Abstract
Background
Primary analysis of the multicenter, open-label, single-arm, phase 2 DESTINY-Breast01 trial (median follow-up, 11.1 months) demonstrated durable antitumor activity with trastuzumab deruxtecan (T-DXd) in patients with human epidermal growth factor receptor 2 (HER2)–positive metastatic breast cancer (mBC) previously treated with trastuzumab emtansine (T-DM1). We report updated cumulative survival outcomes with median follow-up of 26.5 months (data cutoff, March 26, 2021).
Patients and Methods
Patients with HER2-positive mBC resistant or refractory to T-DM1 received T-DXd 5.4 mg/kg intravenously every 3 weeks until disease progression, unacceptable adverse events, or withdrawal of consent. The primary end point was confirmed objective response rate by independent central review. Secondary end points included overall survival, duration of response, progression-free survival, and safety.
Results
The objective response rate by independent central review was 62.0% (95% CI, 54.5–69.0) in patients who received T-DXd 5.4 mg/kg every 3 weeks (n = 184). Median overall survival was 29.1 months (95% CI, 24.6–36.1). Median progression-free survival and duration of response were 19.4 months (95% CI, 14.1–25.0) and 18.2 months (95% CI, 15.0 months–not evaluable), respectively. Drug-related treatment-emergent adverse events (TEAEs) were observed in 183 patients (99.5%), and 99 patients (53.8%) had 1 or more grade ≥ 3 TEAEs. Adjudicated drug-related interstitial lung disease/pneumonitis occurred in 15.8% of patients (n = 29), of which 2.7% (n = 5) were grade 5.
Conclusions
These updated results provide further evidence of sustained antitumor activity of T-DXd with a consistent safety profile in heavily pretreated patients with HER2-positive mBC.
Keywords: HER2-positive, metastatic breast cancer, trastuzumab deruxtecan, overall survival
Introduction
The phase 2 DESTINY-Breast01 trial assessed trastuzumab deruxtecan (T-DXd) 5.4 mg/kg in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (mBC) with promising efficacy results. The confirmed objective response rate (ORR) was 60.9% (95% CI, 53.4–68.0) in the primary data cutoff (DCO; August 1, 2019) and 61.4% (95% CI, 54.0–68.5) in the initial update (DCO, June 8, 2020).1, 2 Primary results of DESTINY-Breast01 supported approval of T-DXd in third-line settings (December 2019) by the US Food and Drug Administration.3, 4 Based on results from DESTINY-Breast03, the indication for T-DXd was updated to include patients with HER2-positive mBC who received a prior anti–HER2-based regimen in the metastatic setting or developed disease recurrence during/within 6 months after therapy in the neoadjuvant/adjuvant setting.4
Median overall survival (OS) was not reached in the primary analysis of DESTINY-Breast01. We report updated cumulative survival outcomes (DCO, March 26, 2021).
Methods
Study Design and Patients
The study design was previously published.2 Women aged ≥18 years with pathologically documented HER2-positive mBC resistant or refractory to trastuzumab emtansine (T-DM1) with an Eastern Cooperative Oncology Group performance status score of 0 or 1 were included. HER2 positivity (centrally confirmed on archival tissue) was defined according to American Society of Clinical Oncology/College of American Pathologists guidelines. Patients with a history of noninfectious interstitial lung disease (ILD)/pneumonitis treated with glucocorticoids, current or suspected ILD/pneumonitis, or untreated/symptomatic brain metastases were excluded. The study was approved by the institutional review board at each participating site. All patients provided written informed consent.
Procedures
Patients received 5.4 mg/kg of T-DXd intravenously every 3 weeks until disease progression, unacceptable adverse events, or withdrawal of consent.
End Points
Primary end point was confirmed ORR by independent central review (ICR). Additional end points included duration of response (DoR), progression-free survival (PFS), OS, and time to response (TTR). Treatment-emergent adverse events (TEAEs) were categorized using the Medical Dictionary for Regulatory Activities, version 23.0, and graded according to National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03. ILD/pneumonitis was evaluated by an independent adjudication committee. Statistical analyses were previously described.2
Results
Patients
Between October 2017 and September 2018, 184 patients received at least 1 dose of T-DXd 5.4 mg/kg (Fig 1). At DCO, 28 patients remained on treatment. Main reasons for treatment discontinuation were disease progression (46.2%), adverse events (19.0%), and withdrawal of consent (6.0%). Median follow-up and treatment duration were 26.5 months (range, 0.7–39.1) and 10.1 months (range, 0.7–39.6), respectively. Table 1 lists patients’ baseline characteristics.
