Abstract
Ubiquitin-conjugation enzyme E2C (UBE2C) is a crucial component of the ubiquitin-proteasome system that is involved in numerous cancers. In this study, we find that UBE2C expression is significantly increased in mouse embryos, a critical stage during skeletal muscle development. We further investigate the function of UBE2C in myogenesis. Knockdown of UBE2C inhibits C2C12 cell differentiation and decreases the expressions of MyoG and MyHC, while overexpression of UBE2C promotes C2C12 cell differentiation. Additionally, knockdown of UBE2C, specifically in the tibialis anterior muscle (TA), severely impedes muscle regeneration in vivo. Mechanistically, we show that UBE2C knockdown reduces the level of phosphorylated protein kinase B (p-Akt) and promotes the degradation of Akt. These findings suggest that UBE2C plays a critical role in myoblast differentiation and muscle regeneration and that UBE2C regulates myogenesis through the Akt signaling pathway.
Keywords: UBE2C, muscle, myoblast differentiation, Akt
Introduction
Skeletal muscle is a highly complex and heterogeneous tissue that has a broad range of functions. Among these processes, myogenesis and skeletal muscle regeneration are two processes involved in muscle generation, including myoblast proliferation, differentiation, and fusion [ 1, 2] . These coordinated events depend on highly complex molecular regulatory networks [3]. The myogenic regulatory factors (MRFs), including myogenic differentiation 1 (MyoD), myogenic factor 5 (Myf5), myogenin (MyoG), and myogenic regulator factor 4 (MRF4), play well-defined roles in muscle development and regeneration [ 4, 5] . In addition, numerous key genes that participate in muscle development have been described in recent years [ 6– 9] . Moreover, signaling pathways are the key link in the regulatory network of muscle differentiation [ 10, 11] . The PI3K/Akt/mTOR pathway was confirmed to be a critical regulator of skeletal muscle differentiation and growth [ 12, 13] . Akt contributes to muscle hypertrophy and myofiber growth in adult muscle without activating the proliferation of muscle satellite cells [14] and stimulates myogenesis by promoting the expressions of MyoD [15] and MyoG [16].
Ubiquitination is an important type of posttranslational modification of proteins that requires ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin-ligase enzymes (E3), and through three-step sequential actions, it transfers the activated ubiquitin from the E2 to the substrate [ 17, 18] . The ubiquitin-conjugating enzyme E2C is a ubiquitin-binding enzyme that accepts ubiquitin from E1 and transfers it to a substrate associated with E3. Previous studies confirmed that UBE2C plays an important role in various malignancies [ 19, 20] and affects cancers through the PI3K/Akt/mTOR signaling pathways [ 21– 23] . However, little is known about the function and molecular mechanisms of UBE2C in skeletal muscle.
In the present study, our findings indicated that knockdown of UBE2C impedes myoblast differentiation and muscle regeneration. Specifically, inhibiting UBE2C in C2C12 cells results in reduced level of phosphorylated Akt (p-Akt) and accelerated Akt degradation. These observations provide compelling evidence for the critical involvement of UBE2C in the regulatory mechanisms underlying myogenesis by regulating the Akt signaling pathway.
Materials and Methods
Cell culture
C2C12 myoblasts were purchased from the American Type Culture Collection (ATCC, Manassas, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning, New York, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 U/mL growth medium (GM). To induce differentiation, the cells were switched to DMEM supplemented with 2% horse serum (differentiation medium, DM) after they reached 100% confluence. All cells were cultured in a 37°C incubator with 5% CO 2.
Animals
Eight-week-old female C57BL/6 mice were housed under SPF conditions with a 12/12-h dark/light cycle and ad libitum access to food and water. All the experimental procedures were approved by the Animal Care and Use Committee of Guangdong Province and were carried out according to ethical standards. The approval ID is SYSU-IACUC-2020-B0614.
