Abstract
Background
Whooping cough is a highly contagious respiratory disease. Infants are at highest risk of severe disease and death. Erythromycin for 14 days is currently recommended for treatment and contact prophylaxis but its benefit is uncertain.
Objectives
To assess the risks and benefits of antibiotic treatment of and contact prophylaxis against whooping cough in children and adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL Issue 4, 2010), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, the Database of Abstracts of Reviews of Effects (DARE Issue 4, 2010), MEDLINE (1966 to January Week 1, 2011) and EMBASE (1974 to 18 January 2011).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of antibiotics for treatment of and contact prophylaxis against whooping cough in children and adults.
Data collection and analysis
Three to four review authors independently extracted data and assessed the quality of each trial.
Main results
Thirteen trials with 2197 participants met the inclusion criteria: 11 trials investigated treatment regimens; two investigated prophylaxis regimens. The quality of the trials was variable. For eradicating Bordetella pertussis (B. pertussis) from the nasopharynx, short‐term antibiotics (azithromycin for three to five days, or clarithromycin or erythromycin for seven days) were as effective as long‐term (erythromycin for 10 to 14 days) (risk ratio (RR) 1.01; 95% confidence interval (CI) 0.98 to 1.04), but had fewer side effects (RR 0.66; 95% CI 0.52 to 0.83). Trimethoprim/sulphamethoxazole for seven days was also effective. Nor were there differences in clinical outcomes or microbiological relapse between short and long‐term antibiotics. For preventing infection by treating contacts older than six months of age, antibiotics did not significantly improve clinical symptoms, nor the number of cases developing culture‐positive B. pertussis. Side effects were reported with antibiotics and they varied from one antibiotic to another.
Authors' conclusions
Although antibiotics were effective in eliminating B. pertussis, they did not alter the subsequent clinical course of the illness. There is insufficient evidence to determine the benefits of prophylactic treatment of pertussis contacts.
Keywords: Humans; Infant; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Azithromycin; Azithromycin/therapeutic use; Bordetella pertussis; Clarithromycin; Clarithromycin/therapeutic use; Contact Tracing; Erythromycin; Erythromycin/therapeutic use; Erythromycin Estolate; Erythromycin Estolate/therapeutic use; Erythromycin Ethylsuccinate; Erythromycin Ethylsuccinate/therapeutic use; Randomized Controlled Trials as Topic; Trimethoprim, Sulfamethoxazole Drug Combination; Trimethoprim, Sulfamethoxazole Drug Combination/therapeutic use; Whooping Cough; Whooping Cough/drug therapy; Whooping Cough/prevention & control; Whooping Cough/transmission
Plain language summary
Antibiotics for whooping cough (pertussis)
Whooping cough is a highly contagious disease caused by pertussis bacteria and may lead to death, particularly in infants less than 12 months of age. Although it can be prevented by routine vaccination, it still affects many people. Thirteen trials involving 2197 participants were included in this review. We found that several antibiotic treatments were equally effective in eliminating the bacteria infecting patients, but they did not alter the clinical outcome. There was insufficient evidence to decide whether there is benefit for treating healthy contacts. Side effects were reported with antibiotics and they varied from one antibiotic to another. The result of the review should be interpreted with caution since this review is based on a limited number of trials and some of these trials involved small numbers of participants.
Background
Description of the condition
Whooping cough is an acute respiratory tract infection, first described in the 1500s and endemic in Europe by the 1600s. Bordetella pertussis (B. pertussis) is the sole cause of epidemic whooping cough and the usual cause of sporadic pertussis. Bordetella parapertussis (B. parapertussis) accounts for 5% of isolates of Bordetella species in the United States and characteristically causes a less protracted illness (Heininger 1994; Long 1997).
Whooping cough epidemics in the pre‐vaccine era (that is, before the mid‐1940s) occurred at two to five‐year intervals and these cycles have continued in the vaccine era. Although immunisation has controlled the disease, it has not reduced the transmission of the organism in the population (Cherry 1984). B. pertussis can be prevented by vaccination and since the introduction of routine childhood immunisation whooping cough morbidity and mortality have declined markedly (Cherry 1984). However, despite widespread vaccination the disease has not been eradicated and an increased incidence rate has been reported in the last two decades (Isacson 1993). There are 20 to 40 million cases of whooping cough annually worldwide (WHO 1999). Ninety percent of cases occur in low‐income countries and result in an estimated 200,000 to 300,000 fatalities annually (WHO 1999).
Although adults and older children usually have mild or moderate symptoms, infants younger than six months of age, who are not old enough to have received three doses of diphtheria‐tetanus‐pertussis (DTP) vaccine, and incompletely vaccinated preschool children are at high risk of severe disease and complications including death (CDC 1995; Cherry 1988).
Whooping cough is highly contagious. Between 70% and 100% of susceptible household members and between 50% and 80% of susceptible school contacts become infected following exposure to an acute case (Atkinson 1996). Data from the Centers for Disease Control and Prevention (CDC) for the years 1997 to 2000 showed that among 29,048 persons with whooping cough, 8390 (29%) were aged less than one year; 3359 (12%) were aged one to four years; 2835 (10%) were aged five to nine years; 8529 (29%) were aged 10 to 19 years; and 5935 (20%) were aged over 20 years old. The average annual incidence rates were highest among infants aged less than one year (55.5 cases per 100,000 population). They were lower in children aged one to four years (5.5 cases/100,000), children aged five to nine years (3.6 cases/100,000), individuals aged 10 to 19 years (5.5 cases/100,000), and individuals aged over 20 years old (0.8 cases/100,000) (CDC 2002). The incubation period is thought to be seven to 10 days (range four to 21 days) and, rarely, may be as long as 42 days (Heininger 1998).
Since 1976, reported cases of pertussis in the United States have increased, with a substantial rise among persons aged 10 to 19 years old (CDC 2005). Polymerase chain reaction (PCR)‐confirmed cases make up a substantial proportion of the total number of reported cases in this age group. Exactly how the increase in reported pertussis cases in adolescents reflects a true change in the burden of disease remains unclear (CDC 2005).
Whooping cough is characterised by spasms of severe coughing (paroxysms). The paroxysms are continuous without inspiration until the end and are often followed by the characteristic inspiratory whoop or post‐tussive vomiting or both. The illness onset is insidious, with symptoms similar to those of a minor upper respiratory infection (that is, a catarrhal period). During the first one to two weeks of the illness, coryza (a head cold) with an intermittent non‐productive cough is common. This phase is followed by episodes of paroxysmal coughing which frequently last for several weeks (that is, paroxysmal phase). The disease peaks in severity after one or more weeks of paroxysmal coughing and begins to taper off with an extensive convalescent period of two to six weeks; convalescence may last up to three months in some cases.
Description of the intervention
Whooping cough may cause severe illness in young infants and result in complications such as apnoea, cyanosis, feeding difficulties, pneumonia and encephalopathy. Infants and other patients with severe whooping cough may require hospitalisation for supportive care; for very severe cases, intensive care facilities may be required. Corticosteroids and albuterol (a B2‐adrenergic stimulant) may be effective in reducing paroxysms of coughing but further evaluation is required before their use can be recommended (Bettiol 2010; Broomhall 1984).
Clinical studies have used erythromycin estolate, erythromycin ethylsuccinate or erythromycin stearate for treatment in patients with whooping cough or for prophylaxis. The studies using erythromycin estolate 40 to 50 mg/kg/day in divided doses have reported elimination of B. pertussis from the nasopharynx within seven days and no clinical relapses (Bass 1969; Islur 1975). In contrast, studies with erythromycin ethylsuccinate 50 to 55 mg/kg/day (Halsey 1980) or erythromycin stearate 40 to 50 mg/kg/day (Henry 1981) have reported delay or failure of bacterial eradication, or apparent failure of prophylaxis, in 10% to 30% of cases. This has been explained in part by a higher serum and tissue concentration of the drug achieved following administration of the estolate preparation compared with other esters (Bass 1985).
How the intervention might work
The CDC recommends erythromycin for treatment of whooping cough and contact prophylaxis (CDC 2000). The recommended dose of erythromycin for use in treatment of whooping cough in children is 40 to 50 mg/kg per day (maximum 2 g/day) and in adults 1 to 2 g/day orally in four divided doses for 14 days. Some experts recommend the use of erythromycin estolate because it achieves higher serum levels compared to erythromycin ethylsuccinate or stearate when equal doses are given (CDC 2000; Ginsburg 1986). The antimicrobial agents and dosages used for chemoprophylaxis of contacts are the same as those recommended for treatment of clinical cases (CDC 2000).
The gastrointestinal side effects of erythromycin limit its usefulness in some patients. The erythromycin estolate preparation Ilosone is no longer available in Australia (Thomas 2002) and possibly in other parts of the world, due to discontinuation of manufacture of the drug.
The newly developed macrolides clarithromycin and azithromycin may be superior to erythromycin because of improved absorption, a longer half‐life, good in vitro activity against B. pertussis and a better side effect profile (Aoyama 1996; Lebel 2001). Roxithromycin has not been well studied in the treatment of whooping cough. Based on a few studies, trimethoprim‐sulphamethoxazole (TMP‐SMZ) also appears to be effective in eradicating B. pertussis and it is currently recommended as an alternative antibiotic treatment for patients who cannot tolerate erythromycin (CDC 1991).
Why it is important to do this review
The recommended therapy for treatment of and prophylaxis against whooping cough infection is inconvenient and prolonged and it is likely that compliance is often poor (CDI 1997). The optimal duration of treatment is uncertain (Halperin 1997; Hoppe 1988). There is also some controversy as to whether prophylaxis of contacts is effective and, therefore, worthwhile (De Serres 1995). To date, there has not been a systematic review of the literature regarding the antibiotic treatment of and contact prophylaxis against whooping cough.
Objectives
To study the risks and benefits of antibiotic treatment of, and contact prophylaxis against, whooping cough.
Treatment
Do antibiotics achieve microbiological eradication of B. pertussis?
Do antibiotics improve the clinical illness of whooping cough?
The appropriate dose and duration of therapy.
The side effects profile of antibiotics used to treat whooping cough.
Contact prophylaxis
Do antibiotics achieve microbiological eradication of B. pertussis?
Do antibiotics prevent the clinical illness of whooping cough?
The appropriate dose and duration of therapy.
The side effects profile of antibiotics used for prophylaxis of whooping cough.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs comparing two or more antibiotics or antibiotics versus placebo or no treatment for the treatment or prophylaxis (prevention) of whooping cough. Quasi‐RCTs are those studies which are intended to be randomised by using methods of allocation such as alternation, date of birth or case record number (Higgins 2011).
Types of participants
Patients: children and adults with whooping cough, diagnosed clinically or by laboratory means (therapeutic regimen).
Contacts: children and adults who had contact with individual(s) with proven whooping cough but have not developed clinical whooping cough (prophylactic regimen).
Types of interventions
This review will address the following comparisons in both treatment and prophylaxis groups:
antibiotic versus placebo or no intervention;
one type of antibiotic versus another type of antibiotic; and
one antibiotic regimen (dose or duration or both) versus another regimen of the same antibiotic.
Types of outcome measures
Primary outcomes
Mortality from any cause.
Clinical assessment of whooping cough: assessment of severity including a decrease in frequency of paroxysmal coughing, frequency of whoop, severity of the cough, mean duration of symptoms and development of complications, for example otitis media and respiratory complications.
Complete remission (clinical cure).
Number of contacts that develop clinical whooping cough (in prophylactic studies).
Laboratory outcome measures, for example microbiological eradication and microbiological relapse of B. pertussis organisms.
Secondary outcomes
Antibiotic side effects/adverse events.
Patient compliance and tolerance to antibiotics.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews of Effects (DARE) 2010, Issue 4, part of The Cochrane Library,www.thecochranelibrary.com (accessed 18 January 2011), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (March 2007 to January Week 1, 2011) and EMBASE (March 2007 to 18 January 2011). Details of the previous search are in Appendix 1.
We searched MEDLINE and CENTRAL using the following terms. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE; sensitivity maximising version (2008 revision); Ovid format (Lefebvre 2009). We modified the search EMBASE (Appendix 2).
MEDLINE (OVID)
1 exp Whooping Cough/ 2 whoop$.mp. 3 exp Bordetella pertussis/ 4 pertus$.mp. 5 or/1‐4 6 exp Anti‐Bacterial Agents/ 7 antibiotic$.mp. 8 antimicrob$.mp. 9 or/6‐8 10 5 and 9
Searching other resources
We scanned reference lists of medical journal articles and reviews relevant to the use of antibiotics in pertussis. We also searched conference abstracts and reference lists of articles. We approached study investigators and pharmaceutical companies for additional information (published or unpublished studies). There were no language restrictions.
Data collection and analysis
The inclusion of studies in this systematic review was influenced mainly by the method of allocation concealment. Empirical research has shown that lack of adequate allocation concealment is associated with bias (Chalmers 1983; Schulz 1995). Indeed, concealment has been found to be more important in preventing bias than other components of allocation such as the generation of the allocation sequence (for example, computer, random number table or alternation). Thus studies can be judged on the method of allocation concealment (Higgins 2011).
Selection of studies
Three review authors (SA, RK, JM) independently screened the titles and abstracts from the literature searches. The full paper was obtained for further screening if it was felt that the trial could possibly meet the inclusion criteria.
Data extraction and management
Four review authors (SA, RK, JM, NC) independently assessed study eligibility using the above criteria. Disagreements among review authors were resolved by discussion and consensus.
Assessment of risk of bias in included studies
Four review authors (SA, RK, JM, NC) independently assessed study eligibility using the following criteria: participant randomisation, allocation concealment, blinding of participants, investigators and outcome assessors, intention‐to‐treat (ITT) analysis and completeness of follow up. We assessed criteria separately and did not combine them to give a quality score. The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) highlights that involvement of at least two review authors to conduct a systematic review has an important effect on reducing the possibility that relevant reports will be discarded and may also limit bias, minimise errors and improve reliability of findings.