Fig 1.
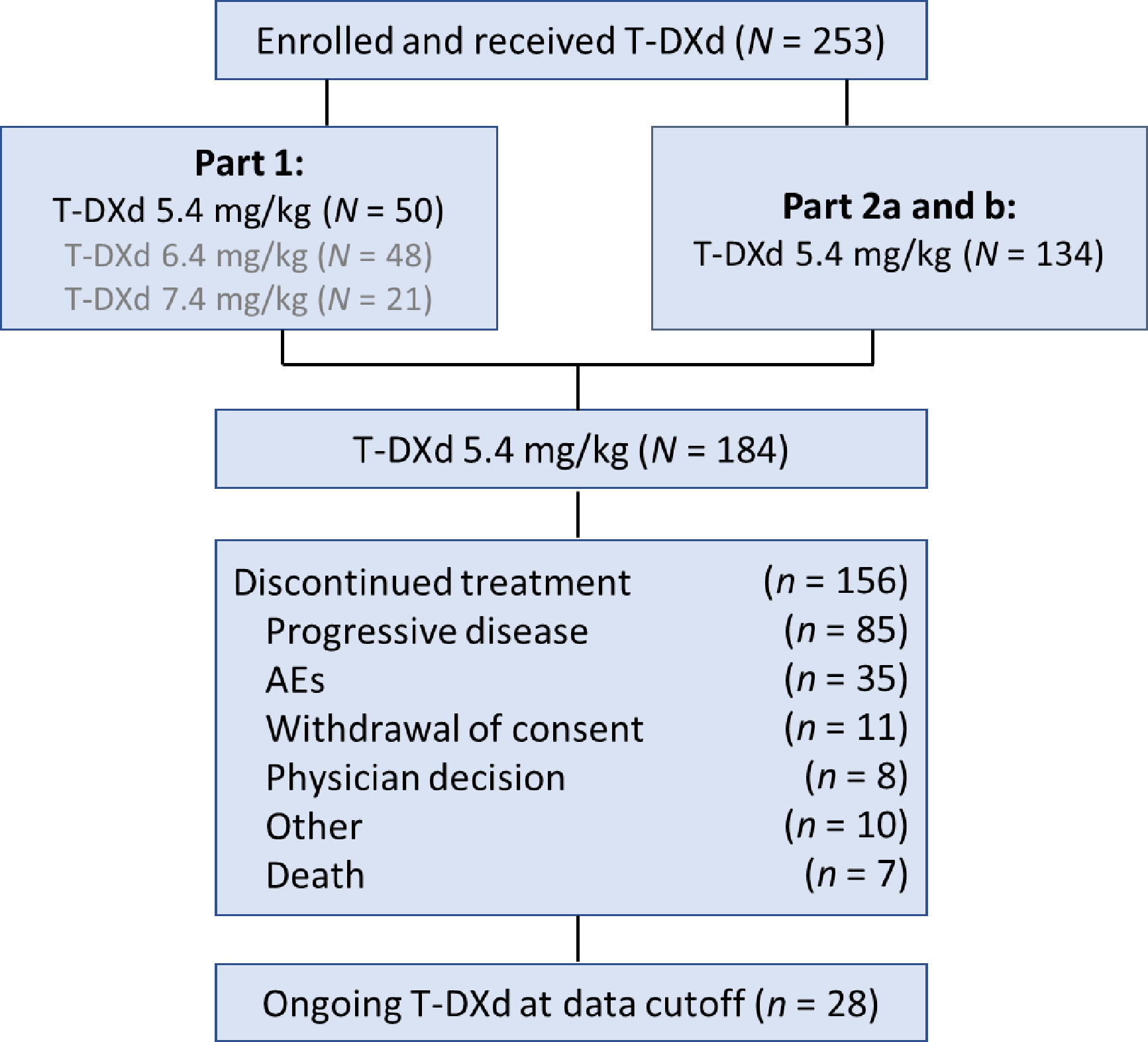
Patient disposition. Part 1 of the study consisted of 2 sequential stages: pharmacokinetics and dose finding. Based on these results, a dose of T-DXd 5.4 mg/kg was recommended for part 2 of the study. Part 2 consisted of an evaluation of the efficacy and safety of T-DXd in patients treated at the recommended dose who had tumor progression during or after the administration of T-DM1 (part 2a) and in those who had discontinued T-DM1 for reasons other than progressive disease (part 2b).
