Abstract
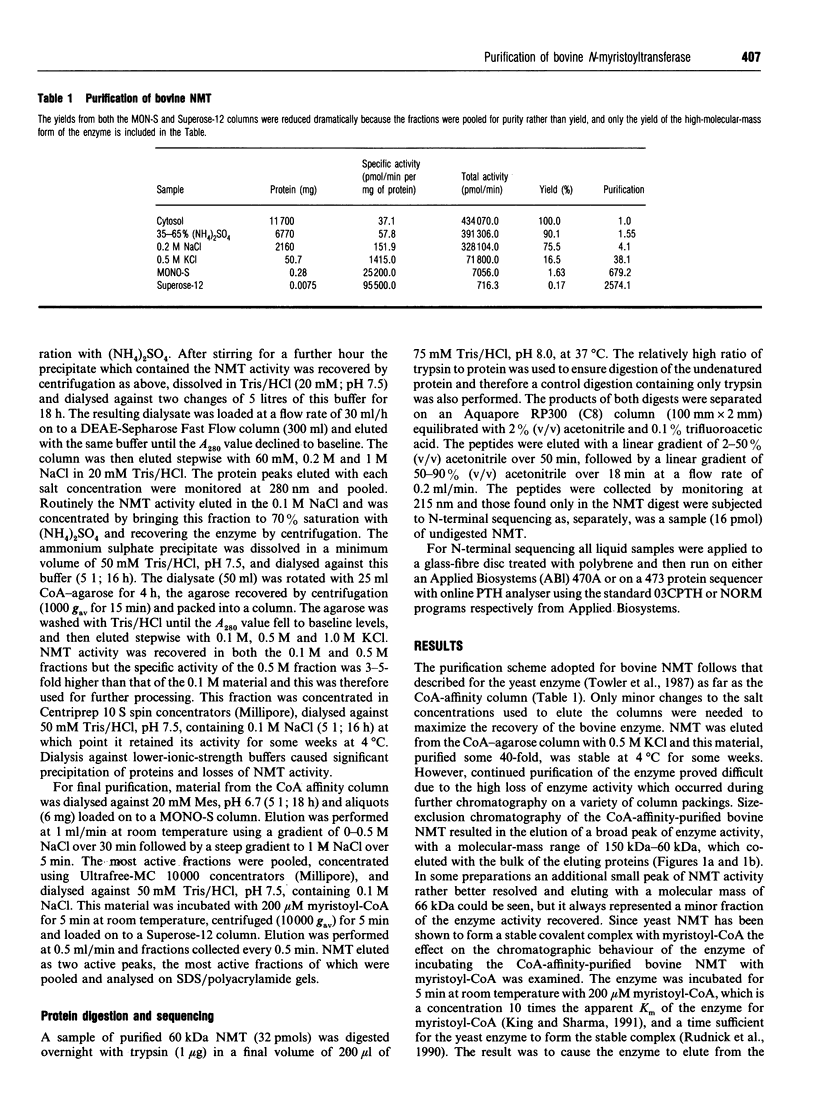
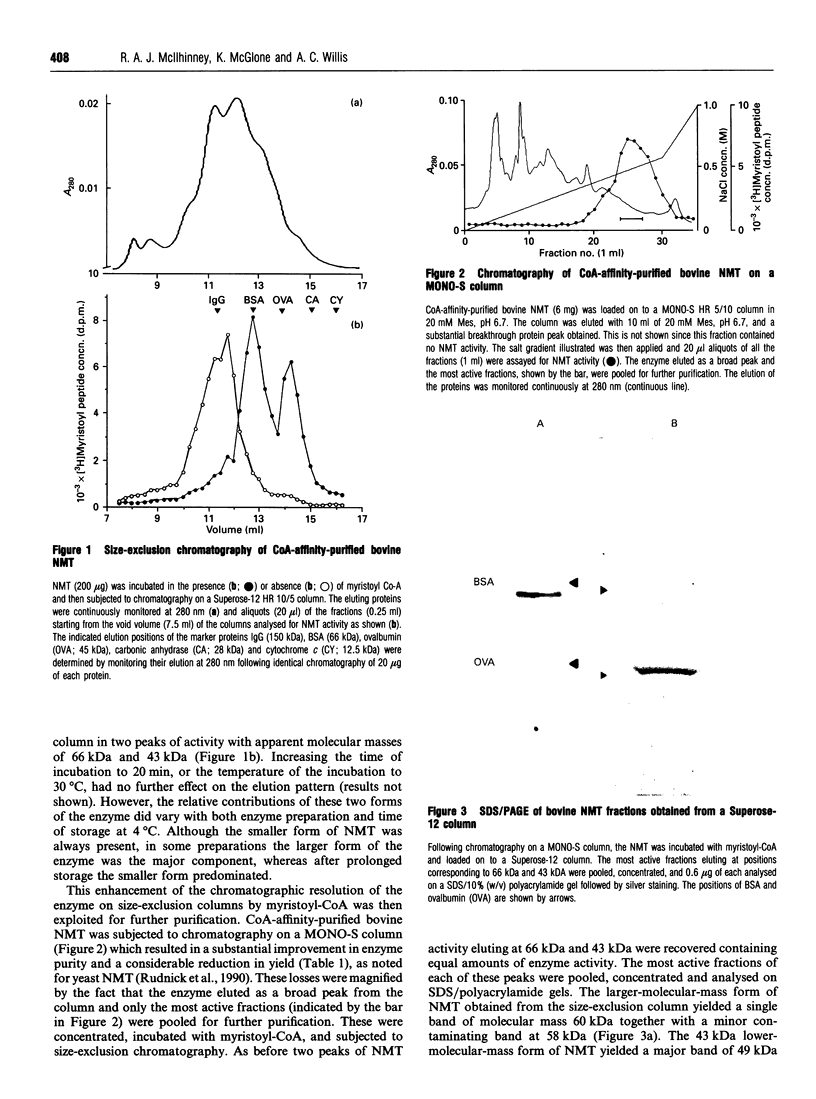
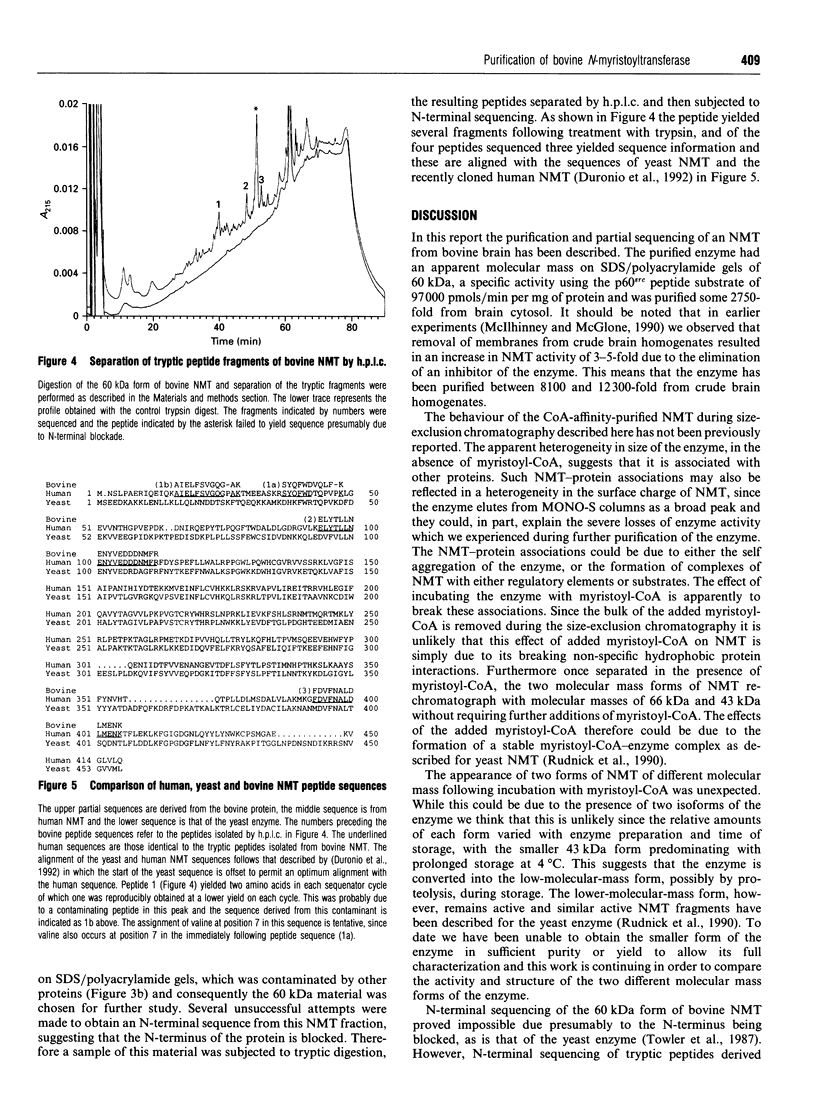
The enzyme myristoyl-CoA:protein N-myristoyltransferase (NMT; EC 2.3.1.97) catalyses the transfer of myristic acid to the N-terminal glycine residue of cell and viral proteins. In this report the purification and partial sequencing of this enzyme from bovine brain is described. Using a combination of ammonium sulphate precipitation, chromatography on DEAE-Sepharose and affinity chromatography on CoA-agarose the enzyme was purified some 40-fold. Size-exclusion chromatography of this material in the presence of myristoyl-CoA yielded two peaks of enzyme activity with apparent molecular masses of 66 kDa and 43 kDa. Chromatography of the CoA-affinity-purified material on MONO-S followed by size-exclusion chromatography in the presence of myristoyl-CoA resulted in the isolation of the large form of the enzyme purified 3000-fold. Analysis by SDS/PAGE of this material showed a major 60 kDa silver-stained band. Similar analysis of the 43 kDa enzyme fraction from the same separation showed that this fraction contained several proteins including a major component with an apparent molecular mass of 49 kDa. Attempts at N-terminal sequencing of the 66 kDa form of the enzyme were unsuccessful and therefore this material was digested with trypsin and the resulting peptides separated by reverse-phase h.p.l.c. N-terminal protein sequencing of these peptides yielded sequences which show sequence similarity to those of yeast N-myristoyl-transferase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Heuckeroth R. O., Kimata J. T., Ratner L., Gordon J. I. Replication of human immunodeficiency virus 1 and Moloney murine leukemia virus is inhibited by different heteroatom-containing analogs of myristic acid. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8655–8659. doi: 10.1073/pnas.86.22.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Clark B., Desselberger U. Myristylation of rotavirus proteins. J Gen Virol. 1988 Oct;69(Pt 10):2681–2686. doi: 10.1099/0022-1317-69-10-2681. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio R. J., Reed S. I., Gordon J. I. Mutations of human myristoyl-CoA:protein N-myristoyltransferase cause temperature-sensitive myristic acid auxotrophy in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4129–4133. doi: 10.1073/pnas.89.9.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Gordon J. I., Blackshear P. J. Myristoylated and nonmyristoylated forms of a protein are phosphorylated by protein kinase C. Science. 1989 Oct 27;246(4929):503–506. doi: 10.1126/science.2814478. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. J., Sharma R. K. N-myristoyl transferase assay using phosphocellulose paper binding. Anal Biochem. 1991 Dec;199(2):149–153. doi: 10.1016/0003-2697(91)90082-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marc D., Drugeon G., Haenni A. L., Girard M., van der Werf S. Role of myristoylation of poliovirus capsid protein VP4 as determined by site-directed mutagenesis of its N-terminal sequence. EMBO J. 1989 Sep;8(9):2661–2668. doi: 10.1002/j.1460-2075.1989.tb08406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlhinney R. A., McGlone K. A simplified assay for the enzyme responsible for the attachment of myristic acid to the N-terminal glycine residue of proteins, myristoyl-CoA: glycylpeptide N-myristoyltransferase. Biochem J. 1989 Oct 15;263(2):387–391. doi: 10.1042/bj2630387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlhinney R. A., McGlone K. Characterisation of a myristoyl CoA:glycylpeptide N-myristoyl transferase activity in rat brain: subcellular and regional distribution. J Neurochem. 1990 Jan;54(1):110–117. doi: 10.1111/j.1471-4159.1990.tb13289.x. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A. The fats of life: the importance and function of protein acylation. Trends Biochem Sci. 1990 Oct;15(10):387–391. doi: 10.1016/0968-0004(90)90237-6. [DOI] [PubMed] [Google Scholar]
- Molnar E., Varga S., Martonosi A. Differences in the susceptibility of various cation transport ATPases to vanadate-catalyzed photocleavage. Biochim Biophys Acta. 1991 Sep 10;1068(1):17–26. doi: 10.1016/0005-2736(91)90056-e. [DOI] [PubMed] [Google Scholar]