Measures of treatment effect
We used Review Manager 5.1 software (RevMan 2011) to analyse data. We performed statistical analysis for dichotomous outcomes and expressed results as a risk ratio (RR) with 95% confidence interval (CI). We used the mean difference (MD) between groups and 95% CI where continuous scales of measurement were used to assess the effect of treatment. We used the standardised mean difference (SMD) and 95% CI to compare different measurement scales.
Unit of analysis issues
Participating individuals in the individually randomised trials were the unit of analysis.
Dealing with missing data
We contacted trial authors of primary studies when necessary, to clarify data and to provide missing information.
Assessment of heterogeneity
When possible we used the Cochrane Chi2 test (Q‐test) to assess heterogeneity. We considered a P value < 0.10 statistically significant.
Assessment of reporting biases
It was not possible to undertake a funnel plot graph for publication bias because of the heterogeneity of the included studies and inability to perform a meta‐analysis.
Data synthesis
We used fixed‐effect models for outcomes without statistically significant heterogeneity and random‐effects models for outcomes with significant heterogeneity (P < 0.10).
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis comparing short‐term (three to seven days) to long‐term (14 days) treatment with antibiotics. Other subgroup analyses to determine potential causes of variability amongst treatment effects were not possible because of the difficulties of obtaining enough detailed data from studies of those various subgroups.
Sensitivity analysis
We performed a sensitivity analysis in which poorer quality studies (unknown or inadequate allocation concealment) were excluded when studies differed considerably in quality. Important sensitivity analyses may include method of randomisation (excluding studies with inadequate allocation concealment), chronology of RCTs (to distinguish between RCTs by their place in time) and size of RCTs (to distinguish between RCTs by the number of participants).
Results
Description of studies
Results of the search
A total of 27 search results were obtained when the search was updated in August 2009 (26 results from MEDLINE, two from CENTRAL and six search results from Embase.com). Seven more search results were obtained when the search was updated again in January 2011. However no new trials were identified.
We identified 13 RCTs for inclusion in this review, published between 1953 and 2004. Eight of the RCTs were found by a MEDLINE search, two studies in EMBASE (Henry 1981; Lebel 2001), one in CENTRAL (Cruickshank 1953), one by screening reference lists (Adcock 1972), and one by searching conference abstracts on a medical web site (http://www.icmask.org) and then contacting the authors (Bace 2002). We identified no unpublished RCTs by contacting drug companies (Table 1).
1. Sources of included studies.
| Trials | MEDLINE | EMBASE | The Cochrane Library | Reference lists | Conference abstracts | Personal contacts | Drugs company |
| Adcock 1972 | + | ||||||
| Bace 2002 | + | + | |||||
| Bass 1969 | + | + | |||||
| Cruickshank 1953 | + | ||||||
| Degn 1981 | + | + | + | ||||
| Grob 1981 | + | + | |||||
| Halperin 1997 | + | + | + | ||||
| Halperin 1999 | + | + | |||||
| Henry 1981 | + | + | |||||
| Hoppe 1992 | + | + | |||||
| Langley 2004 | + | + | |||||
| Lebel 2001 | + | ||||||
| Strangert 1969 | + |
Included studies
There were 11 studies on the treatment of whooping cough that met the inclusion criteria (Adcock 1972; Bace 2002; Bass 1969; Cruickshank 1953; Degn 1981; Halperin 1997; Henry 1981; Hoppe 1992; Langley 2004; Lebel 2001; Strangert 1969). Of these, 10 were RCTs and one was a quasi‐RCT (Strangert 1969).
There were two studies on prophylaxis against whooping cough infection (Grob 1981; Halperin 1999) that met the inclusion criteria. The two studies were conducted in household contacts of children who were culture‐positive for B. pertussis.
Ten studies of treatment of whooping cough compared one antibiotic with another antibiotic (Adcock 1972; Bace 2002; Cruickshank 1953; Degn 1981; Halperin 1997; Henry 1981; Hoppe 1992; Langley 2004; Lebel 2001; Strangert 1969) and one study compared antibiotics versus no treatment (Bass 1969). The two studies of contact prophylaxis were placebo‐controlled (Grob 1981; Halperin 1999).
Immunisation status was reported in five of the studies on the treatment of whooping cough (Bass 1969; Halperin 1997; Hoppe 1992; Langley 2004; Lebel 2001) and in one study of contact prophylaxis (Grob 1981). Bass (Bass 1969) reported that only 9/50 (18%) children studied had received any previous pertussis vaccine injection and only 2/50 (4%) children had previously received three DTP injections; their illness appeared milder than in non‐immunised children. Grob (Grob 1981) found that 60/91 (66%) children were vaccinated, with 32 vaccinated children in the erythromycin group and 28 in the placebo group. Halperin (Halperin 1997) reported that 65/74 (88%) of the seven days of erythromycin estolate group and 88% (83/94) of the 14 days of erythromycin estolate group had received three or more doses of the vaccine. Hoppe (Hoppe 1992) found that pertussis vaccination status was similar in both study groups: 115/190 (60.5%) of patients had not been vaccinated at all ‐ 56/93 (60.2%) in the erythromycin estolate group and 59/97 (60.8%) in the erythromycin ethylsuccinate group. Langley (Langley 2004) reported previous number of pertussis vaccine doses received by children who were assigned to erythromycin or azithromycin (mean 4.4 versus 4.1). Lebel (Lebel 2001) reported that 68/76 (89%) of children had received whooping cough vaccination in the clarithromycin treatment group and 69/77 (90%) in the erythromycin control group.
Outcome measures used to assess efficacy of antibiotic treatment or prophylaxis varied between trials. Most trials considered clinical improvement (for example, decreased frequency of cough, whoop and complete remission) or microbiological eradication or both. Mortality was reported in two trials (Bass 1969; Cruickshank 1953).
Clinical assessment was reported in almost all the included randomised trials but sufficient data for analysis were available for six trials only. However, in these trials (Adcock 1972; Grob 1981; Halperin 1997; Halperin 1999; Hoppe 1992; Lebel 2001) the clinical assessment was reported differently; for example, in some trials complete remission was assessed and in other trials frequency of whoop/paroxysmal cough were assessed. Complications due to whooping cough were reported in two trials (Cruickshank 1953; Lebel 2001). The number of cases that resulted in clinical whooping cough in contacts (attack rate) was evaluated in one study (Halperin 1999).
Ten trials reported microbiological eradication (defined as B. pertussis negative culture at the end of treatment) (Adcock 1972; Bace 2002; Bass 1969; Degn 1981; Halperin 1997; Henry 1981; Hoppe 1992; Langley 2004; Lebel 2001; Strangert 1969).
Microbiological relapse (defined as a positive culture one week post‐completion of therapy after a negative end‐of‐therapy culture) was reported in two trials (Halperin 1997; Langley 2004).
Drug side effects (such as abdominal pain and diarrhoea) were stated in nine trials (Bace 2002; Cruickshank 1953; Halperin 1997; Halperin 1999; Henry 1981; Hoppe 1992; Langley 2004; Lebel 2001; Strangert 1969). Patient compliance (measured as the mean percent of drug taken or in other ways such as measurement of antimicrobial activity in the urine) was reported in five trials (Halperin 1997; Halperin 1999; Hoppe 1992; Langley 2004; Lebel 2001). Analysis of compliance in Halperin 1997 was not possible because data were presented as a mean percent of drugs taken without the standard deviation.
For further details, please see the Characteristics of included studies and Characteristics of excluded studies tables.
Excluded studies
We excluded seven trials from this review. These trials might have provided some useful information if they were included (for example, efficacy of antibiotics used and occurrence of side effects). On the other hand, these studies had many methodological errors including the use of historical control patients, large numbers of dropout participants, poor quality methods or analysis and non‐interpretable results. Inclusion of such studies might lead to several forms of bias (for example, performance bias, attrition bias) and hence misleading conclusions. Management of secondary respiratory infections in patients with whooping cough was not part of the inclusion criteria of this systematic review. Intervention with symptomatic drugs such as steroids, bronchodilators and cough syrups for whooping cough was also not part of the inclusion criteria as another Cochrane Review on symptomatic treatment in whooping cough is has been published (Bettiol 2010).
Risk of bias in included studies
Not only was the number of RCTs small but those included were undertaken over 20 years ago and are of poor methodological quality. All but one were undertaken in high‐income countries. The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
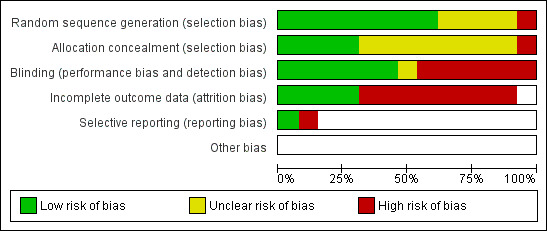
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
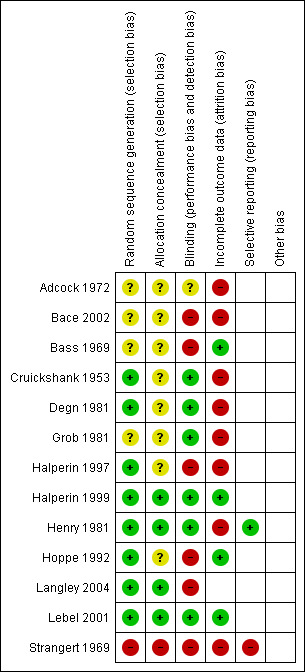
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In only four trials (Halperin 1999; Henry 1981; Langley 2004; Lebel 2001) was the treatment assignment adequately concealed prior to allocation. Allocation concealment was unclear in eight studies (Adcock 1972; Bace 2002; Bass 1969; Cruickshank 1953; Degn 1981; Grob 1981; Halperin 1997; Hoppe 1992) and in one quasi‐RCT the allocation concealment was inadequate (Strangert 1969).
Blinding
There were three double‐blinded trials (Cruickshank 1953; Degn 1981; Halperin 1999), four single‐blinded trials (patients or investigators) (Adcock 1972; Grob 1981; Henry 1981; Lebel 2001), three open (unblinded) trials (Halperin 1997; Hoppe 1992; Langley 2004) and in three trials neither intervention nor outcome assessments were blinded to treatment (Bace 2002; Bass 1969; Strangert 1969). Intention‐to‐treat (ITT) analysis was reported in two trials (Langley 2004; Lebel 2001).
Incomplete outcome data
Follow up was complete for children admitted to hospital. Follow up was incomplete for children who were not admitted to hospital. The duration of follow up varied from one study to another (up to 40 days after discharge from hospital). In four out of 13 trials, patients were considered to have completed their follow up (Bass 1969; Halperin 1999; Hoppe 1992; Lebel 2001).
Selective reporting
Included studies were too heterogeneous with regards to intervention and outcomes to allow pooling of results. In only three trials was meta‐analysis possible, comparing short‐term versus long‐term antibiotic treatment of whooping cough (subgroup analysis).
Other potential sources of bias
The method of randomisation was described in eight of the 13 trials (computer‐generated random lists, random number book or random sequence) (Cruickshank 1953; Degn 1981; Halperin 1997; Halperin 1999; Henry 1981; Hoppe 1992; Langley 2004; Lebel 2001). In four trials the method of randomisation was not stated (Adcock 1972; Bace 2002; Bass 1969; Grob 1981) and in one quasi‐RCT (Strangert 1969) alternation of patients (each second child admitted with whooping cough was treated with ampicillin) was used.
Effects of interventions
Antibiotics for treatment of whooping cough
Mortality
Mortality was reported in two trials (Bass 1969; Cruickshank 1953). Neither trial showed a statistically significant difference in mortality. In the trial by Cruickshank (Cruickshank 1953) one child died in the aureomycin group (1/96) and one died in the chloramphenicol group (1/98). In detail, in the aureomycin group a male aged seven months developed convulsions on day 14 of observation and died the following day; in the chloramphenicol group a female aged one year developed widespread atelectasis of the lungs on day 7 of observation and died on day 11 of observation. Authors of the trial reported that none of the complicated or fatal cases occurred in patients treated within eight days of the onset of symptoms.
In the trial by Bass (Bass 1969) one child died in the ampicillin group (1/10) compared to none in the untreated group (0/10), oxytetracycline (0/10), chloramphenicol (0/10) or erythromycin (0/10) groups. The child who died was two months old and with B. pertussis proven on a nasopharyngeal (NP) specimen. He was on penicillin V for seven days in the catarrhal stage before admission; during admission he was on ampicillin (100 mg/kg/day) for about 20 days. He was also given three doses of whooping cough hyperimmune globulin but remained B. pertussis culture‐positive and died in the paroxysmal stage. No further details were reported regarding his death.
Clinical cure (complete remission)/improvement
Clinical cure/improvement was worded and defined differently between studies, therefore, we analysed results separately for each study and according to the definition used by the trial authors. In the trial by Hoppe (Hoppe 1992) clinical cure (according to parents' judgement after the completion of antimicrobial treatment and as compared with the onset) was 4/97 (4%) in the erythromycin ethylsuccinate (14 days) group and 13/92 (14%) in the erythromycin estolate (14 days) group. The results showed that erythromycin estolate was superior to erythromycin ethylsuccinate (RR 3.43; 95% CI 1.16 to 10.13) (Analysis 1.2.1). Clinical improvement after one week of treatment was not statistically different when tetracycline was compared to trimethoprim/sulphamethoxazole (Adcock 1972). Decreased frequency of cough at 14 days of treatment was reported by Hoppe (Hoppe 1992) and was 72/97 (74%) in the erythromycin ethylsuccinate (14 days) and 72/92 (78%) in the erythromycin estolate (14 days) group with no statistically significant difference between these two esters (i.e. erythromycin ethylsuccinate and erythromycin estolate).