AEs, adverse events; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | T-DXd 5.4 mg/kg N = 184a |
|---|---|
|
| |
| Median age (range), years | 55.0 (28–96) |
|
| |
| Age group, n (%) | |
| <65 years | 140 (76.1) |
| ≥65 years | 44 (23.9) |
|
| |
| Female, n (%) | 184 (100.0) |
|
| |
| Region, n (%) | |
| Asia | 63 (34.2) |
| North America | 53 (28.8) |
| Europe | 68 (37.0) |
|
| |
| ECOG PS, n (%) | |
| 0 | 102 (55.4) |
| 1 | 81 (44.0) |
| 2 | 1 (0.5) |
|
| |
| Hormone receptor, n (%) | |
| Positive | 97 (52.7) |
| Negative | 83 (45.1) |
| Unknown | 4 (2.2) |
|
| |
| HER2 expression,b n (%) | |
| IHC 3+ | 154 (83.7) |
| IHC 2+/IHC 1+, ISH+ | 28 (15.2) |
| Not evaluable | 2 (1.1) |
|
| |
| History of visceral disease at baseline, n (%) | 169 (91.8) |
|
| |
| History of brain metastases,c n (%) | 24 (13.0) |
| Presence of brain metastases at baseline,c n (%) | 7 (3.8) |
|
| |
| Sum of diameters of target lesions, median (range), cm2 | 5.8 (1.2–24.5) |
|
| |
| Median number of prior treatments for metastatic disease (range) | 6 (2–27) |
|
| |
| Prior treatment for metastatic disease, n (%) | |
| Trastuzumab | 184 (100.0) |
| T-DM1 | 184 (100.0) |
| Pertuzumab | 121 (65.8) |
| Other anti-HER2 | 100 (54.3) |
| Hormone therapy | 90 (48.9) |
| Other systemic therapy | 183 (99.5) |
|
| |
| Best response to prior T-DM1 therapy, n (%) | |
| CR | 5 (2.7) |
| PR | 35 (19.0) |
| SD | 39 (21.2) |
| PD | 66 (35.9) |
| Not evaluable | 39 (21.2) |
CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; PD, progressive disease; PR, partial response; SD, stable disease; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Data cutoff March 26, 2021.
All 184 patients received 1 or more dose of T-DXd.
HER2 status was centrally assessed on the most recent archival tissue according to the American Society of Clinical Oncology/College of American Pathologists guidelines.
Includes treated and asymptomatic brain metastases.
Median prior regimens was 6.0 (range, 2–27). All patients previously received trastuzumab and T-DM1; 54.3% received other HER2-targeted treatments, 65.8% received pertuzumab, and 48.9% received hormone therapy.
Efficacy
Confirmed ORR by ICR was 62.0% (95% CI, 54.5–69.0), with 54.9% and 7.1% of patients achieving partial and complete responses, respectively (Table S1). Median OS (mOS) was 29.1 months (95% CI, 24.6–36.1), and 51.6% of patients had OS events (Fig 2A). Median PFS was 19.4 months (95% CI, 14.1–25.0), and 41.3% of patients had PFS events (Fig 2B).
Fig 2.

Efficacy analyses showing (A) overall survival, (B) progression-free survival, and (C) best percentage change from baseline in target lesions in DESTINY-Breast01 (N = 184). DCO March 26, 2021. Yellow line denotes progressive disease and green line denotes partial response. Blue line represents overall duration of response probability and gray lines represent 95% CI upper and lower limits.
aBy independent central review. A total of 169 patients had both baseline and postbaseline target lesion assessments by independent central review and were included in this analysis. DCO, data cutoff; OS, overall survival; PFS, progression-free survival.
Most patients’ tumors reduced in size following T-DXd treatment (Fig 2C). Median confirmed DoR was 18.2 months (95% CI, 15.0 months–not evaluable; Fig 3), and median TTR was 1.6 months (95% CI, 1.4–2.7).
Fig 3.

Duration of Response. DCO March 26, 2021. Blue line represents overall DoR probability and gray lines represent 95% CI upper and lower limits.
CR, complete response; DCO, data cutoff; DoR, duration of response; NE, not evaluable; PR, partial response.
