulum phosphatidate phosphohydrolase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augert G., Blackmore P. F., Exton J. H. Changes in the concentration and fatty acid composition of phosphoinositides induced by hormones in hepatocytes. J Biol Chem. 1989 Feb 15;264(5):2574–2580. [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Carter H. A., Patterson M. K., Jr Catalytic formation of epsilon-(gamma-glutamyl)lysine in guinea pig liver transglutaminase. Biochem Biophys Res Commun. 1977 Sep 9;78(1):1–7. doi: 10.1016/0006-291x(77)91213-x. [DOI] [PubMed] [Google Scholar]
- Campbell C. R., Fishman J. B., Fine R. E. Coated vesicles contain a phosphatidylinositol kinase. J Biol Chem. 1985 Sep 15;260(20):10948–10951. [PubMed] [Google Scholar]
- Cubitt A. B., Geras-Raaka E., Gershengorn M. C. Thyrotropin-releasing hormone receptor occupancy determines the fraction of the responsive pool of inositol lipids hydrolysed in rat pituitary tumour cells. Biochem J. 1990 Oct 15;271(2):331–336. doi: 10.1042/bj2710331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. P., Yeaman S. J. Physical evidence for the presence of two forms of phosphatidate phosphohydrolase in rat liver. Biochim Biophys Acta. 1992 Jul 9;1127(1):87–94. doi: 10.1016/0005-2760(92)90205-a. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Stone M. A. Lithium-induced reduction in intracellular inositol supply in cholinergically stimulated parotid gland. Biochem J. 1986 Feb 15;234(1):199–204. doi: 10.1042/bj2340199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Raeburn C. A. The interaction of lithium with thyrotropin-releasing hormone-stimulated lipid metabolism in GH3 pituitary tumour cells. Enhancement of stimulated 1,2-diacylglycerol formation. Biochem J. 1984 Nov 15;224(1):129–136. doi: 10.1042/bj2240129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., Gates J., Hauser G. The mechanism of modification by propranolol of the metabolism of phosphatidyl-CMP (CDP-diacylglycerol) and other lipids in the rat pineal gland. Biochim Biophys Acta. 1979 Apr 27;573(1):90–106. doi: 10.1016/0005-2760(79)90176-0. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P. Potentiation by lithium of CMP-phosphatidate formation in carbachol-stimulated rat cerebral-cortical slices and its reversal by myo-inositol. Biochem J. 1989 Mar 1;258(2):621–624. doi: 10.1042/bj2580621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Harden T. K., Petch L. A., Traynelis S. F., Waldo G. L. Agonist-induced alteration in the membrane form of muscarinic cholinergic receptors. J Biol Chem. 1985 Oct 25;260(24):13060–13066. [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Metabolism and role of phosphatidylinositol in acetylcholine-stimulated membrane function. Adv Exp Med Biol. 1977;83:429–446. doi: 10.1007/978-1-4684-3276-3_40. [DOI] [PubMed] [Google Scholar]
- Hoover R. K., Toews M. L. Evidence for an agonist-induced, ATP-dependent change in muscarinic receptors of intact 1321N1 cells. J Pharmacol Exp Ther. 1989 Oct;251(1):63–70. [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Independent phosphatidylinositol synthesis in pituitary plasma membrane and endoplasmic reticulum. Nature. 1987 Feb 19;325(6106):726–728. doi: 10.1038/325726a0. [DOI] [PubMed] [Google Scholar]
- Jamal Z., Martin A., Gomez-Muñoz A., Brindley D. N. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J Biol Chem. 1991 Feb 15;266(5):2988–2996. [PubMed] [Google Scholar]
- Jamil H., Yao Z. M., Vance D. E. Feedback regulation of CTP:phosphocholine cytidylyltransferase translocation between cytosol and endoplasmic reticulum by phosphatidylcholine. J Biol Chem. 1990 Mar 15;265(8):4332–4339. [PubMed] [Google Scholar]
- Kinney A. J., Carman G. M. Enzymes of phosphoinositide synthesis in secretory vesicles destined for the plasma membrane in Saccharomyces cerevisiae. J Bacteriol. 1990 Jul;172(7):4115–4117. doi: 10.1128/jb.172.7.4115-4117.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Michell R. H., Hems D. A. Phosphatidylinositol metabolism in rat hepatocytes stimulated by vasopressin. Biochem J. 1981 Jan 15;194(1):155–165. doi: 10.1042/bj1940155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R. N., Hemer M. R. Physiologic 1,2-diacylglycerol levels induce protein kinase C-independent translocation of a regulatory enzyme. J Biol Chem. 1990 Jul 5;265(19):10900–10904. [PubMed] [Google Scholar]
- Koréh K., Monaco M. E. The relationship of hormone-sensitive and hormone-insensitive phosphatidylinositol to phosphatidylinositol 4,5-bisphosphate in the WRK-1 cell. J Biol Chem. 1986 Jan 5;261(1):88–91. [PubMed] [Google Scholar]