Western blot analysis
Total proteins were extracted from C2C12 cells or mouse tissues using RIPA buffer supplemented with 1 mM PMSF (GenStar, Beijing, China). The collected proteins were subjected to 10% or 12% SDS-PAGE and transferred to 0.22-μm or 0.45-μm PVDF membranes (Millipore, Billerica, USA), which were blocked with 3% bovine serum albumin (BSA) in 0.1% TBS-Tween for 1 h at room temperature and then incubated with primary antibodies overnight at 4°C. After being incubated with appropriate HRP-conjugated secondary antibodies, the blots were visualized with an enhanced chemiluminescence (ECL) detection kit (FDbio, Hangzhou, China). The antibodies used are described in Supplementary Table S1.
Cardiotoxin (CTX) injury and intramuscular transfection of siRNAs
The intramuscular transfection of siRNAs was performed using an Entranster- in vivo kit (Engreen, Beijing, China). The sequences of the siRNAs and the siRNA transfection system used in vivo are shown in Supplementary Tables S2 and S3. The hindlimbs of six 8-week-old female mice were cleaned with 75% alcohol. Then, the mixture containing si-UBE2C was injected into the left TA muscles, and the mixture containing NC was injected into the right TA muscles as a negative control.
CTX (Sigma, St Louis, USA) was dissolved in sterile saline to a final concentration of 20 mM. One day after siRNA injection, the hindlimbs of the mice were cleaned with alcohol, and the tibialis anterior (TA) muscles were intramuscularly injected with 50 μL of CTX via a hypodermic syringe. To maintain the long-term effects of siRNA, si-UBE2C or NC was injected into the TA muscle every 2 days. Regenerating TA muscles were isolated at 3, 7, and 14 days after CTX injection.
RNA extraction and real-time quantitative PCR
Total RNA was extracted from C2C12 cells and mouse tissues with Trizol reagent (Invitrogen Carlsbad, USA) and reverse transcribed to cDNA using StarScript II First-strand cDNA Synthesis Mix (GenStar). Real-time quantitative PCR was performed using 2× RealStar Green Power Mixture (GenStar) on a QuantStudio 7 Flex (ABI, Foster City, USA). GAPDH was used as an internal control for normalization. The primers used for qPCR are listed in Supplementary Table S4.
Transfection of plasmids and siRNA
Three siRNAs targeting UBE2C (si-UBE2C-1, si-UBE2C-2, and si-UBE2C-3) were purchased from Invitrogen. The sequences of all siRNAs are listed in Supplementary Table S5. For the UBE2C overexpression vector, the coding sequences (CDSs) of the mouse UBE2C gene were inserted into the pcDNA3.1 vector (Invitrogen). C2C12 cells were seeded into 12-well plates 24 h before treatment and then transfected with siRNAs using Lipofectamine 3000 (Invitrogen) or with overexpression plasmids using Y40 (Invitrogen).
Immunofluorescence (IF) staining
C2C12 cells were fixed with 4% paraformaldehyde for 10 min, permeabilized in 0.5% Triton X-100 for 15 min, blocked in 3% BSA/PBST for 1 h, incubated with indicated primary antibodies and the corresponding fluorescein-linked secondary antibody listed in Supplementary Table S1, and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000 in PBS). Images were captured with a fluorescence reverse microscope (Nikon, Tokyo, Japan). The differentiation rate was calculated as the percentage of nuclei in MyHC-positive cells. Nine images of immunofluorescence from three replicates (three images from each replicate) were randomly selected for analysis in each group.
Use of SC79 and LY294002
C2C12 cells were treated with siRNA or overexpressed plasmid, and cultured on growth medium. The 10 μM SC79 (Selleck, Housto, USA) and 20 μM LY294002 (Selleck) were added to the interfering or overexpressed cells 12 h later, respectively. After cells reached 100% confluence the cells were switched to differentiation medium to induce differentiation.
5-Ethynyl-2′-deoxyuridine (EDU) assay
The EdU assay was performed using an EDU kit (RiboBio, Guangzhou, China). C2C12 cells cultured in GM for 36 h were treated with 50 mM EdU for 2 h. Then, the C2C12 cells were fixed, permeabilized, and stained with Apollo 567 (RiboBio) according to the manufacturer’s protocol. The nuclei were stained with DAPI. Images were captured with a fluorescence reverse microscope (Nikon).