- Moscufo N., Simons J., Chow M. Myristoylation is important at multiple stages in poliovirus assembly. J Virol. 1991 May;65(5):2372–2380. doi: 10.1128/jvi.65.5.2372-2380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick D. A., McWherter C. A., Adams S. P., Ropson I. J., Duronio R. J., Gordon J. I. Structural and functional studies of Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase produced in Escherichia coli. Evidence for an acyl-enzyme intermediate. J Biol Chem. 1990 Aug 5;265(22):13370–13378. [PubMed] [Google Scholar]
- Rudnick D. A., McWherter C. A., Rocque W. J., Lennon P. J., Getman D. P., Gordon J. I. Kinetic and structural evidence for a sequential ordered Bi Bi mechanism of catalysis by Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase. J Biol Chem. 1991 May 25;266(15):9732–9739. [PubMed] [Google Scholar]
- Saermark T., Kleinschmidt A., Wulff A. M., Andreassen H., Magee A., Erfle V. Characterization of N-myristoyl transferase inhibitors and their effect on HIV release. AIDS. 1991 Aug;5(8):951–958. doi: 10.1097/00002030-199108000-00005. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acylation of proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):411–426. doi: 10.1016/0304-4157(89)90013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Tsai S. C., Kung H. F., Oroszlan S., Moss J., Vaughan M. Hydroxylamine-stable covalent linkage of myristic acid in G0 alpha, a guanine nucleotide-binding protein of bovine brain. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1234–1239. doi: 10.1016/0006-291x(87)90780-7. [DOI] [PubMed] [Google Scholar]
- Shoji S., Tashiro A., Kubota Y. Antimyristoylation of gag proteins in human T-cell leukemia and human immunodeficiency viruses with N-myristoyl glycinal diethylacetal. J Biochem. 1988 May;103(5):747–749. doi: 10.1093/oxfordjournals.jbchem.a122340. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Myristoyl CoA:protein N-myristoyltransferase activities from rat liver and yeast possess overlapping yet distinct peptide substrate specificities. J Biol Chem. 1988 Feb 5;263(4):1784–1790. [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]