- Lutton J. K., Frederich R. C., Jr, Perkins J. P. Isolation of adenylate cyclase-enriched membranes from mammalian cells using concanavalin A. J Biol Chem. 1979 Nov 25;254(22):11181–11184. [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Richardson C. N., Glomset J. A. Regulation of diacylglycerol kinase reaction in Swiss 3T3 cells. Increased phosphorylation of endogenous diacylglycerol and decreased phosphorylation of didecanoylglycerol in response to platelet-derived growth factor. J Biol Chem. 1988 Jan 25;263(3):1575–1583. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Meeker R. B., Harden T. K. Muscarinic cholinergic receptor-mediated activation of phosphodiesterase. Mol Pharmacol. 1982 Sep;22(2):310–319. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Maccallum S. H., Hunt P. A. Inositol lipids: receptor-stimulated hydrolysis and cellular lipid pools. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):239–246. doi: 10.1098/rstb.1988.0074. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Adelson J. R. Evidence for coupling of resynthesis to hydrolysis in the phosphoinositide cycle. Biochem J. 1991 Oct 15;279(Pt 2):337–341. doi: 10.1042/bj2790337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco M. E. Inositol metabolism in WRK-1 cells. Relationship of hormone-sensitive to -insensitive pools of phosphoinositides. J Biol Chem. 1987 Sep 25;262(27):13001–13006. [PubMed] [Google Scholar]
- Monaco M. E. The phosphatidylinositol cycle in WRK-1 cells. Evidence for a separate, hormone-sensitive phosphatidylinositol pool. J Biol Chem. 1982 Mar 10;257(5):2137–2139. [PubMed] [Google Scholar]
- Morand J. N., Kent C. A one-step technique for the subcellular fractionation of total cell homogenates. Anal Biochem. 1986 Nov 15;159(1):157–162. doi: 10.1016/0003-2697(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Cook H. W., Byers D. M., Spence M. W., Palmer F. B. Phosphoinositide metabolism in cultured glioma and neuroblastoma cells: subcellular distribution of enzymes indicate incomplete turnover at the plasma membrane. Biochim Biophys Acta. 1990 Mar;1022(3):339–347. doi: 10.1016/0005-2736(90)90283-t. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J. Phosphorylation, transbilayer movement, and facilitated intracellular transport of diacylglycerol are involved in the uptake of a fluorescent analog of phosphatidic acid by cultured fibroblasts. J Biol Chem. 1985 Feb 10;260(3):1909–1916. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Imai A., Gershengorn M. C. Phosphatidylinositol depletion in GH3 rat pituitary cells inhibits sustained responses to thyrotropin-releasing hormone. Reversal with myo-inositol. Mol Endocrinol. 1987 Nov;1(11):802–807. doi: 10.1210/mend-1-11-802. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. J., Downes C. P. Lithium treatment of affective disorders: effects of lithium on the inositol phospholipid and cyclic AMP signalling pathways. Biochim Biophys Acta. 1992 Jan 16;1138(1):46–52. doi: 10.1016/0925-4439(92)90150-l. [DOI] [PubMed] [Google Scholar]
- Sleight R. G., Abanto M. N. Differences in intracellular transport of a fluorescent phosphatidylcholine analog in established cell lines. J Cell Sci. 1989 Jun;93(Pt 2):363–374. doi: 10.1242/jcs.93.2.363. [DOI] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Downes C. P. Metabolic and structural evidence for the existence of a third species of polyphosphoinositide in cells: D-phosphatidyl-myo-inositol 3-phosphate. Biochem J. 1989 Apr 1;259(1):267–276. doi: 10.1042/bj2590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercé F., Record M., Tronchère H., Ribbes G., Chap H. Reversible translocation of cytidylyltransferase between cytosol and endoplasmic reticulum occurs within minutes in whole cells. Biochem J. 1992 Mar 1;282(Pt 2):333–338. doi: 10.1042/bj2820333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. K., Fisher S. K. Preferential coupling of cell surface muscarinic receptors to phosphoinositide hydrolysis in human neuroblastoma cells. J Biol Chem. 1991 Mar 15;266(8):5004–5010. [PubMed] [Google Scholar]
- Tijburg L. B., Houweling M., Geelen M. J., Van Golde L. M. Inhibition of phosphatidylethanolamine synthesis by glucagon in isolated rat hepatocytes. Biochem J. 1989 Feb 1;257(3):645–650. doi: 10.1042/bj2570645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Paridon P. A., Gadella T. W., Jr, Somerharju P. J., Wirtz K. W. On the relationship between the dual specificity of the bovine brain phosphatidylinositol transfer protein and membrane phosphatidylinositol levels. Biochim Biophys Acta. 1987 Sep 18;903(1):68–77. doi: 10.1016/0005-2736(87)90156-8. [DOI] [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]
- Zhu X., Eichberg J. A myo-inositol pool utilized for phosphatidylinositol synthesis is depleted in sciatic nerve from rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9818–9822. doi: 10.1073/pnas.87.24.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]