Safety
As of March 26, 2021, investigator-reported drug-related TEAEs had occurred in 99.5% of patients; 53.8% of those TEAEs were grade ≥3 (Table 2). Most common TEAEs were generally gastrointestinal or hematologic (Table S2). Cumulative drug-related TEAEs associated with drug discontinuation, dose reduction, or dose interruption were reported in 33 (17.9%), 43 (23.4%), and 60 patients (32.6%), respectively. Drug-related TEAEs associated with death were reported by investigators in 3 patients (1.6%); 2 deaths were due to pneumonitis, and 1 death was due to respiratory failure. No new cases of left ventricular dysfunction were reported.
Table 2.
Overall Safety of T-DXd
| Type of Adverse Event,a n (%) | T-DXd 5.4 mg/kg N = 184 |
|---|---|
|
| |
| Any grade TEAEs | 183 (99.5) |
| Drug-related | 183 (99.5) |
|
| |
| Grade ≥3 TEAEs | 116 (63.0) |
| Drug-related | 99 (53.8) |
|
| |
| TEAEs associated with drug discontinuation | 35 (19.0) |
| Drug-related | 33 (17.9) |
|
| |
| TEAEs associated with dose reduction | 46 (25.0) |
| Drug-related | 43 (23.4) |
|
| |
| TEAEs associated with dose interruption | 77 (41.8) |
| Drug-related | 60 (32.8) |
|
| |
| TEAEs associated with deathb | 10 (5.4) |
| Drug-related | 3 (1.6) |
T-DXd, trastuzumab deruxtecan; TEAE, treatment-emergent adverse events.
Data cutoff March 26, 2021.
Relationship to study drug was determined by the treating investigator.
Each of the following TEAEs was associated with a fatal outcome: respiratory failure, acute respiratory failure, disease progression, general physical health deterioration, lymphangitis, pneumonia, pneumonitis, shock hemorrhagic; 1 patient had 2 TEAEs associated with death: acute kidney injury and acute hepatic failure.
Adjudicated drug-related ILD/pneumonitis was reported in 29 patients (15.8%) (Table S3), including 1 new case (grade 3) since the June 8, 2020 DCO, which was ongoing and managed according to ILD/pneumonitis management guidelines; the patient was withdrawn from treatment.5 The outcome of the patient with the grade 3 ILD event was reported as recovered/resolved. A total of 4 patients received T-DXd retreatment after recovering from grade 1 ILD; 3 of these patients did not experience recurrence of ILD and 1 patient had recurrence of grade 1 ILD, which was reported as recovered. 5 deaths (2.7%) were attributed to drug-related ILD/pneumonitis by an independent adjudication committee.
Discussion
In this update of DESTINY-Breast01, mOS was 4.5 months longer (29.1 months; March 26, 2021) than the previous DCO (24.6 months; June 8, 2020), demonstrating durable antitumor activity and prolonged survival with T-DXd in pretreated patients with HER2-positive mBC. ORR, DoR, and PFS were consistent with previous DCOs.1, 5 The results of this study support the use of T-DXd in heavily pretreated patients with HER2-positive mBC.
The use of T-DXd in patients resistant or refractory to T-DM1 is further supported by results from the phase 3 DESTINY-Breast02 trial, in which T-DXd demonstrated superiority to conventional chemotherapy-based treatment with a mOS of 39.2 months compared to 26.5 months with treatment of physician’s choice (hazard ratio, 0.66 [95% CI, 0.50–0.86]; P = 0.0021) at a median follow-up of 21.5 months.6 Both DESTINY-Breast01 and DESTINY-Breast02 trials further support the clinical benefit of T-DXd after use of a previous antibody-drug conjugate (T-DM1). The results of these trials add to the body of evidence for T-DXd in the treatment of HER2-positive mBC following its approval for use as second-line or later therapy based on DESTINY-Breast03.3, 4
Other trials in patients with 2 or more prior lines of therapy have reported mOS between 21.6 and 24.7 months in patients with heavily pretreated HER2-positive mBC, however, cross-trial comparisons should be interpreted carefully given study differences.7–10 In the TH3RESA trial, mOS was 22.7 months with T-DM1 in patients with HER2-positive advanced breast cancer (BC) previously treated with trastuzumab and lapatinib (advanced setting), a taxane (any setting), and 2 or more HER2-directed regimens (advanced setting).7 In the HER2CLIMB trial, mOS with tucatinib or placebo plus trastuzumab and capecitabine in patients with HER2-positive mBC previously treated with trastuzumab, pertuzumab, and T-DM1 (any setting) was 24.7 and 19.2 months, respectively.8 Similarly, mOS was 21.6 months with chemotherapy plus margetuximab in the SOPHIA trial, compared with 19.8 months with chemotherapy plus trastuzumab in patients with progressive disease following 2 or more prior lines of HER2-targeted therapy and 1 to 3 lines of nonhormonal mBC therapy.9 In the NALA trial, mean OS with capecitabine plus neratinib or lapatinib was 24.0 and 22.2 months, respectively, in patients who had previously received 2 or more HER2-directed regimens.10