The presence of any signs or symptoms of whooping cough at the completion of treatment was similar whether participants were treated with erythromycin estolate for seven or 14 days (Halperin 1997). Clinical outcomes in the study by Degn (Degn 1981) were not possible to analyse since tables were reported as medians. However, the clinical course of the disease as estimated by the number of bouts of coughing per day was identical in the two groups. The clinical course of illness in the study by Bass (Bass 1969) was presented per individual patients and it was not possible to analyse these data accurately. However, the author reported that there was no significant difference in the subsequent course of illness in those groups receiving antimicrobial therapy when compared with the untreated control group.
Microbiological eradication
Microbiological eradication was reported in 10 trials involving 811 participants and varied from 0% to 100%. Meta‐analysis of microbiological eradication as an outcome in these trials was not possible because of the difference in type of antibiotics used. For this reason, we analysed results separately for each study.
In the study by Bass (Bass 1969) there was microbiological eradication on day seven of treatment in the oxytetracycline (8/10) and erythromycin (9/10) treatment groups over the untreated group (RR 17; 95% CI 1.11 to 259.87 and RR 19; 95% CI 1.25 to 287.92, respectively) (Analysis 1.6.2 and Analysis 1.6.4). Microbiological eradication was not statistically significant in the chloramphenicol treatment group (7/10) compared with the untreated group (0/10); and microbiological eradication was not achieved in the ampicillin treatment group (0/10) compared with the untreated group (0/10). No statistically significant benefit was found with one antibiotic compared with another antibiotic, with regard to microbiological eradication in nine trials (Adcock 1972; Bace 2002; Degn 1981; Halperin 1997; Henry 1981; Hoppe 1992; Langley 2004; Lebel 2001; Strangert 1969).
Microbiological relapse
In a study by Halperin (Halperin 1997) microbiological relapse (defined as a positive culture one week post‐completion of therapy after a negative end‐of‐therapy culture) was reported in 1/72 (1.4%) with erythromycin estolate (seven days) compared to 0/83 (0%) with erythromycin estolate (14 days) (RR 3.45; 95% CI 0.14 to 83.44) (Analysis 1.7.5). In the study by Langley (Langley 2004) no bacterial recurrence was demonstrated in the 51 patients in the azithromycin group or the 53 patients in the erythromycin group with one‐week post‐treatment cultures available.
Complications
Respiratory complications (defined as development of bronchopneumonia, lobar pneumonia or bronchitis complications) of whooping cough were reported in one trial (Cruickshank 1953): 7/96 (7%) in the Aureomycin group compared to 5/98 (5%) in the chloramphenicol group. Otitis media as a complication of whooping cough was reported in the (Lebel 2001) trial with 0/76 (0%) developing otitis media in the clarithromycin (seven days) group and 6/77 (8%) in the erythromycin estolate (14 days) group. There was no significant difference in complications in either trial.
Side effects
Side effects were reported in six trials involving 975 participants. Meta‐analysis of side effects in these trials was not possible because of the difference in the types of antibiotics used. We therefore analysed results separately for each study. Fewer side effects were noted with azithromycin (three days) compared with erythromycin (14 days) (RR 0.38; 95% CI 0.19 to 0.75) (Analysis 1.10.2) in Bace 2002; and with clarithromycin (seven days) compared with erythromycin estolate (14 days) (RR 0.72; 95% CI 0.53 to 0.97) (Analysis 1.10.3) (Lebel 2001). No significant difference in side effects of one antibiotic over another was found in four trials (Cruickshank 1953; Halperin 1997; Hoppe 1992; Strangert 1969). In the study by Langley (Langley 2004) fewer gastrointestinal adverse effects were noted with azithromycin (five days) compared with erythromycin estolate (10 days) (RR 0.46; 95% CI 0.34 to 0.62) (Analysis 1.11.2). In the study by Lebel (Lebel 2001) 24/76 (32%) had gastrointestinal adverse effects with clarithromycin (seven days) and in 34/77 (44%) with erythromycin estolate (14 days) but this was not statistically different. Diarrhoea as a drug‐related side effect was reported by Henry (Henry 1981): in 2/10 (20%) with erythromycin stearate (seven days) compared to 1/12 (10%) with co‐trimoxazole (seven days) but this was not statistically different.
Compliance
The study participant compliance with medication was reported in three trials (Hoppe 1992; Langley 2004; Lebel 2001). In the study by Hoppe (Hoppe 1992) better compliance was achieved in those receiving erythromycin ethylsuccinate compared to those receiving erythromycin estolate (RR 0.80; 95% CI 0.69 to 0.94) (Analysis 1.13.1). Compliance was better in those children who received azithromycin compared to those who received erythromycin estolate (RR 1.63; 95% CI 1.45 to 1.85) (Analysis 1.14.1) (Langley 2004). In the study by Lebel (Lebel 2001) those receiving clarithromycin had better compliance than those receiving erythromycin estolate (MD 9.90; 95% CI 5.34 to 14.46) (Analysis 1.15.1).
Antibiotics for short‐term (three to seven days) versus long‐term (10 to 14 days) treatment of whooping cough (subgroup analysis)
Clinical improvement
In the study by Halperin (Halperin 1997) the presence of any sign or symptom of whooping cough was reported in 73/74 (99%) with erythromycin estolate (seven days) compared to 93/94 (99%) with erythromycin estolate (14 days). There was no difference in clinical improvement with 14‐day treatment duration compared to the seven‐day duration with erythromycin estolate (RR 1.00; 95% CI 0.96 to 1.03) (Analysis 2.1.1).
Microbiological eradication
Four trials involving 358 participants compared the efficacy of antibiotics in the microbiological eradication of B. pertussis (Halperin 1997; Langley 2004; Lebel 2001; Bace 2002). Meta‐analysis showed that there was no significant benefit of long‐term antibiotic treatment (10 to 14 days with erythromycin estolate or unspecified salt of erythromycin) compared to short‐term antibiotic treatment (azithromycin for three to five days, erythromycin estolate for seven days, or clarithromycin for seven days) in microbiological eradication of B. pertussis (RR 1.01; 95% CI 0.98 to 1.04) (Analysis 2.2).
2.2. Analysis.
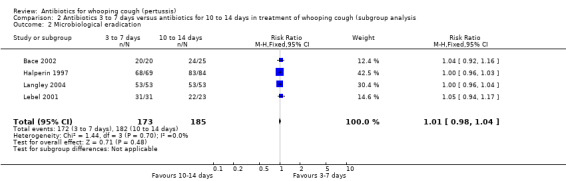
Comparison 2 Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis, Outcome 2 Microbiological eradication.
We performed sensitivity analysis by excluding the Bace 2002 study, which to date has only been published as an abstract. This again showed that there was no significant benefit of long‐term antibiotic treatment over short‐term antibiotic treatment in the microbiological eradication of B. pertussis (RR 1.01; 95% CI 0.98 to 1.04) (Analysis 2.3) (Halperin 1997; Langley 2004; Lebel 2001).
2.3. Analysis.
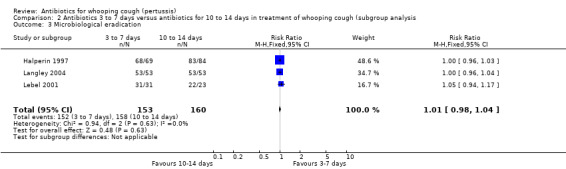
Comparison 2 Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis, Outcome 3 Microbiological eradication.
Microbiological relapse
Microbiological relapse was reported in two trials involving 259 participants (Langley 2004; Halperin 1997). In the study by Langley (Langley 2004) no bacterial relapse was demonstrated in the 51 patients in the azithromycin group or the 53 patients in the erythromycin group with one‐week post‐treatment cultures available. In the study by Halperin (Halperin 1997) microbiological relapse was reported in 1/72 (1.4%) of patients receiving erythromycin estolate for seven days compared to 0/83 (0%) in the group receiving erythromycin estolate for 14 days. However, there was no significant difference in microbiological relapse between seven days treatment with erythromycin estolate and 14 days treatment with the same antibiotic (RR 3.45; 95% CI 0.14 to 83.44) (Analysis 2.4.1).
Side effects
Three trials involving 443 participants reported side effects (Bace 2002; Halperin 1997; Lebel 2001). Meta‐analysis showed that fewer side effects were reported in those receiving short‐term antibiotic treatment compared to those receiving long‐term antibiotic treatment (14 days of erythromycin) (RR 0.66; 95% CI 0.52 to 0.83) (Analysis 2.5). We performed sensitivity analysis excluding the Bace 2002 study. Meta‐analysis again showed significantly fewer side effects reported in those receiving short‐term antibiotic treatment compared to those receiving long‐term antibiotic treatment (RR 0.73; 95% CI 0.57 to 0.93) (Analysis 2.6) (Halperin 1997; Lebel 2001). Other subgroup analyses to determine potential causes of variability amongst treatment effects were not possible because it was not possible to obtain enough detailed data from studies of various patient subgroups.
2.5. Analysis.
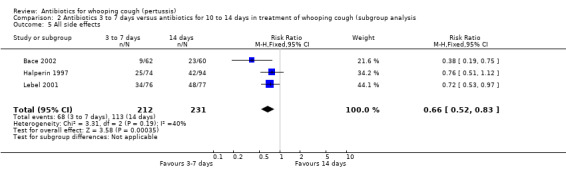
Comparison 2 Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis, Outcome 5 All side effects.
2.6. Analysis.
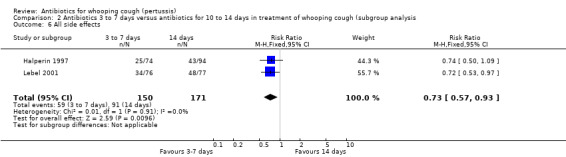
Comparison 2 Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis, Outcome 6 All side effects.
Antibiotics for prophylaxis against whooping cough
Mortality
No mortality was reported in any of the included prophylaxis trials in this review.
Clinical improvement
In the prophylaxis trials, clinical symptoms, frequency of whooping cough and frequency of paroxysmal cough in the household contacts were slightly less in the treatment group compared to placebo (not statistically significant) (Grob 1981; Halperin 1999). Frequency of whooping cough in the vaccinated contacts was reported by Grob (Grob 1981). No vaccinated child had whooping cough: in the erythromycin ethylsuccinate group (0/32) or in the placebo group (0/28) (RR not estimable). The frequency of whooping cough in the unvaccinated contacts in the same trial was 4/20 (20%) in the treatment group and 2/11 (18%) in the placebo group with no benefit of antibiotic over the placebo (RR 1.10; 95% CI CI 0.24 to 5.08) (Analysis 3.7.1).
Number of contacts that became culture‐positive or developed clinical pertussis (attack rate)
In the study by Halperin 1999 the number of cases that became culture positive for B. pertussis after prophylaxis was slightly less in the erythromycin group 3/142 (2.1%) compared to placebo 8/158 (5.1%) group but the difference was not statistically significant (RR 0.42; 95% CI 0.11 to 1.54) (Analysis 3.8.1). Culture positivity or development of two weeks of paroxysmal cough after prophylaxis occurred in 6/124 (4.8%) contacts in the erythromycin estolate group and 8/132 (6.1%) contacts in the placebo group. There was no significant benefit of erythromycin estolate over placebo in contacts (RR 0.80; 95% CI 0.29 to 2.24) (Analysis 3.9.1) (Halperin 1999).
Side effects
Any side effects which were reported by the participants (including nausea, vomiting, diarrhoea, abdominal pain) were more frequently reported by participants in the erythromycin estolate group 49/144 (34%) than in the placebo group 26/166 (16%) (RR 2.17; 95% CI 1.43 to 3.31) (Analysis 3.10.1) (Halperin 1999).
Compliance
Compliance (greater than 90% of doses taken by the participants) was assessed in one study by Halperin (Halperin 1999). It was better in the placebo group 78/144 (54.2%) than in the erythromycin estolate group 108/166 (65.1%) although this difference was borderline for statistical significance (RR 0.83; 95% CI 0.69 to 1.00) (Analysis 3.11.1).
Discussion
Summary of main results
Only 13 studies met our inclusion criteria in our literature search between 1953 and 2011. Eleven of these addressing treatment included 1796 patients (1628 children and 168 adults) or household contacts; and two addressing contact prophylaxis of 401 household contacts with children culture‐positive for B. pertussis.
Heterogeneity of studies
Included studies were too heterogeneous with regard to intervention and outcomes to allow pooling of results. In only three trials was meta‐analysis possible, comparing short‐term versus long‐term antibiotic treatment of whooping cough (subgroup analysis).
The studies varied greatly in timing of B. pertussis cultures for participants (for example, catarrhal stage versus paroxysmal stage), types of antibiotic used, dose regimes and duration of treatment with antibiotics. In general, nasopharyngeal aspirates were taken at the beginning of the study and repeated after the completion of treatment. The end‐of‐treatment cultures varied according to the planned duration of therapy. For example, in the study by Strangert (Strangert 1969) the duration of treatment in both treatment groups was six days while in a study by Lebel (Lebel 2001) the duration of treatment was seven days with clarithromycin compared to 14 days with erythromycin estolate. Nasopharyngeal cultures were taken at seven days (for clarithromycin group) and at 14 days (for the erythromycin group) but not at seven and 14 days for both. Furthermore, cultures taken one or two weeks post‐completion of therapy, that might indicate microbiological relapses, were missing in many studies.
Interestingly, many studies initially enrolled larger number of patients based on the clinical diagnosis of pertussis (for example, Bace 2002 and Hoppe 1992) but subsequently only 30% to 40% were found to be B. pertussis culture‐positive. This can be attributed to many factors:
(i) the organism can usually be recovered during the catarrhal stage but not two or three weeks after the onset of paroxysms (Krugman 1992); (ii) isolation of B. pertussis depends on correct collection of samples, careful transport and efficient processing of the samples obtained for culture; isolation is enhanced if the clinical microbiologist is experienced with the organism (Krugman 1992); (iii) although B. pertussis is the sole cause of epidemic pertussis, other organisms such as B. parapertussis and Bordetella bronchiseptica (B. bronchiseptica) are occasional causes of pertussis (Long 1997).