Hematoxylin and eosin (H&E) staining
TA muscles were fixed in 4% formalin for 24 h, dehydrated with graded ethanol, embedded in paraffin, and sectioned at 4 μm. Muscle sections were dewaxed using xylene and then rehydrated with graded ethanol and double distilled water. TA muscle paraffin sections were stained using an H&E staining kit (Xiuwei, Guangzhou, China) according to the manufacturer’s instructions. Images were captured with a confocal microscope (Leica, Wetzlar, Germany).
Statistical analysis
Data are presented as the mean±SEM, and the significance of differences was analyzed using the unpaired two-tailed Student’s t test. P<0.05 was considered to indicate statistical significance.
Results
The expression of UBE2C is downregulated during myogenesis
To determine the role of UBE2C in myogenesis, the expression profiles of muscle samples from the longissimus dorsi at five developmental stages were analyzed. qPCR analysis revealed that the expression level of UBE2C was greater during the embryonic stage than that in the postnatal stage and displayed a trend similar to that of Myf5 ( Figure 1A). Additionally, both the mRNA and protein levels of UBE2C were gradually decreased from proliferation to differentiation in C2C12 cells ( Figure 1B,C). These observations collectively suggested that the expression pattern of UBE2C may be involved in myogenesis.
Figure 1 .
Expression pattern of UBE2C
(A) The mRNA expressions of UBE2C and myogenic markers were measured in the longissimus dorsi of mice at five developmental stages. GAPDH was used as an internal control for normalization. E denotes the embryonic period, while P indicates post-birth. (B) The mRNA levels of UBE2C and myogenic markers were evaluated at five time points during C2C12 differentiation. (C) The protein expression levels of UBE2C and myogenic markers were evaluated at five time points during C2C12 differentiation (left). Grayscale scanning for quantifying protein expression (right). The cells were cultured in growth medium (GM) at sub-confluent densities. When the cells reached 100% confluence, it was defined as day 0 (D0), and the growth medium was changed to differentiation medium. Data are presented as the mean±SEM ( n=3 per group).
UBE2C is essential for C2C12 differentiation
Given that UBE2C is highly expressed in embryonic longissimus dorsi and proliferating cells ( Figure 1), UBE2C may affect the proliferation of myoblasts. However, the real-time cell proliferation assay, EdU labelling, and Ki67 immunofluorescence staining all indicated no significant difference in proliferation between C2C12 cells treated with siRNA and control cells ( Supplementary Figure S1C–E). Notably, we observed an obvious decrease in the expression of Myf5, the myogenic regulatory factor expressed at the earliest stage [24], after UBE2C knockdown ( Supplementary Figure S1A,B), suggesting a potential role for UBE2C in myogenesis.
Meanwhile, decreased mRNA and protein levels of MyoG and MyHC were observed ( Figure 2A,B). Immunofluorescence staining for myoG and MyHC revealed a reduction in the number of myoG +cells and myotubes, respectively ( Figure 2C,D). Conversely, when UBE2C was overexpressed in C2C12 cells, the expression levels of MyoG and MyHC increased ( Figure 2E). Additionally, UBE2C overexpression led to enhanced C2C12 cell differentiation, as evidenced by a significant increase in the number of multinucleated myotubes ( Figure 2F). Collectively, these results indicated that UBE2C promotes myoblast differentiation.
Figure 2 .