A confirmed ORR was achieved in 62% of patients in DESTINY-Breast01. This is supported by results from DESTINY-Breast02, in which a confirmed ORR was reported in 70% of patients in the T-DXd group versus 29% of patients in the treatment of physician’s choice group.6 Trials of other therapies have reported rates ranging from 14%–41%.9–12 However, cross-trial comparisons must be interpreted cautiously given differences in patient populations; TH3RESA, SOPHIA, and NALA enrolled patients with a median of 2–4 prior lines of HER2-targeted therapies, whereas DESTINY-Breast01 enrolled patients with a median of 6 prior lines of HER2-targeted therapies. HER2CLIMB enrolled a higher proportion of patients with active and nonactive brain metastases compared to the other trials.9, 10, 12 In addition, the proportion of patients with previous exposure to T-DM1 and pertuzumab therapies varied across these trials (100% and 100% [HER2CLIMB], 91% and 100% [SOPHIA], and 19%–36% and 7%–36% [NALA]).9, 10, 12
Safety results were consistent with the established safety profile of T-DXd in BC, with no new safety signals.1, 2, 5, 13 Two additional grade ≥3 drug-related TEAEs and 4 drug-related TEAEs leading to dose reductions were reported since June 8, 2020, with no new reports of drug discontinuation, dose interruption, or death due to drug-related TEAEs. As previously detailed, patients were proactively monitored for signs and symptoms of ILD and underwent diagnostic tests to rule out other potential etiologies. Cases of suspected or detected ILD/pneumonitis were managed with established ILD management guidelines, including supportive care and immediate steroid treatment, with dose modification of T-DXd as recommended.14 Most cases were grade 1/2,2 and there was 1 additional case of ILD/pneumonitis adjudicated as drug-related (grade 3) reported since June 8, 2020 (this patient was withdrawn from T-DXd). Most TEAEs, including ILD/pneumonitis cases, were observed within the first 12 months of T-DXd treatment, which was consistent with previous reports in this population.2, 5, 15
In conclusion, this updated analysis of DESTINY-Breast01 provides evidence of sustained antitumor activity with T-DXd in heavily pretreated patients with HER2-positive mBC. Further confirmation of the results observed in DESTINY-Breast01 are supported by DESTINY-Breast02.6
Supplementary Material
Highlights.
T-DXd was investigated in patients with HER2-positive mBC who had prior T-DM1 treatment in DESTINY-Breast01
Primary analysis showed a confirmed objective response rate of 60.9%; median overall survival was not reached
This updated analysis showed a confirmed objective response rate of 62.0% and median overall survival of 29.1 months
Safety was consistent with the primary analysis and the established safety profile of T-DXd
These updated results further support T-DXd for the treatment of patients with heavily pretreated HER2-positive mBC
Acknowledgements
This study was sponsored by Daiichi Sankyo Co, Ltd., and AstraZeneca. We thank the patients who participated in this study, as well as their families and caregivers. We also thank the staff and investigators at all the study sites. Under the guidance of the authors, assistance in medical writing and editorial support was provided by Selene Jarrett, PhD, and Cindy M. Rigby, PhD, of ApotheCom, and was funded by Daiichi Sankyo Co, Ltd.
Funding
This work was supported by Daiichi Sankyo Co, Ltd and AstraZeneca. Grant number is not applicable.
Disclosures
CS reports consulting or advisory roles for AstraZeneca, AX’Consulting, Byondis B.V., Daiichi Sankyo, Eisai, Exact Sciences, Exeter Pharma, F. Hoffmann-La Roche Ltd, ISSECAM, Medical Statistics Consulting, MediTech, Merck Sharp & Dohme, Novartis, Pfizer, Philips, Pierre Fabre, PintPharma, Puma, Roche Farma, Seagen, and Zymeworks and a leadership role for SOLTI.