Clinical diagnosis varied from one study to another. In the study by Adcock (Adcock 1972) the clinical diagnosis of whooping cough was based on presence of typical 'whoop' and relative or absolute lymphocytosis; while in the study by Hoppe (Hoppe 1992) clinical pertussis meant non‐specific cough at a time when pertussis was prevalent in the community, or early paroxysmal stage. Other studies provided no clear definition of clinical pertussis.
Types of antibiotics used in trials for treatment or contact prophylaxis were different. Antibiotic choice varied in every aspect: (i) type of antibiotic used, (ii) dose, (iii) salt preparation and (iv) duration of treatment (that is, from three to 14 days or more). It was difficult to find even two studies that used similar antibiotic regimens. These differences made it difficult to undertake quantitative meta‐analysis for most aspects of the study and recommendations, therefore, were finally made on the basis of individual studies.
It is noticeable that there was only one study included in the treatment regimen that compared antibiotics with no treatment (Bass 1969). Hoppe 1992 reported that in Germany it would be considered unethical by most physicians to withhold appropriate antimicrobial treatment from a child with proven or strongly suspected pertussis. This view might also be applicable in many other parts of the world. However, from a purely scientific perspective the lack of such knowledge makes it hard to know the true effect of antibiotic therapy.
The age of participants enrolled in the studies was not mentioned in most of the included studies, the one exception being the study by Bass (Bass 1969) where findings were stratified according to individual patient age. It is known that almost 90% of the reported deaths caused by whooping cough occur in non‐immunised infants younger than one year of age (Hoppe 2000).
Further difficulties were encountered in the contact prophylaxis studies. The unit of randomisation in the two contact prophylactic studies (Grob 1981; Halperin 1999) was the household rather than individuals. All household members were allocated to the same treatment group (either antibiotic or placebo). Halperin 1999 reported that households were used as the unit of randomisation and in analysis because of concern that the risk of second cases of pertussis within a household were dependent not only on the index case but potentially on the other household contacts. Grob 1981 reported that individual household member randomisation was not achievable and it was simpler to use the household as the unit of randomisation. Household randomisation is not equivalent to individual randomisation. Although it might be easier for the investigator to use the household as a unit for randomisation, such a method of randomisation made it difficult to know the 'real' effect of the antibiotic on individuals because all household members received either erythromycin or placebo. As a result of household randomisation it was not possible to determine the age of participants; assess the effectiveness of antibiotics in different age subgroups; or assess the effectiveness of antibiotics on immunised, partially immunised or non‐immunised children.
The prophylaxis studies were only relevant to children older than six months of age. In the study by Halperin (Halperin 1999) children younger than six months of age were excluded from the study and in the study by Grob (Grob 1981) there were no clear data about the inclusion of younger infants. Children below six months of age (who are incompletely immunised) have a higher rate of whooping cough and are at considerable risk of morbidity and mortality. A low incidence of culture‐positive pertussis was found in the study by Halperin (Halperin 1999) in both erythromycin and placebo groups, perhaps due to the high rate of immunisation and this led to the study being insufficiently powered to detect any significant difference.
Unfortunately, information on immunisation status was deficient in 6/10 (60%) of trials in the treatment of whooping cough and in 1/2 (50%) trials of contact prophylaxis. Immunisation status and type of immunisation (that is, passive or active) of individuals is valuable data because it may influence the apparent efficacy of antibiotics, particularly in contact prophylaxis. The proportion of individuals protected against clinical pertussis by full immunisation with the whole‐cell vaccine is high but not as high as the proportion protected by natural disease (Krugman 1992). Vaccine failure occurs in approximately 10% of individuals with some variation perhaps caused by the intensity of exposure to wild pertussis (Krugman 1992). Comparative efficacy trials of acellular versus whole‐cell vaccine for primary immunisation have been conducted in several countries. Products containing multiple pertussis components were superior to simple vaccines and compared favourably with whole‐cell diphtheria‐tetanus‐pertussis (DTP) vaccines. Reactogenicity of acellular DTP was significantly less (Long 1997).
Immunity to whooping cough has been shown to wane five to 10 years after vaccination with whole‐cell pertussis vaccines. Waning immunity following vaccination with acellular pertussis vaccines may also occur but data are currently limited (CDC 2000). This apparent loss of immunity has become particularly evident in recent years because of an increase in the incidence of reported cases of whooping cough in adolescents and young adults (Krugman 1992).
There was a lack of uniformity in the monitoring of side effects and compliance of patients. The studies varied in types and clear definitions of the side effects, microbiological relapse and recurrence. Compliance was also poorly estimated in most of the studies. It was reported in only three studies and these varied in their measures of compliance. In the study by Hoppe (Hoppe 1992) compliance was measured by the detection of antimicrobial activity in the urine whereas in the studies by Lebel (Lebel 2001) and Halperin (Halperin 1999) compliance was measured by the amount of the drug taken by patients.
A cost‐benefit analysis was not part of this review but it is an important factor for healthcare services when considering choice of treatment. Unfortunately cost information was not provided in any of the included studies. In general, the cost of treatment with antibiotics varies from one country to another and many drugs are no longer patented. Calculation of the exact cost for each drug was not, therefore, undertaken in this review. In addition, modest benefits of antibiotics must be weighed up against the cost and inconvenience of therapy and the risk of side effects.
Methodological quality of included studies
This review identified 13 randomised controlled trials investigating antibiotics for treatment and contact prophylaxis of whooping cough. The methodological quality of these trials was variable. Four randomised controlled trials (RCTs) reported adequate randomisation; in eight RCTs the method of allocation was unclear. Only in one trial (Strangert 1969) was the method of allocation concealment considered inadequate. Blinding was reported in seven of the 12 trials. Most trials compared one antibiotic with another antibiotic. The two prophylactic trials were placebo‐controlled (Grob 1981; Halperin 1999).
Data analysis
We carried out a subgroup analysis comparing short‐term (three to seven days) with long‐term (14 days) treatment with antibiotics. Other subgroup analyses, to determine potential causes of variability amongst treatment effects, were not possible because obtaining enough detailed data from studies of various subgroups was not possible. Important subgroups that should be addressed are adults versus children, early antibiotic treatment (that is, catarrhal stage) versus late antibiotic treatment (that is, paroxysmal or convalescent stage), antibiotics versus placebo and immunised versus non‐immunised children. It is hoped that further data may become available to permit such analyses.
Although we performed sensitivity analysis by excluding one study (Bace 2002), which to date has been published as an abstract only, this did not alter the conclusions significantly. Some of the planned analyses were constrained owing to heterogeneity of the included studies and the inability to perform a quantitative meta‐analysis.
Limitations of the systematic review
All 11 of the included RCTs of treatment involved children only and no trials were found specifically in adults. The two prophylaxis trials, which were done in household contacts, did not report separate data for adults and, therefore, subgroup analysis for adults was not possible. There were some differences between the studies regarding definition of whooping cough, patient diagnosis, inclusion criteria, interventions (that is, various types of antibiotics, doses used, duration) and outcome measures (that is, clinical cure, clinical improvement, microbiological eradication); which did not allow quantitative meta‐analysis for most of the outcome measures.
In addition, there was minimal information on immunisation status of participants, microbiological relapse, definition of clinical cure or improvement and timing of intervention (for example, catarrhal stage, paroxysmal stage). There was a lack of blinding in some studies (for example, Bass 1969, Halperin 1997 and Hoppe 1992).
Recommendation for treatment of and contact prophylaxis against whooping cough
This systematic review of RCTs examining the treatment of whooping cough has found that antibiotic treatment is effective in eliminating B. pertussis from the nasopharynx and thus rendering participants non‐infectious, but does not alter the clinical course of the illness. Prophylaxis with antibiotic was significantly associated with side effects; it did not significantly improve clinical symptoms, prevent the development of culture‐positive B. pertussis, nor paroxysmal cough for more than two weeks, in contacts older than six months of age.
Information from other sources
Special precaution is needed when treating or providing prophylaxis for newborns because infantile hypertrophic pyloric stenosis (IHPS) in neonates has been reported following the use of erythromycin. In one case, pyloric stenosis developed in a breast‐fed infant whose mother took erythromycin (CDC 2000; Honein 1999; Stang 1986).
Although short‐term treatment of azithromycin successfully eradicated B. pertussis from the nasopharynx (Bace 2002), it may affect carriage of Streptococcus pneumoniae (S. pneumoniae) in the nasopharynx. Leach (Leach 1997) reported in a prospective study that community‐based treatment with azithromycin may result in the appearance of azithromycin‐resistant strains of S. pneumoniae in the nasopharynx. No data are available regarding the effect of clarithromycin on S. pneumoniae nasopharyngeal carriage.
The results of this review suggest that tetracycline and chloramphenicol are also effective antibiotics for the clearance of B. pertussis from the nasopharynx but these drugs have a number of serious side effects. The American Academy of Pediatrics (AAP) does not recommend the use of tetracycline in children less than eight years old (Ray 1977), based on the fact that administration of tetracycline during tooth development (last half of pregnancy, infancy and childhood to the age of eight years) may cause permanent discolouration of the teeth (AAP 1975). Tetracycline may also result in photosensitivity and hepatotoxicity, and is contraindicated in pregnancy and breast‐feeding (Smilack 1999). The most serious side effect of chloramphenicol is aplastic anaemia; it increases the relative risk of this disorder 13‐fold (Wallerstein 1969). Grey baby syndrome is another potentially fatal adverse reaction to chloramphenicol, occurring mainly in neonates (Weiss 1960). Dose‐related association between the use of chloramphenicol and the development of acute lymphocytic and non‐lymphocytic leukaemias has also been reported (Shu 1987). With the availability of other effective antibiotics it seems unnecessary to use these antibiotics for treatment of whooping cough.
Roxithromycin is a very popular antibiotic in Australia and is frequently used as an alternative to erythromycin but this macrolide antibiotic has not been studied in whooping cough. In vitro studies show that roxithromycin is generally two‐ to four‐fold less active than erythromycin against B. pertussis organisms (Kucers 1997). However, there are no clinical studies of the efficacy of this antibiotic in the treatment or prophylaxis of whooping cough.
Overall completeness and applicability of evidence
The results should be interpreted with caution because of the heterogeneity between studies.
Quality of the evidence
This review may be subject to bias because the summary results are based on a limited number of trials and some of these trials involved small numbers of patients.
Potential biases in the review process
There is a possibility of publication and selection bias in this systematic review. However, a comprehensive literature search was conducted using a systematic strategy to avoid bias. Attempts to find unpublished trials were carried out by consulting experts in the field, searching abstracts from recent conferences and corresponding with the authors of the included studies.
Agreements and disagreements with other studies or reviews
This is the first systematic review of RCTs for treatment and prophylaxis of whooping cough (pertussis).
Authors' conclusions
Implications for practice.
Antibiotics for treatment of pertussis
The findings of this review suggest that administration of antibiotics for the treatment of whooping cough is effective in eliminating B. pertussis from patients with the disease to render them non‐infectious but does not alter the subsequent clinical course of the illness. The effective regimens include:
three days of azithromycin (10 mg/kg as a single dose);
five days of azithromycin (10 mg/kg on the first day of treatment and 5 mg/kg once daily on the second day to fifth days of treatment);
seven days of clarithromycin (7.5 mg/kg/dose twice daily);
seven to 14 days of erythromycin (40 mg/kg/day in three divided doses);
14 days of erythromycin (60 mg/kg/day in three divided doses);
seven days of oxytetracycline (50 mg/kg/day in four divided doses); or
seven days of chloramphenicol (50 mg/kg/day in four divided doses).
The best regimens for microbiological clearance, with fewer side effects, are:
three days of azithromycin (10 mg/kg as a single dose);
five days of azithromycin (10 mg/kg on the first day of treatment and 5 mg/kg once daily on the second day to fifth days of treatment); or
seven days of clarithromycin (7.5 mg/kg/dose twice daily).
Seven days of trimethoprim/sulphamethoxazole (20 mg trimethoprim with 100 mg sulphamethoxazole per dose, twice daily, for children under six months of age; double this dose for older children) appears to be effective in eradicating B. pertussis from the nasopharynx and may serve as an alternative antibiotic treatment for patients who can not tolerate a macrolide. The use of oxytetracycline or chloramphenicol is not recommended in the treatment of whooping cough because of their potential side effects, especially in children, and because of the availability of other effective and safer antibiotics.
Antibiotics for prophylaxis against whooping cough
There is insufficient evidence to determine the benefit of prophylactic treatment of pertussis contacts. Prophylaxis with antibiotics was significantly associated with side effects and did not significantly improve clinical symptoms, whoop, paroxysmal cough, number of cases who develop culture‐positive B. pertussis or paroxysmal cough for more than two weeks in contacts older than six months of age. Due to the high risk of morbidity and mortality in infants less than six months of age who are incompletely immunised, contact prophylaxis is recommended for families who have an infant less than six months of age. The recommended antibiotics and dosages for contact prophylaxis are the same as those recommended in the treatment of whooping cough.
Implications for research.
General
We would encourage authors of future trials to follow the revised CONSORT guidelines (Consolidated Standards of Reporting Trials), which have been adopted by several leading journals and can be found on the Internet (www.consort‐statement.org). CONSORT comprises a checklist and flow diagram to help improve the quality of reports of RCTs. It offers a standard way for researchers to report trials. The checklist includes descriptions of the randomisation procedure (allocation concealment), the mechanisms of blinding, number of people lost during the follow up, and some details about the analysis made.