UBE2C is essential for C2C12 differentiation
(A) The mRNA expression levels of UBE2C, MyoD, MyoG, and MyHC were quantified using qPCR one day after the induction of differentiation. (B) Western blot analysis was used to determine the protein expression levels of UBE2C, MyoG, and MyHC (left panel). Greyscale scanning of the western blots is shown in the right panel, with GAPDH serving as the loading control. (C) Immunofluorescence staining for MyoG was performed on C2C12 cells one day after induction of differentiation (left panel), and the percentage of MyoG-positive cells was calculated (right panel). (E) Western blot analysis was utilized to assess the protein expression levels of UBE2C, MyoG, and MyHC in cells overexpressing UBE2C (left panel). Greyscale scanning of the western blots is shown in the right panel. Immunofluorescence staining for MyHC was conducted three days after induction of differentiation in cells with UBE2C knockdown (D) or overexpression (F). The nuclei were counterstained with DAPI. Scale bar: 200 μm. Data are presented as the mean±SEM, n=3 per group. * P<0.05, ** P<0.01, *** P<0.001, and ns indicates no significant difference.
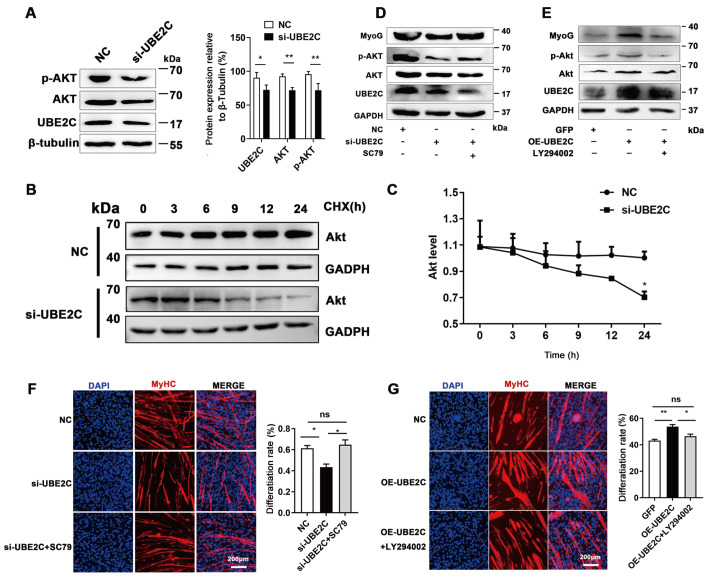
UBE2C promotes myoblast differentiation by regulating Akt phosphorylation and degradation
The PI3K/Akt signaling pathway is widely recognized as one of the pivotal pathways involved in myogenesis [ 25, 26] . In this study, inhibiting UBE2C expression led to a decrease in Akt expression and phosphorylation ( Figure 3A). Furthermore, we evaluated the protein stability of Akt using cycloheximide (CHX) treatment. The results demonstrated that UBE2C inhibition promoted the degradation of the Akt protein in C2C12 cells ( Figure 3B,C), suggesting that UBE2C knockdown reduced Akt protein stability.
Figure 3 .
UBE2C regulates Akt phosphorylation and degradation
(A) The protein levels of Akt and p-Akt were analyzed by western blot analysis. (B) C2C12 cells were transfected with NC or si-UBE2C, followed by incubation with CHX. The cells were harvested at the designated time points for western blot analysis to evaluate Akt protein levels. (C) Greyscale scanning of the western blots was used to quantify the Akt protein levels. (D) C2C12 cells were transfected with siRNA and treated with SC79. The expression levels of MyoG, MyHC, Akt, and p-Akt were detected by western blot analysis. (E) C2C12 cells were transfected with plasmids to overexpress UBE2C and treated with LY294002. The expression levels of MyoG, MyHC, Akt, and p-Akt were analyzed by western blot analysis. (F,G) Immunofluorescence staining was performed on C2C12 cells treated as described in (D,E), with detection specifically targeting MyHC. Scale bar: 200 μm. Data are presented as the mean±SEM, n=3 per group. * P<0.05, and ** P<0.01. ns indicates no significant difference.