SM reports consulting or advisory roles for AstraZeneca, Daiichi Sankyo, Genentech, GlaxoSmithKline, Macrogenics, Novartis, and Seagen; research funding from AstraZeneca (inst) and Daiichi Sankyo (inst); and honoraria for AstraZeneca, Daiichi Sankyo, Genentech, Macrogenics, Seagen.
IK reports consulting or advisory roles for AstraZeneca, Bristol Meyers Squibb, Daiichi Sankyo, Genentech, Macrogenics, Merck, Novartis, Roche, Seagen, and Taiho Oncology; research funding from Genentech (inst), Macrogenics (inst), Pfizer (inst), and Roche (inst); honoraria from AstraZeneca; and a leadership role with PureTech.
YHP reports consulting or advisory roles for AstraZeneca, Daiichi Sankyo, Eisai, Roche, MSD, Novartis, Pfizer, and Lilly; research funding from AstraZeneca, Gencurix, Novartis, Pfizer, and Roche; and honoraria from Pfizer, Roche, MSD, and Novartis.
S-BK reports consulting or advisory roles for AstraZeneca, Beigene, Dae Hwa Pharmaceutical Co, Ltd, Daiichi Sankyo, IUS Abxis, Lilly, and Novartis and research funding from DongKook Pharm (inst), Novartis (inst), and Sanofi-Aventis (inst).
KT has declared no conflicts of interest.
HI reports consulting or advisory roles for AstraZeneca, Chugai, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, and Sanofi and honoraria from AstraZeneca, Chugai, Daiichi Sankyo, Lilly, MSD, and Pfizer.
JT reports consulting or advisory roles for AstraZeneca and Daiichi Sankyo; research funding from Chugai (inst), Daiichi Sankyo (inst), Eisai (inst), Lilly (inst), Nihon Kayaku (inst), and Taiho (inst); honoraria from Chugai, Daiichi Sankyo, Eisai, Lilly, Kyowa Kirin, and Taiho; travel, accommodations, expenses from Chugai, Daiichi Sankyo, and Eisai; a leadership role with West Japan Oncology Group; and receipt of equipment, materials, drugs, medical writing, or other services from Daiichi Sankyo.
JS reports research funding from AstraZeneca (inst), Boehringer Ingelheim (inst), Lilly (inst), GSK (inst), MSD (inst), Novartis (inst), Pfizer (inst), Roche (inst), and Sanofi (inst) and stock for Daiichi Sankyo.
EM, YL, JC, and JS report employment and stock and other ownership interest with Daiichi
TY reports research funding from Chugai (inst), Kyowa Kirin (inst), Nippon Kayaku (inst), and Taiho (inst) and honoraria from AstraZeneca, Chugai, Daiichi Sankyo, MSD, Eisai, Lilly, Nippon Kayaku, Kyowa Kirin, Novartis, Pfizer Japan, Taiho.
Footnotes
Prior Presentation: This study was presented in part at the 2021 European Society for Medical Oncology (ESMO) congress [Saura CM, et al. Ann Oncol. 2021;32:S485–S486].
Data Sharing Statement
Anonymized individual participant data (IPD) on completed studies and applicable supporting clinical trial documents may be available upon request at https://vivli.org. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures. Daiichi Sankyo, Inc, will continue to protect the privacy of our company and our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo.
References
- 1.Modi S, Saura C, Yamashita T et al. Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd ) in HER2 positive metastatic breast cancer. Am Assoc Cancer Res. 2021; 81:Abstract PD3–06. [Google Scholar]
- 2.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. [DOI] [PubMed] [Google Scholar]
- 4.Rugo HS, Bianchini G, Cortes J, et al. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 2022;7(4):100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saura C Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): Updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol. 2021;32(suppl 5):S457–S515. Abstract 279P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André F, Hee Park Y, Kim S-B, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401(10390):1773–1785. [DOI] [PubMed] [Google Scholar]
- 7.Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–754. [DOI] [PubMed] [Google Scholar]
- 8.Curigliano G, Mueller V, Borges V, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–329. [DOI] [PubMed] [Google Scholar]
- 9.Rugo HS, Im SA, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-Positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. [DOI] [PubMed] [Google Scholar]
- 12.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. [DOI] [PubMed] [Google Scholar]
- 14.Modi S, Saura C, Yamashita T et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020; 382 (7): 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4):100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data (IPD) on completed studies and applicable supporting clinical trial documents may be available upon request at https://vivli.org. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures. Daiichi Sankyo, Inc, will continue to protect the privacy of our company and our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo.