Specific
Given the growing importance of pertussis in infants and adolescents, there seems to be an urgent need for larger RCTs for treatment of and prophylaxis against whooping cough. Future trials should incorporate simple and clear indices for clinical outcomes (such as clinical cure, duration of symptoms, severity and improvement), microbiological eradication, microbiological relapse, side effects, compliance and attack rate (in prophylaxis trials). Further therapeutic studies of appropriate size are needed based on age, immunological status, duration of disease and cost/benefit ratios from both patients and contacts. Special emphasis on the effectiveness of antibiotics, compared to placebo, for treatment of or prophylaxis against whooping cough in vaccinated and unvaccinated participants is needed. Short‐duration trials with newer macrolides such as azithromycin, clarithromycin and perhaps roxithromycin are desirable.
Feedback
Feedback, 16 October 2012
Summary
Comment: In the section “Antibiotics for prophylaxis against whooping cough” it states:
"In the study by Halperin 1999 the number of cases that became culture positive for B. pertussis after prophylaxis was slightly less in the erythromycin group 3/142 (2.1%) compared to placebo 8/158 (5.1%) group but the difference was not statistically significant (RR 0.42; 95%CI 0.11 to 1.54) (Analysis 3.8.1). Culture positivity or development of two weeks of paroxysmal cough after prophylaxis occurred in 6/124 (4.8%) contacts in the erythromycin estolate group and 8/132 (6.1%) contacts in the placebo group. There was no significant benefit of erythromycin estolate over placebo in contacts (RR 0.80; 95% CI 0.29 to 2.24) (Analysis 3.9.1) (Halperin 1999)." (Altunaiji et al, 2011, p.11)
The original paper by Halperin et al (http://pediatrics.aappublications.org/content/104/4/e42.abstract ) randomised families by household to receive erythromycin (n=73 households) or placebo (n=79 households). They reported that 4 households in the erythromycin group and 15 households in the placebo group had a member who developed a positive culture for pertussis. They then analysed these results by household, by protocol rather than intent to treat. They reported a significant difference between the groups (p=0.026). (They also reported no differences between groups when analysed by self‐reported symptoms, arguably reflecting the low sensitivity of symptoms for differentiating pertussis from other respiratory infections.)
Calculating the relative risk of a secondary case of pertussis within an erythromycin compared to placebo household by ITT gives: 4/73 (5.5%) divided by 15/79 (18.9%) = RR 0.3 (95%CI 0.1, 0.8); NNT=8. Thus, in contrast to what is cited in the quote above, the primary analysis by Halperin et al does show a statistically significant effect of treatment.
The data cited in the quote above was actually carried out as a post‐hoc analysis (contained in table four of Halperin et al). This analysis only included those individuals who did not report symptoms at the start of treatment. As well as being an unplanned secondary analysis, it does not take into account the clustered nature of the data.
This Cochrane Review has already been used in the formulation of guidelines in the UK (www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1287142671506 ). In this case, the guideline does recommend chemoprophylaxis for a selected population of household contacts.
However I would request that the Review team revisit the original Halperin et al paper, and consider reporting the primary outcome analysis rather than the post‐hoc analysis when the Cochrane Review is next updated. At the present time there is a risk that those using the Review to inform guidelines may take the reported findings at face value, may not take the time to read the original trial report, and may not realise that there is evidence to support antibiotic chemoprophylaxis to prevent secondary cases of whooping cough.
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Chris Littlejohn Specialty Registrar in Public Health Aberdeen UK
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2012 | Feedback has been incorporated | Feedback comment added to the review |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 18 January 2011 | New search has been performed | Searches conducted. No new trials were included or excluded in this update. |
| 3 August 2009 | New search has been performed | Searches conducted. |
| 21 August 2008 | Amended | Converted to new review format. |
| 4 March 2007 | New search has been performed | In this 2007 substantive update: (1) A single new randomised controlled trial (RCT) was included (Langley 2004) for treatment of whooping cough. (2) No new RCT was found for prophylaxis of whooping cough. (3) This Cochrane Review has been considerably revised and updated. |
| 20 February 2004 | New search has been performed | Searches conducted. |
Acknowledgements
Sultan Altunaiji would like to acknowledge the United Arab Emirates (U.A.E.) for sponsoring his postgraduate study (Master of Medicine degree) at Melbourne University and the Royal Children's Hospital.
Appendices
Appendix 1. Previous search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), the Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2007, Issue 1), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1966 to March 2007) and EMBASE (January 1974 to March 2007). (See Appendix 2 for the EMBASE search strategy).
MEDLINE (OVID)
1 exp Whooping Cough/ 2 whoop$.mp. 3 exp Bordetella pertussis/ 4 pertus$.mp. 5 or/1‐4 6 exp Anti‐Bacterial Agents/ 7 antibiotic$.mp. 8 antimicrob$.mp. 9 or/6‐8 10 RANDOMIZED CONTROLLED TRIAL.pt. 11 CONTROLLED CLINICAL TRIAL.pt. 12 RANDOMIZED CONTROLLED TRIALS.sh. 13 RANDOM ALLOCATION.sh. 14 DOUBLE BLIND METHOD.sh. 15 SINGLE‐BLIND METHOD.sh. 16 or/10‐15 17 Animals/ 18 Humans/ 19 17 not 18 20 16 not 19 21 CLINICAL TRIAL.pt. 22 exp Clinical Trials/ 23 (clin$ adj25 trial$).ti,ab. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 25 PLACEBOS.sh. 26 placebo$.ti,ab. 27 random$.ti,ab. 28 or/21‐27 29 28 not 19 30 20 or 29 31 and/5,9,30
Appendix 2. Embase.com search strategy
13. #9 AND #12 12. #10 OR #11 11. random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR placebo*:ab,ti OR (doubl* NEXT/1 blind*):ab,ti OR (singl* NEXT/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti 10. 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'randomised controlled trial'/exp OR 'crossover procedure'/exp 9. #5 AND #8 8. #6 OR #7 7. antibiotic*:ab,ti OR antimicrobial*:ab,ti 6. 'antibiotic agent'/exp 5. #1 OR #2 OR #3 OR #4 4. pertus*:ab,ti 3. 'bordetella pertussis'/exp 2. whoop*:ab,ti 1. 'pertussis'/exp
Data and analyses
Comparison 1. Antibiotics for treatment of whooping cough.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ampicillin (treatment) versus untreated control group, oxytetracycline, chloramphenicol or erythromycin | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 1.2 Aureomycin (treatment) versus chloramphenicol (control) | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.09] |
| 2 Complete remission (clinical cure) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Erythromycin estolate for 14 days (treatment) versus erythromycin ethylsuccinate for 14 days (control) | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.16, 10.13] |
| 3 Clinical improvement (better condition) after one week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Trimethoprim/sulfamethoxazole (treatment) versus tetracycline (control) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.70, 1.83] |
| 4 Decreased frequency of cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Erythromycin estolate for 14 days (treatment) versus erythromycin ethylsuccinate for 14 days (control) | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.24] |
| 5 Presence of any sign or symptoms of whooping cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Erythromycin estolate for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.03] |
| 6 Microbiological eradication | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Ampicillin (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Oxytetracycline (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [1.11, 259.87] |
| 6.3 Chloramphenicol (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [0.97, 231.84] |
| 6.4 Erythromycin (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.0 [1.25, 287.92] |
| 6.5 Erythromycin estolate for 14 days (treatment) versus erythromycin ethylsuccinate for 14 days (control) | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.03] |
| 6.6 Erythromycin stearate for 7 days (treatment) versus co‐trimoxazole for 7 days (control) | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.65, 1.90] |
| 6.7 Erythromycin estolate for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.03] |
| 6.8 Azithromycin (treatment) for 3 days versus erythromycin for 14 days (control) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.16] |
| 6.9 Azithromycin (treatment) for 5 days versus erythromycin estolate for 10 days (control) | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.96, 1.04] |
| 6.10 Clarithromycin for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.17] |
| 6.11 Trimethoprim/sulphamethoxazole for 7 days (treatment) versus tetracycline (control) for 7 days | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.94, 1.06] |
| 6.12 Sulfadiazine/trimethoprim for 6 days (treatment) versus chloramphenicol for 6 days (control) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 6.13 Ampicillin (treatment) versus chloramphenicol (control) | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 7 Bacteriological relapse | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Ampicillin (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.81] |
| 7.2 Oxytetracycline (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.69] |
| 7.3 Chloramphenicol (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.81] |
| 7.4 Erythromycin (treatment) versus untreated group (control) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.69] |
| 7.5 Erythromycin estolate for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.14, 83.44] |
| 7.6 Azithromycin (treatment) for 5 days versus erythromycin estolate for 10 days (control) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Respiratory complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Aureomycin (treatment) versus chloramphenicol (control) | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.47, 4.35] |
| 9 Complications (otitis media) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Clarithromycin for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.36] |
| 10 All side effects | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Erythromycin estolate for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.12] |
| 10.2 Azithromycin (treatment) for 3 days versus erythromycin for 14 days (control) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.19, 0.75] |
| 10.3 Clarithromycin for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.97] |
| 10.4 Ampicillin for 6 days (treatment) versus chloramphenicol for 6 days (control) | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.94, 3.48] |
| 10.5 Erythromycin estolate for 14 days (treatment) versus erythromycin ethylsuccinate for 14 days (control) | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.46] |
| 10.6 Aureomycin (chlortetracycline) (treatment) versus chloramphenicol (control) | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.63, 2.58] |
| 11 Gastro‐intestinal system side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Clarithromycin for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.08] |
| 11.2 Azithromycin (treatment) for 5 days versus erythromycin estolate for 10 days (control) | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.62] |
| 12 Side effects (diarrhoea) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Erythromycin stearate for 7 days (treatment) versus co‐trimoxazole for 7 days (control) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [0.25, 22.75] |
| 13 Compliance (detected by antimicrobial activity in urine) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Erythromycin estolate for 14 days (treatment) versus erythromycin ethylsuccinate for 14 days (control) | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.94] |
| 14 Compliance (presented as number of children who took 100% of prescribed doses) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Azithromycin (treatment) for 5 days versus erythromycin estolate for 10 days (control) | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.45, 1.85] |
| 15 Compliance (presented as percentage of drugs taken) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Clarithromycin for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [5.34, 14.46] |
1.1. Analysis.
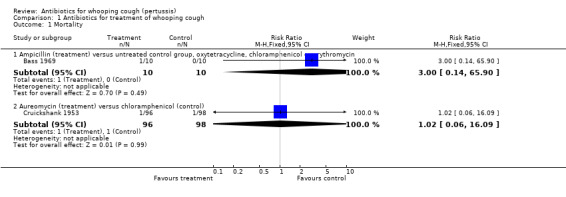
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 1 Mortality.
1.2. Analysis.
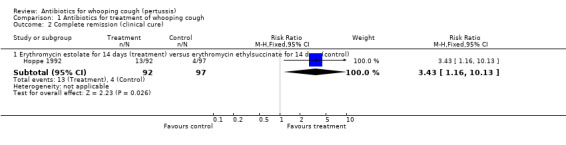
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 2 Complete remission (clinical cure).
1.3. Analysis.
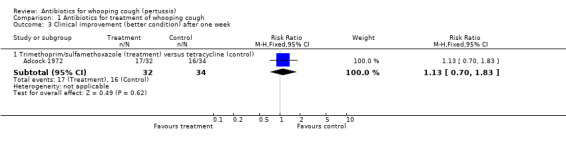
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 3 Clinical improvement (better condition) after one week.
1.4. Analysis.
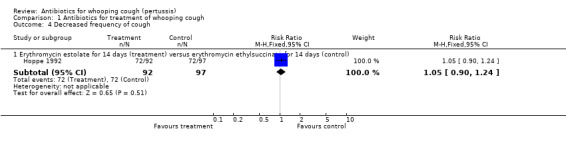
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 4 Decreased frequency of cough.
1.5. Analysis.
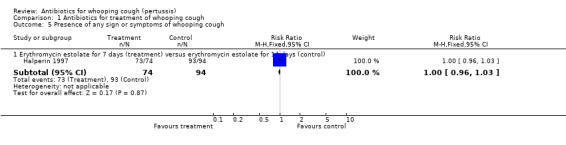
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 5 Presence of any sign or symptoms of whooping cough.
1.6. Analysis.
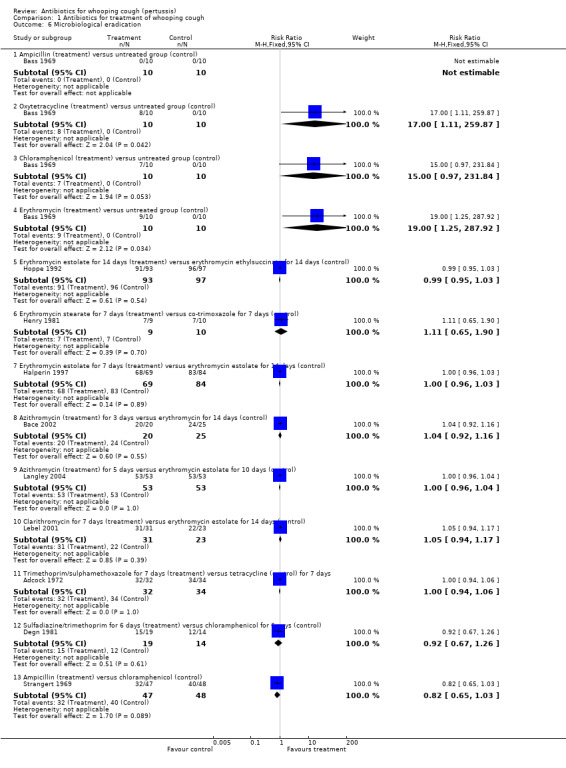
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 6 Microbiological eradication.