To further investigate whether UBE2C regulates myoblast differentiation through the modulation of Akt phosphorylation, C2C12 cells were treated with SC79, an agonist of the PI3K/Akt signaling pathway, and LY294002, a PI3K signaling inhibitor. The results showed that SC79 restored MyoG expression and rescued the defects in myoblast differentiation caused by UBE2C knockdown ( Figure 3D,F). Conversely, the promoting effects of UBE2C overexpression on MyoG expression and myoblast differentiation were abolished by LY294002 treatment ( Figure 3E,G). These findings collectively indicate that Akt phosphorylation facilitates myoblast differentiation and suggest that UBE2C knockdown inhibits myogenic differentiation by impairing Akt phosphorylation and enhancing Akt degradation.
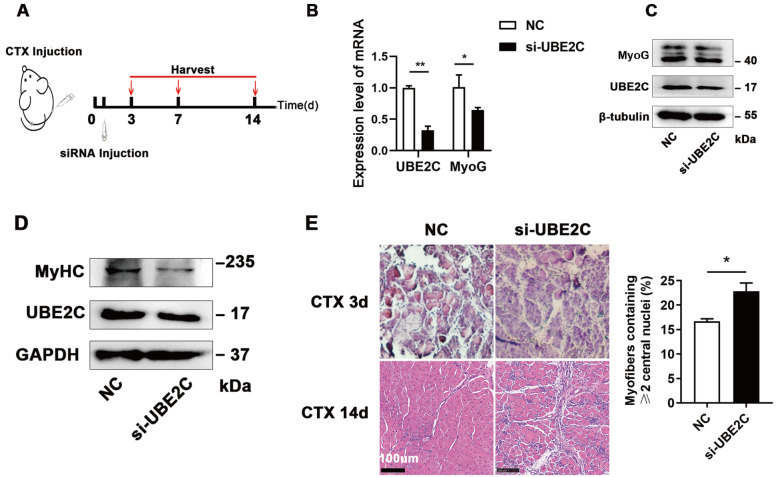
UBE2C knockdown blocks muscle regeneration
To investigate whether the functions of UBE2C in C2C12 cells can be repeated in vivo, we utilized a CTX-mediated muscle regeneration model. Si-UBE2C or negative control (NC) was injected into TA muscles every 2 days to maintain UBE2C knockdown efficiency. Subsequently, the TA muscles were harvested on days 3, 7, and 14 ( Figure 4A). The expression of MyoG was significantly lower in the TA muscles of the si-UBE2C-treated group than in those of the NC group on day 7 ( Figure 4B,C), which is consistent with the in vitro experimental results ( Figure 2B). Additionally, UBE2C knockdown resulted in a reduction in MyHC protein level on day 14 ( Figure 4D). H&E staining revealed impeded formation of myofibers on day 14 in the UBE2C inhibition group ( Figure 4E). These findings align with the in vitro observations, indicating that UBE2C knockdown inhibits MyoG expression and myoblast differentiation, consequently leading to delayed muscle tissue repair and regeneration.
Figure 4 .
UBE2C knockdown restrains skeletal muscle regeneration
(A) The mouse model of TA tissue injury is illustrated in the schematic diagram. (B,C) After 7 days, the mRNA (B) and protein (C) expression levels of the given genes were evaluated in mice subjected to TA treatment with CTX. (D) The protein expression level of MyHC was assessed after 14 days of CTX treatment. (E) H&E staining was conducted on TA samples harvested at 3 d and 14 d after CTX treatment (left panel), and the proportion of muscle fibers with two or more central nuclei were quantified (right panel). Scale bar: 100 μm. Data are presented as the mean±SEM, n=3 per group. * P<0.05, and ** P<0.01.
Discussion
Skeletal muscle originates from the embryonic paraxial mesoderm [ 2, 4] . Myofiber formation in the embryonic stage is crucial for the growth of limbs and trunks [27] and the muscle mass of livestock and poultry [28]. In this study, we observed that the expression of UBE2C in embryonic skeletal muscle was greater than that in postnatal skeletal muscle in mice. Furthermore, UBE2C was found to be expressed at a higher level during proliferation rather than terminal differentiation. These findings suggest a potential role for UBE2C in myoblast proliferation. However, real-time cell proliferation assays, EdU labelling and Ki67 immunofluorescence staining all showed that knockdown of UBE2C did not affect proliferation, although there was a significant decrease in Myf5 expression. This prompted us to explore the function of UBE2C in differentiation.