1.7. Analysis.
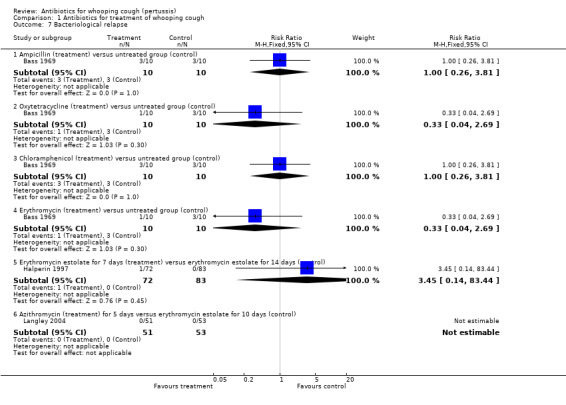
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 7 Bacteriological relapse.
1.8. Analysis.
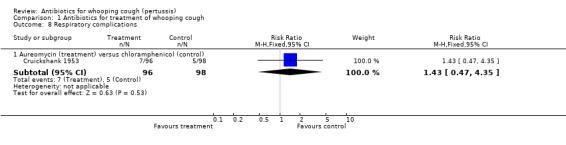
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 8 Respiratory complications.
1.9. Analysis.
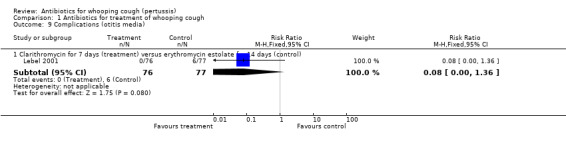
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 9 Complications (otitis media).
1.10. Analysis.
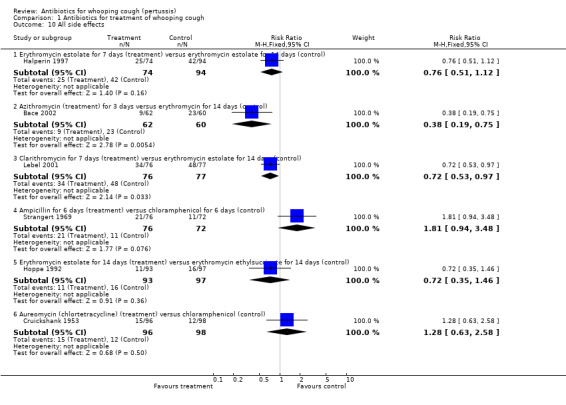
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 10 All side effects.
1.11. Analysis.
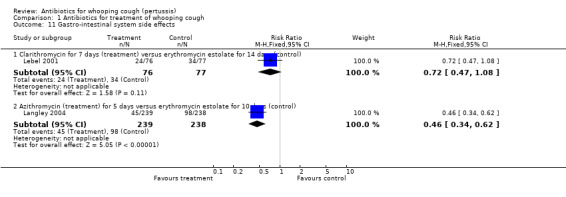
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 11 Gastro‐intestinal system side effects.
1.12. Analysis.
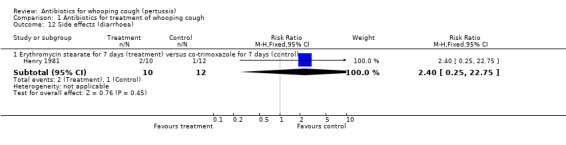
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 12 Side effects (diarrhoea).
1.13. Analysis.
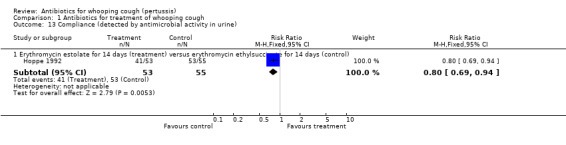
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 13 Compliance (detected by antimicrobial activity in urine).
1.14. Analysis.
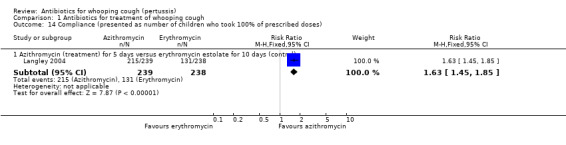
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 14 Compliance (presented as number of children who took 100% of prescribed doses).
1.15. Analysis.
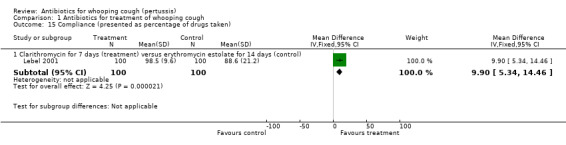
Comparison 1 Antibiotics for treatment of whooping cough, Outcome 15 Compliance (presented as percentage of drugs taken).
Comparison 2. Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Presence of any sign or symptoms of whooping cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Erythromycin estolate for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.03] |
| 2 Microbiological eradication | 4 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
| 3 Microbiological eradication | 3 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
| 4 Bacteriological relapse | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.14, 83.44] |
| 4.1 Erythromycin estolate for 7 days (treatment) versus erythromycin estolate for 14 days (control) | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.14, 83.44] |
| 4.2 Azithromycin (treatment) for 5 days versus erythromycin estolate for 10 days (control) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 All side effects | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.83] |
| 6 All side effects | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.93] |
2.1. Analysis.
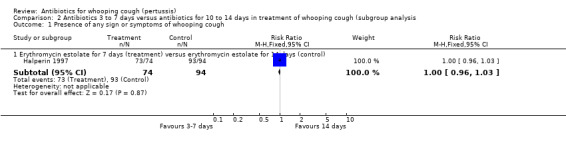
Comparison 2 Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis, Outcome 1 Presence of any sign or symptoms of whooping cough.
2.4. Analysis.
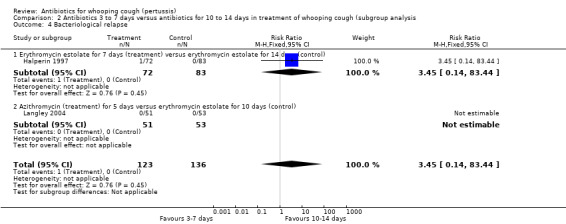
Comparison 2 Antibiotics 3 to 7 days versus antibiotics for 10 to 14 days in treatment of whooping cough (subgroup analysis, Outcome 4 Bacteriological relapse.
Comparison 3. Antibiotic for prophylaxis of whooping cough.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All (any) clinical symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 2 Presence of all (any) cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 3 Paroxysmal cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.31] |
| 4 Frequency of whoop in contacts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.07] |
| 5 Frequency of whooping cough in contacts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Erythromycin ethyl succinate (treatment) versus identical placebo (control) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.78] |
| 6 Frequency of whooping cough in vaccinated contacts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Erythromycin ethyl succinate (treatment) versus identical placebo (control) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Frequency of whooping cough in unvaccinated contacts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Erythromycin ethyl succinate (treatment) versus identical placebo (control) | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.24, 5.08] |
| 8 Culture‐positive after prophylaxis in contacts (attack rate post‐prophylaxis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.54] |
| 9 Culture‐positive or paroxysmal cough > 2 weeks after prophylaxis in contacts (attack rate post‐prophylaxis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.24] |
| 10 All (any) side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.43, 3.31] |
| 11 Compliance (> 90% of doses) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Erythromycin estolate (treatment) versus identical placebo (control) | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.69, 1.00] |
3.1. Analysis.
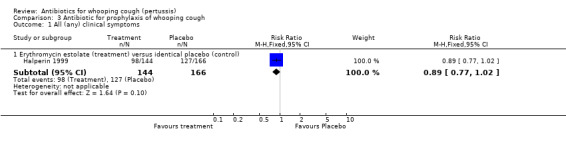
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 1 All (any) clinical symptoms.
3.2. Analysis.
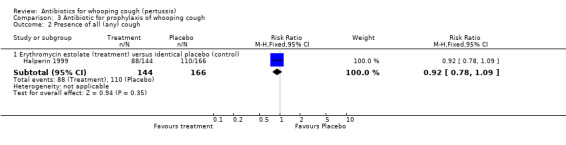
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 2 Presence of all (any) cough.
3.3. Analysis.
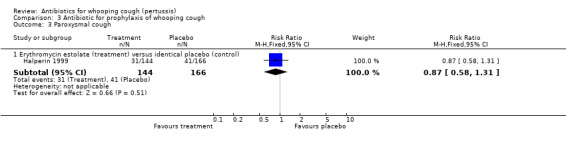
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 3 Paroxysmal cough.
3.4. Analysis.
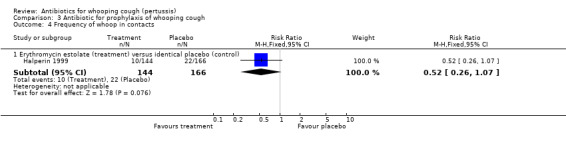
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 4 Frequency of whoop in contacts.
3.5. Analysis.
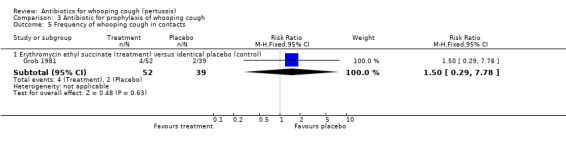
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 5 Frequency of whooping cough in contacts.
3.6. Analysis.
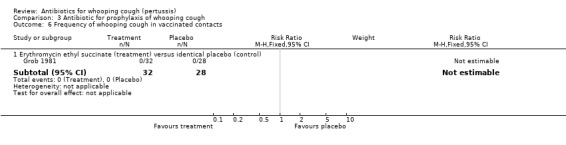
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 6 Frequency of whooping cough in vaccinated contacts.
3.7. Analysis.
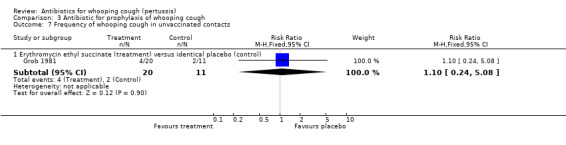
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 7 Frequency of whooping cough in unvaccinated contacts.
3.8. Analysis.
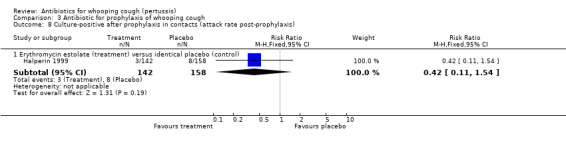
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 8 Culture‐positive after prophylaxis in contacts (attack rate post‐prophylaxis).
3.9. Analysis.
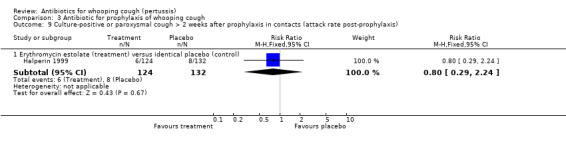
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 9 Culture‐positive or paroxysmal cough > 2 weeks after prophylaxis in contacts (attack rate post‐prophylaxis).
3.10. Analysis.
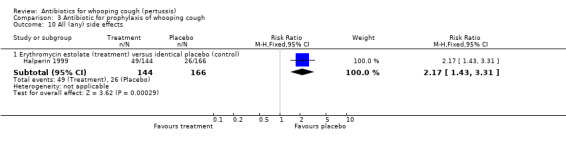
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 10 All (any) side effects.
3.11. Analysis.
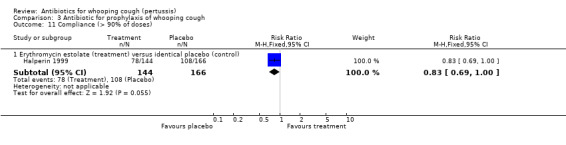
Comparison 3 Antibiotic for prophylaxis of whooping cough, Outcome 11 Compliance (> 90% of doses).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adcock 1972.
| Methods | Randomised, single‐blinded, controlled trial | |
| Participants | 88 children with isolated B. pertussis from nasopharynx or with a typical 'whooping' cough and a relative and absolute lymphocytosis No account was taken of previous vaccination history Exclusion criteria not stated Tetracycline group N = 44 (55% male); 0 to 4 years: 22 males, 19 females; 5 to 10 years: 2 males, 1 female Trimethoprim/sulphamethoxazole group N = 44 (36% male); 0 to 4 years: 15 males, 24 females; 5 to 10 years: 1 male, 4 females | |
| Interventions | Treatment group: received tetracycline: children under 2 years old were given 62.5 mg tetracycline 6‐hourly and older children 125 mg 6‐hourly for 1 week Control group received trimethoprim/sulphamethoxazole: children under 6 months old were given 20 mg trimethoprim with 100 mg sulphamethoxazole twice daily for one week; older children received double this dose All children received phenobarbitone 15 mg t.d.s until vomiting and spasmodic cough had ceased, and were also given a simple linctus for use as required | |
| Outcomes | Primary outcome: microbiological eradication of B. pertussis Secondary outcome: clinical improvement after one week of treatment | |
| Notes | Children were treated at home by their carers, who were asked to bring the children back after 1 week for assessment to the same doctor who saw the children at first attendance. This assessment was based on a full clinical examination, detailed history concerning cough, sleep pattern, vomiting, feeding and general behaviour. Pernasal swabs were taken on first and second attendance Out of 88 patients only 66 returned for follow up. Missed (dropout) patients = 22 (25%). Immunisation status not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation: not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: unclear. Blinding of outcome measure: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
Bace 2002.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: hospitalised children aged 0 to 18 months with symptoms and signs of pertussis were enrolled in the study 122 children; 84 (69%) had pertussis confirmed by bacteriological or serological findings or both, and 38 (31%) had pertussis syndrome caused by other pathogens | |
| Interventions | Treatment group received single dose of 10 mg/kg azithromycin for 3 days Controlled group received 50 mg/kg of erythromycin 3 times daily for 14 days | |
| Outcomes | Microbiological eradication Clinical scores and adverse reactions were also recorded | |
| Notes | This is a conference abstract. Full article is not yet available. We tried to contact the authors but did not receive a reply Clinical examination were scheduled at baseline and 72 hours, 7, 14 and 21 days after the start of the therapy. Immunisation status not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation: not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no. Blinding of outcome measure: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
Bass 1969.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: hospitalised children with clinical pertussis and initial nasopharyngeal swap positive for B. pertussis both by culture and by fluorescent microscopy. Patients who had received previous antimicrobial therapy or those who had previous immunisation against pertussis were not excluded Exclusion criteria: those whose cultures subsequently were negative were withdrawn | |
| Interventions | Children were assigned to 1 of 5 study groups, each group consisted of 10 patients. Group 1 received no antimicrobial agents (control group). Group 2 received ampicillin 100 mg/kg/day. Group 3 received oxytetracycline 50 mg/kg/day. Group 4 received chloramphenicol 50 mg/kg/day. Group 5 received erythromycin (estolate) 50 mg/kg/day. All antimicrobial drugs were administered in 4 divided doses at 6‐hourly intervals for at least 7 days, by oral route except in those who were comatose or with severe vomiting in which parenteral route was used Pertussis hyperimmune globulin was administered to some of the patients according to physician's preference | |
| Outcomes | Microbiological eradication, clinical improvement, duration of the illness | |
| Notes | The second part of the study regarding antimicrobial prophylaxis against pertussis was excluded since it was not a randomised controlled trial Immunisation status: only 9 (19%) of the 50 children studied had received any previous injection of pertussis vaccine. Only 2 children out of 50 studies had previously received 3 DTP injections and their illness appeared milder than non‐immunised children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation: not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of Intervention: no. Blinding of outcome measure: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
Cruickshank 1953.