Our results verified that UBE2C is essential for myoblast differentiation. UBE2C knockdown decreased the expression of MyoG and suppressed myoblast differentiation in C2C12 cells, while UBE2C overexpression had the opposite effect. Consistently, intramuscular injection of si-UBE2C suppressed MyoG expression and blocked myoblast differentiation during CTX-mediated muscle regeneration.
The PI3K/Akt/mTOR signaling pathway is a well-established pathway involved in various cellular processes, including apoptosis, proliferation, differentiation, and metabolism [29]. In this study, we observed that knockdown of UBE2C resulted in a decrease in the expression levels of both total Akt and phosphorylated Akt. After UBE2C was overexpressed in C2C12 cells, the total and phosphorylated Akt levels increased. Previous studies have extensively reported the involvement of UBE2C in cancer through the Akt/mTOR signalling pathway. These studies consistently demonstrated that UBE2C knockdown is accompanied by inhibition of p-Akt [ 21– 23] . The precise activation and degradation of Akt play important roles in maintaining diverse biological responses [ 30, 31] . Two distinct ubiquitination systems have been reported to regulate Akt signalling [32]. Wei et al. [33] reported that dephosphorylation of p-Akt could accelerate its degradation through the ubiquitin-proteasome pathway. In our study, inhibition of UBE2C facilitated the protein degradation of Akt. Based on these findings, we propose that UBE2C may regulate myogenesis by modulating the stability and phosphorylation of Akt.
In conclusion, our study provides novel evidence supporting the crucial role of UBE2C in myoblast differentiation and skeletal muscle regeneration ( Figure 5). Specifically, we elucidated that UBE2C enhances Akt phosphorylation and stabilizes Akt, thereby promoting C2C12 cell differentiation.
Figure 5 .
Schematic diagram of UBE2C regulating myogenesis
UBE2C promotes myoblast differentiation and muscle regeneration by regulating Akt phosphorylation and stability.
Supporting information
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinica online.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No. 32072697), the Rural Revitalization Special Project of Guangdong Province (No. 2021-440000-24010202-8887), the Laboratory of Lingnan Modern Agriculture Project (No. NZ2021006), and the Earmarked Fund for CARS-35.
References
- 1.Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol. . 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf F, Brand-Saberi B. Myogenesis and muscle regeneration. Histochem Cell Biol. . 2012;138:187–199. doi: 10.1007/s00418-012-0972-x. [DOI] [PubMed] [Google Scholar]
- 3.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. . 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 4.Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. . 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez-Hernandez JM, Garcia-Gonzalez EG, Brun CE, Rudnicki MA. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. . 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai S, Zhu Q, Guo C, Yuan R, Zhang X, Nie Y, Chen L, et al. MLL1 promotes myogenesis by epigenetically regulating Myf5 . Cell Prolif. . 2020;53:e12744. doi: 10.1111/cpr.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie Y, Cai S, Yuan R, Ding S, Zhang X, Chen L, Chen Y, et al. Zfp422 promotes skeletal muscle differentiation by regulating EphA7 to induce appropriate myoblast apoptosis. Cell Death Differ. . 2020;27:1644–1659. doi: 10.1038/s41418-019-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Q, Liang F, Cai S, Luo X, Duo T, Liang Z, He Z, et al. KDM4A regulates myogenesis by demethylating H3K9me3 of myogenic regulatory factors. Cell Death Dis. . 2021;12:514. doi: 10.1038/s41419-021-03799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai S, Wang X, Xu R, Liang Z, Zhu Q, Chen M, Lin Z, et al. KLF4 regulates skeletal muscle development and regeneration by directly targeting P57 and myomixer. Cell Death Dis. . 2023;14:612. doi: 10.1038/s41419-023-06136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. . 2005;16:612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. . 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Jiang B, Ensign W Y, Vogt P K, Han J. Myogenic differentiation requires signalling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell Signal. . 2000;12:751–757. doi: 10.1016/S0898-6568(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 13.Gardner S, Anguiano M, Rotwein P. Defining akt actions in muscle differentiation. Am J Physiol Cell Physiol. . 2012;303:C1292–C1300. doi: 10.1152/ajpcell.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, et al. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. . 2009;23:3896–3905. doi: 10.1096/fj.09-131870. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Liu L, Yang XJ, Wu Z. Akt binds prohibitin 2 and relieves its repression of MyoD and muscle differentiation. J Cell Sci. . 2004;117:3021–3029. doi: 10.1242/jcs.01142. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem. . 2000;275:36750–36757. doi: 10.1074/jbc.M005030200. [DOI] [PubMed] [Google Scholar]
- 17.Wijk SJL, Timmers HTM. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. . 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- 18.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. . 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 19.Xie C, Powell C, Yao M, Wu J, Dong Q. Ubiquitin-conjugating enzyme E2C: a potential cancer biomarker. Int J Biochem Cell Biol. . 2014;47:113–117. doi: 10.1016/j.biocel.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Kariri Y, Toss MS, Alsaleem M, Elsharawy KA, Joseph C, Mongan NP, Green AR, et al. Ubiquitin-conjugating enzyme 2C (UBE2C) is a poor prognostic biomarker in invasive breast cancer. Breast Cancer Res Treat. . 2022;192:529–539. doi: 10.1007/s10549-022-06531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Song Y, Liu X, Wang Q, Wang Y, Li L, Kang C, et al. UBE2C induces EMT through Wnt/β-catenin and PI3K/Akt signaling pathways by regulating phosphorylation levels of aurora-A. Int J Oncol. . 2017;50:1116–1126. doi: 10.3892/ijo.2017.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu ZN, Song J, Sun TH, Sun G. UBE2C affects breast cancer proliferation through the AKT/mTOR signaling pathway. Chin Med J. . 2021;134:2465–2474. doi: 10.1097/CM9.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao JZ, Nie G, Hu H, Zhang X, Ni CM, Huang ZP, Qiao GL, et al. UBE2C promotes the progression of pancreatic cancer and glycolytic activity via EGFR stabilization-mediated PI3K-Akt pathway activation. J Gastrointest Oncol. . 2022;13:1444–1453. doi: 10.21037/jgo-22-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. Early expression of the myogenic regulatory gene, myf-5 , in precursor cells of skeletal muscle in the mouse embryo . Development. . 1991;111:1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- 25.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo . Nat Cell Biol. . 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 26.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. . 2004;14:395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 27.Bismuth K, Relaix F. Genetic regulation of skeletal muscle development. Exp Cell Res. . 2010;316:3081–3086. doi: 10.1016/j.yexcr.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Ashmore CR, Addis PB, Doerr L. Development of muscle fibers in the fetal pig. JAnim Sci. . 1973;36:1088–1093. doi: 10.2527/jas1973.3661088x. [DOI] [PubMed] [Google Scholar]
- 29.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. . 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suizu F, Hiramuki Y, Okumura F, Matsuda M, Okumura AJ, Hirata N, Narita M, et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated akt. Dev Cell. . 2009;17:800–810. doi: 10.1016/j.devcel.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Su CH, Lan KH, Li CP, Chao Y, Lin HC, Lee SD, Lee WP. Phosphorylation accelerates geldanamycin-induced Akt degradation. Arch Biochem Biophys. . 2013;536:6–11. doi: 10.1016/j.abb.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi M, Hirata N, Suizu F. The links between AKT and two intracellular proteolytic cascades: ubiquitination and autophagy. Biochim Biophys Acta Rev Cancer. . 2014;1846:342–352. doi: 10.1016/j.bbcan.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Zhou J, Yu H, Jin X. AKT phosphorylation sites of Ser473 and Thr308 regulate AKT degradation. Biosci Biotechnol Biochem. . 2019;83:429–435. doi: 10.1080/09168451.2018.1549974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.