| Methods | Randomised, double‐blinded, controlled trial | |
| Participants | Inclusion criteria: children 0 to 5 years of age admitted to the 8 hospitals with uncomplicated clinical pertussis within 21 days after the onset of the earliest symptoms were included in the study Exclusion criteria children with complicated pertussis | |
| Interventions | Treatment group: Group 1: Aureomycin (chlortetracycline) 0 to 11 months: 1 g daily, 12 to 35 months: 1.5 g and children aged 36 to 59 months 2 g daily in 2 divided doses daily for 7 days Second group: chloramphenicol doses are given in similar doses as in Aureomycin for 7 days Control group: children were given a mixture of lactose and quinine | |
| Outcomes | 1. Mortality rate 2. Respiratory complications 3. All side effects 4. Bacteriological eradication: cannot be assessed for each group 5. Clinical assessment cannot be assessment for each group | |
| Notes | On admission the patients were divided by sex and placed in one of 3 age groups 0 to 11 months, 12 to 35 months, and 36 to 59 months, then they were allocated to one of the 3 treatment groups by a randomly determined sequence. Immunisation status: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: randomly determined sequence |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes. Blinding of outcome measure: yes. Double‐blinded trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
Degn 1981.
| Methods | Randomised, double‐blinded, controlled trial | |
| Participants | Inclusion criteria children 1 to 12 months old with a body weight above 4 kg, and with clinical pertussis or B. pertussis culture positive or both Exclusion criteria children with another infectious disease, with bronchial asthma, cerebrally injured patients, with radiologically proven lung infiltration and patients previously exhibiting allergic manifestations to treatment of sulpha‐preparations or chloramphenicol | |
| Interventions | Treatment group sulfadiazine/trimethoprim 30 mg/6 mg per kg per day in 4 divided dose for 6 days followed by observational period of further 6 days Control group chloramphenicol 50 mg/kg /day in 4 divided doses for 6 days | |
| Outcomes | Microbiological eradication Median number of paroxsymal coughs per day | |
| Notes | The article was in Danish language and was translated by The Cochrane Collaboration. Immunisation status not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: sequence of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes. Blinding of outcome measure: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
Grob 1981.
| Methods | Randomised, single‐blinded, placebo‐controlled study | |
| Participants | Inclusion criteria: symptom‐free family contacts of a B. pertussis culture‐positive child Exclusion criteria: if none of the swabs taken from the children (including the index case) grew B. pertussis the contacts were not included Any contact showing early signs of whooping cough was excluded This study was in general practice and children were living in good social circumstances in South‐West Thames region in the UK The children were visited frequently by a nurse who recorded progress and took swabs | |
| Interventions | Treatment group erythromycin (ethylsuccinate) 50 mg/kg/day in 4 divided doses. The dosage schedule was 125 mg before meals 4 times a day for contacts under 2 years. 250 mg before meals 4 times a day for those aged 2 to 8 years both for 14 days. Controlled group: identical placebo syrup | |
| Outcomes | Frequency of whooping cough in vaccinated and non‐vaccinated contacts. Microbiological eradication result is unclear | |
| Notes | This is a prophylactic erythromycin placebo‐controlled study in whooping cough contacts. Immunisation status: 60 (66%) children were vaccinated out of 91 children included in the trial. No vaccinated child had whooping cough | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation: not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes. Blinding of outcome measure: unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
Halperin 1997.
| Methods | Open randomised controlled trial | |
| Participants | Inclusion criteria children and their households contacts with culture‐positive pertussis Exclusion criteria allergy to erythromycin, pre‐existing liver disease or pregnancy Immunisation status % >= 3 doses in treatment group = 88.1%, control group = 87.8 | |
| Interventions | Treatment group erythromycin estolate 40 mg/kg/day in 3 divided doses with a maximum of 1000 mg /day for 7 days Control group erythromycin estolate 40 mg/kg/day in 3 divided doses with a maximum of 1000 mg/day for 14 days | |
| Outcomes | Microbiological eradication, clinical assessments, adverse reactions and compliance | |
| Notes | Immunisation status: % >= 3 doses in treatment group = 88.1%, control group = 87.8% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no. Blinding of outcome measure: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
Halperin 1999.
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | Inclusion criteria all household contacts of 152 children with culture‐positive pertussis who provided consent Exclusion criteria pregnancy, age < 6 months, had history of culture‐positive pertussis, already receiving erythromycin‐containing antibiotics, erythromycin allergy or liver disease | |
| Interventions | Treatment group erythromycin estolate 40 mg/kg/day in 3 divided doses, maximum dose 1 g/day for 10 days Control group identical placebo for 10 days | |
| Outcomes | Microbiological eradication, clinical assessments, adverse reactions and compliance. Immunisation status not stated | |
| Notes | This is prophylactic erythromycin placebo‐controlled study in whooping cough households contacts The unit of randomisation was the household, therefore all household members were allocated to the same treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: eligible households members were allocated by the pharmacy department by using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Low risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes. Blinding of outcome measure: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
Henry 1981.
| Methods | Randomised, single‐blinded (patient), controlled trial | |
| Participants | Children with whooping cough and B. pertussis culture‐positive Children who had received antibiotics other than erythromycin or co‐trimoxazole for their illness before their admission and children who had been immunised were included Exclusion criteria: those who received antibiotics by the parenteral route or fluids by the intravenous route | |
| Interventions | Treatment group co‐trimoxazole 6 mg/kg/day of trimethoprim in 2 divided doses orally for 7 days Controlled group erythromycin stearate 40 mg/kg/day in 4 divided doses orally for 7 days | |
| Outcomes | Microbiological eradication, diarrhoea | |
| Notes | Author supplied us with information regarding the article's methodology through personal contact. Immunisation status not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: pharmacy randomly allocated patients according to random number book |
| Allocation concealment (selection bias) | Low risk | Low risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: no. Blinding of outcome measure: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
| Selective reporting (reporting bias) | Low risk | |
Hoppe 1992.
| Methods | Open, randomised, multi centre controlled trial | |
| Participants | Inclusion criteria: ambulatory children with whooping cough and B. pertussis culture‐positive Exclusion criteria: antimicrobial treatment during the 3 days before enrolment of patients, hypersensitivity to macrolide antibiotics, preexisting liver or renal disease, simultaneous treatment of theophylline or ergotamine, or body weight > 27.5 kg The pertussis vaccination status was similar in both study groups. 115 patients (60.5%) had not been vaccinated at all (EST, 56 patients (60.2%); ETH, 59 patients (60.8)) | |
| Interventions | Treatment group: erythromycin estolate (EST) 40 mg/kg/day in 2 divided doses orally taken during meal for 14 days Controlled group: erythromycin ethylsuccinate (ETH) 60 mg/kg/day in 3 divided doses orally taken during meals for 14 days | |
| Outcomes | Microbiological eradication, clinical assessment, decrease frequency and severity of cough, improved or cured general condition, adverse reactions, and patients' compliance measured by antimicrobial activity in the urine | |
| Notes | Immunisation status: the pertussis vaccination status was similar in both study groups. 115 patients (60.5%) had not been vaccinated at all (EST, 56 patient (60.2%); ETH, 59 patients (60.8)) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated list of numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no. Blinding of outcome measure: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
Langley 2004.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: children aged 6 months to 16 years and had either culture‐proven B. pertussis infection or cough illness suspected by a physician to be pertussis Exclusion criteria: children with known allergy to any macrolide, immunodeficiency, had hepatic, renal, cardiovascular, haematologic disease or chronic lung disease, had concomitant use of theophylline, phenytoin, digitalis, etc. | |
| Interventions | Treatment group: azithromycin 10 mg/kg (maximum: 500 mg) by mouth on first day of treatment and 5 mg/kg (maximum daily dose: 250 mg) once daily on the second to fifth days of treatment. Control group: 3 doses of erythromycin estolate (40 mg/kg/day: maximum 1 g) by mouth for 10 days | |
| Outcomes | Microbiological eradication, bacteriological relapse, compliance, presence of clinical symptoms, treatment‐associated adverse events | |
| Notes | Immunisation status: mean previous number of pertussis vaccine doses received was 4.4 and 4.1 for erythromycin and azithromycin treatment group respectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Low risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Group assignment was not blinded after randomisation |
Lebel 2001.
| Methods | Randomised, single‐blinded (investigator) controlled trial | |
| Participants | Inclusion criteria: children aged 1 month to 16 years with clinical pertussis Exclusion criteria: the presence of a cough for 21 days or longer, treatment with any antibiotic with known activity against B. pertussis, concomitant therapy with terfenadine, astemizole or zidovudine, concomitant therapy with theophylline, digitalis glycoside, ergotamine, carbamazepine, phenytoin, warfarin therapy, known allergy to macrolide antibiotics, presence of a disease requiring the use of steroid medications, presence of underlying cardiac, hepatic, bronchopulmonary, renal, immunodeficiency, malabsorption disorder or pregnancy Immunisation status: pertussis vaccination: (%) in treatment group = 89, control group = 90 | |
| Interventions | Treatment group: clarithromycin granules for suspension 7.5 mg/kg/dose twice daily (maximum dose 500 mg twice daily) orally for 7 days Control group: erythromycin estolate 13.3 mg/kg/dose (maximum dose, 333 mg 3 times a day) orally for 14 days | |
| Outcomes | Microbiological eradication, clinical cure, adverse reactions, complications (otitis media) and compliance to medications | |
| Notes | The study populations considered in the analysis: the per protocol population included those patients who had a positive culture for B. pertussis at baseline and a. received study drug for a minimum 3 days b. had a post‐treatment culture and clinical assessment c. did not take any interfering concomitant antimicrobial therapy, and d. did not violate the study protocol Immunisation status: pertussis vaccination treatment group = 89%, control group = 90% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Low risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes. Blinding of outcome measure: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
Strangert 1969.
| Methods | Quasi‐randomised controlled trial | |
| Participants | Inclusion criteria: children admitted with clinical pertussis Exclusion criteria: children who received chloramphenicol or ampicillin prior to their stay in hospital or were discharged before the course of therapy had been completed | |
| Interventions | Treatment group: ampicillin 75 to 100 mg/kg/day divided in 4 doses for 6 days Controlled group: chloramphenicol 75 to 100 mg/kg/day divided in 4 doses for 6 days Children under 6 months of age were also given immunoglobulin against whooping cough each other day on altogether 3 occasions | |
| Outcomes | Microbiological eradication, adverse reactions, complications (secondary infections) | |
| Notes | This is quasi‐randomised study for treatment of pertussis. Immunisation status not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation: alternation of patients (each second child admitted with whooping cough was treated with ampicillin) |
| Allocation concealment (selection bias) | High risk | High risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no. Blinding of outcome measure: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no |
| Selective reporting (reporting bias) | High risk | |
DTP: diphtheria‐tetanus‐pertussis EST: erythromycin estolate ETH: erythromycin ethylsuccinate t.d.s: three times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aoyama 1996 | Non‐randomised controlled trial with historical control patients |
| Bergquist 1987 | Excluded as used salbutamol in the control group. Results showed fewer whoops and eradication of B. pertussis in erythromycin treated group (n = 17) compared to the salbutamol control group (n = 21) |
| Di Nola 1974 | RCT excluded as used antibiotics in secondary respiratory infection in childhood pertussis |
| LaBoccetta 1952 | Quasi‐RCT with non‐interpretable results for control group; some of them received antibiotics and others received no antibiotics and results cannot be separated |
| Spencely 1981 | RCT excluded because of insufficient data |
| Torre 1984 | RCT with non‐interpretable results; large numbers of patients were missed for follow up (i.e. on day 15 only 5 out of 16 patients showed up in josamycin group, and 6 out of 19 showed up in erythromycin group for microbiological investigation |
| Trollfors 1978 | Quasi‐RCT. Poor quality study. Randomisation is inadequate. Randomised participants are combined with non‐randomised participants and cannot be analysed separately. Results suggest amoxicillin is poorly effective |
RCT: randomised controlled trial
Contributions of authors
Sultan Altunaiji (SA) conceived the idea for the review. He designed and co‐ordinated the screening of search results, retrieval of papers and screening retrieved papers against inclusion criteria, abstracting data from papers, appraising the quality of papers and trial methodology. He was also responsible for writing to authors for additional information, obtaining and screening data on unpublished studies, data entry into RevMan, analysis of data, clinical perspectives, policy and consumer perspectives and writing and updating the review.
Renata Kukuruzovic (RK), John Massie (JM) and Nigel Curtis (NC) contributed to screening search results, retrieval of papers and screening retrieved papers against inclusion criteria, abstracting data from papers, appraising quality of papers, trial methodology, obtaining and screening data on unpublished studies, analysis of data, policy and consumer perspectives and writing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Acute Respiratory Infections Review Group, Australia.
Declarations of interest
None known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Adcock 1972 {published data only}
- Adcock KJ, Reddy S, Okubadejo OA, Montefiore D. Trimethoprim‐sulphamethoxazole in pertussis: comparison with tetracycline. Archives of Disease in Childhood 1972;47(252):311‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bace 2002 {published data only}
- Bace A, Kuzmanovic N, Novak D, Anic‐Milic T, Radosevic S, Welle A, et al. The efficacy and safety of 3‐day azithromycin vs 14‐day erythromycin in the treatment of pertussis in infants and young children. Abstract presented in ICMASKO 6 in Bologna, Italy. January 23‐25 2002.
Bass 1969 {published data only}
- Bass JW, Klenk EL, Kotheimer JB, Linnemann CC, Smith MH. Antimicrobial treatment of pertussis. Journal of Pediatrics 1969;75(5):768‐81. [DOI] [PubMed] [Google Scholar]
Cruickshank 1953 {published data only}
- Cruickshank R, Anderson T, Benn EC, Bradford H, Christie AB, Joe A, et al. Treatment of whooping‐cough with antibiotics. Lancet 1953;1:1109‐12. [MEDLINE: ; CN‐00341118] [Google Scholar]
Degn 1981 {published data only}
- Degn H, Jacobsen AT, Gjerris A, Rasmussen SN, Helin P, Sorensen SF. Treatment of whooping cough in newborn infants with chloramphenicol and sulfadiazine/trimethoprim. Ugeskrift for Laeger 1981;143(3):107‐9. [PubMed] [Google Scholar]
Grob 1981 {published data only}
- Grob PR. Prophylactic erythromycin for whooping‐cough contacts. Lancet 1981;1(8223):772. [PubMed] [Google Scholar]
Halperin 1997 {published data only}
- Halperin SA, Bortolussi R, Langley JM, Miller B, Eastwood BJ. Seven days of erythromycin estolate is as effective as fourteen days for the treatment of Bordetella pertussis infections. Pediatrics 1997;100(1):65‐71. [DOI] [PubMed] [Google Scholar]
Halperin 1999 {published data only}
- Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, Serres G. A randomized, placebo‐controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture‐positive Bordetella pertussis infection. Pediatrics 1999;104(4):e42. [DOI] [PubMed] [Google Scholar]
Henry 1981 {published data only}
- Henry RL, Dorman DC, Skinner JA, Mellis CM. Antimicrobial therapy in whooping cough. Medical Journal of Australia 1981;2(1):27‐8. [PubMed] [Google Scholar]
Hoppe 1992 {published data only}
- Hoppe JE. Comparison of erythromycin estolate and erythromycin ethylsuccinate for treatment of pertussis. The Erythromycin Study Group. Pediatric Infectious Disease Journal 1992;11(3):189‐93. [DOI] [PubMed] [Google Scholar]
Langley 2004 {published data only}
- Langley JM, Halperin SA, Boucher FD, Smith B. Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics 2004;114(1):96‐101. [DOI] [PubMed] [Google Scholar]
Lebel 2001 {published data only}
- Lebel MH, Mehra S. Efficacy and safety of clarithromycin versus erythromycin for the treatment of pertussis: a prospective, randomized, single blind trial. Pediatric Infectious Disease Journal 2001;20(12):1149‐54. [DOI] [PubMed] [Google Scholar]
Strangert 1969 {published data only}
- Strangert K. Comparison between the effect of chloramphenicol and ampicillin in whooping‐cough. Scandinavian Journal of Infectious Diseases 1969;1(1):67‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aoyama 1996 {published data only}
- Aoyama T, Sunakawa K, Iwata S, Takeuchi Y, Fujii R. Efficacy of short‐term treatment of pertussis with clarithromycin and azithromycin. Journal of Pediatrics 1996;129(5):761‐4. [DOI] [PubMed] [Google Scholar]
Bergquist 1987 {published data only}
- Bergquist SO, Bernander S, Dahnsjo H, Sundelof B. Erythromycin in the treatment of pertussis: a study of bacteriologic and clinical effects. Pediatric Infectious Disease Journal 1987;6(5):458‐61. [DOI] [PubMed] [Google Scholar]
Di Nola 1974 {published data only}
- Nola F, Soranzo ML. Comparative trial of cephacetrile versus cephaloridine in the treatment of secondary respiratory infections in childhood pertussis. Arzneimittel‐Forschung 1974;24(9b):1510‐2. [PubMed] [Google Scholar]
LaBoccetta 1952 {published data only}
- LaBoccetta AC, Dawson KE. Pertussis: treatment with aureomycin; clinical study of eighty‐five patients and seventy‐four controls. American Journal of Diseases of Children 1952;84:184‐90. [Cochrane ARI handsearch] [PubMed] [Google Scholar]
Spencely 1981 {published data only}
- Spencely M, Lambert HP. Prophylactic erythromycin for whooping‐cough contacts. Lancet 1981;1(8223):772‐3. [PubMed] [Google Scholar]
Torre 1984 {published data only}
- Torre D, Maggiolo F, Sampietro C. Comparative clinical study of josamycin and erythromycin in pertussis. Chemioterapia 1984;3(4):255‐7. [PubMed] [Google Scholar]
Trollfors 1978 {published data only}
- Trollfors B. Effect of erythromycin and amoxycillin on Bordetella pertussis in the nasopharynx. Infection 1978;6(5):228‐30. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 1975
- Anonymous. American Academy of Pediatrics. Committee on drugs. Requiem for tetracyclines. Pediatrics 1975;55(1):142‐3. [PubMed] [Google Scholar]
Atkinson 1996
- Atkinson W. Centers for Disease Control and Prevention. 3rd Edition. Atlanta: Dept of Health and Human Services. Epidemiology and prevention of vaccine‐preventable diseases, 1996. [Google Scholar]
Bass 1985
- Bass JW. Erythromycin for pertussis: probable reasons for past failure. Lancet 1985;2:147. [DOI] [PubMed] [Google Scholar]
Bettiol 2010
- Bettiol S, Thompson MJ, Roberts NW, Perera R, Heneghan CJ, Harnden A. Symptomatic treatment of the cough in whooping cough. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003257] [DOI] [PubMed] [Google Scholar]
Broomhall 1984
- Broomhall J, Herxheimer A. Treatment of whooping cough: the facts. Archives of Disease in Childhood 1984;59:185‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 1991
- Centers for Disease Control and Prevention. Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventive measures: recommendations of the Advisory Committee on immunization practices (ACIP). Morbidity and Mortality Weekly Report 1991;40(RR‐10):1‐28. [PubMed] [Google Scholar]
CDC 1995
- Centers for Disease Control and Prevention. Pertussis ‐ United States, January 1992 ‐ June 1995. JAMA 1995;274(6):450‐1. [PubMed] [Google Scholar]
CDC 2000
- Centers for Disease Control and Prevention. Guidelines for the control of pertussis outbreak. Available at http://www.cdc.gov/nip/publications/pertussis/guide.htm 2000 (accessed February 2002).
CDC 2002
- Centers for Disease Control and Prevention. Pertussis deaths ‐ United States, 2000. Morbidity and Mortality Weekly Report 2002;51(4):73‐6. [PubMed] [Google Scholar]
CDC 2005
- Centers for Disease Control and Prevention. Pertussis ‐ United States, 2001 ‐ 2003. Morbidity and Mortality Weekly Report 2005;54(50):1283‐6 (available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5450a3.htm). [PubMed] [Google Scholar]
CDI 1997
- Anonymous. Technical report series guidelines for the control of pertussis in Australia. Communicable Diseases Intelligent (CDI) 1997;1:30. [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Cherry 1984
- Cherry JD. The epidemiology of pertussis and pertussis immunization in the United States: a comparative study. Current Problems in Pediatrics 1984;14(2):1‐78. [DOI] [PubMed] [Google Scholar]
Cherry 1988
- Cherry JD, Brunell PA, Golden GS, Karazon DT. Report of the task force on pertussis and pertussis immunization. Pediatrics 1988;81:939‐84. [Google Scholar]
De Serres 1995
- Serres G, Boulianne N, Duval B. Field effectiveness of erythromycin prophylaxis to prevent pertussis within families. Pediatric Infectious Disease Journal 1995;14(11):969‐75. [DOI] [PubMed] [Google Scholar]
Ginsburg 1986
- Ginsburg CM. Pharmacology of erythromycin in infants and children. Pediatric Infectious Disease Journal 1986;5:124‐9. [DOI] [PubMed] [Google Scholar]
Halsey 1980
- Halsey NA, Welling MA, Lehman RM. Nosocomial pertussis: a failure of erythromycin treatment and prophylaxis. American Journal of Diseases of Children 1980;134(5):521‐2. [DOI] [PubMed] [Google Scholar]
Heininger 1994
- Heininger U, Stehr K, Schmitt‐Grohe S, Lorenz C, Rost R, Christenson PD, et al. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatric Infectious Disease Journal 1994;13(4):306‐9. [DOI] [PubMed] [Google Scholar]
Heininger 1998
- Heininger U, Cherry JD, Stehr K, Schmitt‐Grohé S, Uberall M, Laussucq S, et al. Comparative efficacy of the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine and Lederle whole‐cell component DTP vaccine in German children after household exposure. Pediatrics 1998;102(3):546‐53. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://www.cochrane‐handbook.org. 2011.
Honein 1999
- Honein MA, Paulozzi LJ, Himelright IM, Lee B, Cragan JD, Patterson L, et al. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromycin: a case review and cohort study. Lancet 1999;354(9196):2101‐5. [DOI] [PubMed] [Google Scholar]
Hoppe 1988
- Hoppe JE, Haug A. Treatment and prevention of pertussis by antimicrobial agents (Part II). Infection 1988;16(3):148‐52. [DOI] [PubMed] [Google Scholar]
Hoppe 2000
- Hoppe JE. Neonatal pertussis. Pediatric Infectious Disease Journal 2000;19(3):244‐7. [DOI] [PubMed] [Google Scholar]
Isacson 1993
- Isacson J, Trollfors B, Taranger J, Zackrisson G, Lagergard T. How common is whooping cough in a nonvaccinating country?. Pediatric Infectious Disease Journal 1993;12(4):284‐8. [DOI] [PubMed] [Google Scholar]
Islur 1975
- Islur J, Anglin CS, Middleton PJ. The whooping cough syndrome: a continuing problem. Clinical Pediatrics 1975;14(2):171‐6. [DOI] [PubMed] [Google Scholar]
Krugman 1992
- Krugman S, Katz SL, Greshon AA, Wilfert CM. Pertussis (whooping cough). Infectious Diseases of Children. 9th Edition. St. Louis: Mosby Year Book, 1992:299‐313. [Google Scholar]
Kucers 1997
- Kucers A, Crowe SM, Grayson ML, Hoy JF. The use of antibiotics: a clinical view of antibacterial, antifungal, and antiviral drugs. 5th Edition. Oxford: Butterworth‐Heinemann, 1997:637‐42. [Google Scholar]
Leach 1997
- Leach AJ, Shelby‐James TM, Mayo M, Gratten M, Laming AC, Currie BJ, et al. A prospective study of the impact of community‐based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clinical Infectious Diseases 1997;24(3):356‐62. [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: Wiley‐Blackwell, 2009. [Google Scholar]
Long 1997
- Long SS, Pickering LK, Prober CG. Bordetella pertussis (pertussis) and other species. Principles and Practice of Pediatric Infectious Diseases. Saunders WB Co, 1997:977‐86. [Google Scholar]
Ray 1977
- Ray WA, Federspiel CF, Schaffner W. Prescribing of tetracycline to children less than 8 years old. A two‐year epidemiologic study among ambulatory Tennessee medicaid recipients. JAMA 1977;237(19):2069‐74. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2011.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shu 1987
- Shu XO, Gao YT, Linet MS, Brinton LA, Gao RN, Jin F, et al. Chloramphenicol use and childhood leukaemia in Shanghai. Lancet 1987;2(8565):934‐7. [DOI] [PubMed] [Google Scholar]
Smilack 1999
- Smilack JD. The tetracyclines. Mayo Clinic Proceedings 1999;74(7):727‐9. [DOI] [PubMed] [Google Scholar]
Stang 1986
- Stang H. Pyloric stenosis associated with erythromycin ingested through breastmilk. Minnesota Medicine 1986;69(11):669‐70, 682. [PubMed] [Google Scholar]
Thomas 2002
- Thomas J. Australian Prescription Products Guide. 1st Edition. Australian Pharmaceutical Publishing Company, 2002:1844. [Google Scholar]
Wallerstein 1969
- Wallerstein RO, Condit PK, Kasper CK, Brown JW, Morrison FR. Statewide study of chloramphenicol therapy and fatal aplastic anemia. JAMA 1969;208(11):2045‐50. [PubMed] [Google Scholar]
Weiss 1960
- Weiss CF, Glazko AJ, Weston JK. Chloramphenicol in the newborn infant: a physiologic explanation of its toxicity when given in excess doses. New England Journal of Medicine 1960;262:787‐94. [DOI] [PubMed] [Google Scholar]
WHO 1999
- WHO. WHO position paper. Pertussis Vaccine. Weekly Epidemiological Record 1999; Vol. 74, issue 18:137‐42.
References to other published versions of this review
Altunaiji 2005
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004404.pub3] [DOI] [PubMed] [Google Scholar]
Altunaiji 2007
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004404.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


