Abstract
Background
Weight gain is common for people with schizophrenia and this has serious implications for a patient's health and well being. Switching strategies have been recommended as a management option.
Objectives
To determine the effects of antipsychotic medication switching as a strategy for reducing or preventing weight gain and metabolic problems in people with schizophrenia.
Search methods
We searched key databases and the Cochrane Schizophrenia Group's trials register (January 2005 and June 2007), reference sections within relevant papers and contacted the first author of each relevant study and other experts to collect further information.
We updated this search November 2012 and added 167 new trials to the awaiting classification section.
Selection criteria
All clinical randomised controlled trials comparing switching of antipsychotic medication as an intervention for antipsychotic induced weight gain and metabolic problems with continuation of medication and/or other weight loss treatments (pharmacological and non pharmacological) in people with schizophrenia or schizophrenia‐like illnesses.
Data collection and analysis
Studies were reliably selected, quality assessed and data extracted. For dichotomous data we calculated risk ratio (RR) and their 95% confidence intervals (CI) on an intention‐to‐treat basis, based on a fixed‐effect model. The primary outcome measures were weight loss, metabolic syndrome, relapse and general mental state.
Main results
We included four studies for the review with a total of 636 participants. All except one study had a duration of 26 weeks or less. There was a mean weight loss of 1.94 kg (2 RCT, n = 287, CI ‐3.9 to 0.08) when switched to aripiprazole or quetiapine from olanzapine. BMI also decreased when switched to quetiapine (1 RCT, n = 129, MD ‐0.52 CI ‐1.26 to 0.22) and aripiprazole (1 RCT, n = 173, RR 0.28 CI 0.13 to 0.57) from olanzapine.
Fasting blood glucose showed a significant decrease when switched to aripiprazole or quetiapine from olanzapine. (2 RCT, MD ‐2.53 n = 280 CI ‐2.94 to ‐2.11). One RCT also showed a favourable lipid profile when switched to aripiprazole but these measures were reported as percentage changes, rather than means with standard deviation.
People are less likely to leave the study early if they remain on olanzapine compared to switching to quetiapine or aripiprazole.
There was no significant difference in outcomes of mental state, global state, and adverse events between groups which switched medications and those that remained on previous medication. Three different switching strategies were compared and no strategy was found to be superior to the others for outcomes of weight gain, mental state and global state.
Authors' conclusions
Evidence from this review suggests that switching antipsychotic medication to one with lesser potential for causing weight gain or metabolic problems could be an effective way to manage these side effects, but the data were weak due to the limited number of trials in this area and small sample sizes. Poor reporting of data also hindered using some trials and outcomes. There was no difference in mental state, global state and other treatment related adverse events between switching to another medication and continuing on the previous one. When the three switching strategies were compared none of them had an advantage over the others in their effects on the primary outcomes considered in this review. Better designed trials with adequate power would provide more convincing evidence for using medication switching as an intervention strategy.
Note: the 167 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.
Keywords: Humans, Drug Substitution, Antipsychotic Agents, Antipsychotic Agents/adverse effects, Antipsychotic Agents/therapeutic use, Aripiprazole, Benzodiazepines, Benzodiazepines/adverse effects, Benzodiazepines/therapeutic use, Blood Glucose, Blood Glucose/metabolism, Body Mass Index, Body Weight, Body Weight/drug effects, Dibenzothiazepines, Dibenzothiazepines/therapeutic use, Fasting, Fasting/blood, Olanzapine, Piperazines, Piperazines/therapeutic use, Quetiapine Fumarate, Quinolones, Quinolones/therapeutic use, Schizophrenia, Schizophrenia/blood, Schizophrenia/drug therapy, Weight Gain, Weight Gain/drug effects
Plain language summary
Can changing antipsychotic medication improve side effects like increases in weight, blood sugar and cholesterol?
Weight gain is common among people with schizophrenia. The medication commonly used to treat schizophrenia may cause substantial weight gain. This weight gain could be treated through lifestyle interventions that increase physical activity or change diet; or through using other forms of medication that might help with weight loss. However, an easier alternative might be changing the antipsychotic medication to one that causes less weight gain. This review examines evidence for this possibility. Switching antipsychotic medication did show some reduction in weight and also contributed broader health benefits such as reducing fasting blood glucose. Notably, there were no significant difference in outcomes of mental state, global state and adverse events between groups which switched medications and those that remained on previous medication.
Summary of findings
Summary of findings for the main comparison. SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN compared to CONTINUATION ON PREVIOUS REGIMEN: 1a. DIFFERENT DEPOT from DEPOT‐medium term (3‐12 months) for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems.
| SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN compared to CONTINUATION ON PREVIOUS REGIMEN: 1a. DIFFERENT DEPOT from DEPOT‐medium term (3‐12 months) for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems | ||||||
| Patient or population: patients with people with schizophrenia who have neuroleptic‐induced weight or metabolic problems Settings: in community Intervention: SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN Comparison: CONTINUATION ON PREVIOUS REGIMEN: 1a. DIFFERENT DEPOT from DEPOT‐medium term (3‐12 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| CONTINUATION ON PREVIOUS REGIMEN: 1a. DIFFERENT DEPOT from DEPOT‐medium term (3‐12 months) | SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN | |||||
| Weight: Body weight (kg) ‐ switching to haloperidol decanoate from fluphenazine decanoate Follow‐up: 12 months | The mean Weight: Body weight (kg) ‐ switching to haloperidol decanoate from fluphenazine decanoate in the intervention groups was 2.8 lower (7.04 lower to 1.44 higher) | 19 (1 study) | ⊕⊕⊝⊝ low1,2 | Relevant trial but small study. SD estimated rather than reported directly. | ||
| Weight: BMI not improved | See comment | See comment | Not estimable | 0 (03) | See comment | Outcome of interest ‐ but no study reported this outcome. |
| Physiological measure: Metabolic syndrome | See comment | See comment | Not estimable | 0 (03) | See comment | Outcome of interest ‐ but no study reported this outcome. |
| Global state: Changing dose because of deterioration ‐ switching to haloperidol decanoate from fluphenazine decanoate Follow‐up: 12 months | 222 per 1000 | 40 per 1000 (2 to 744) | RR 0.18 (0.01 to 3.35) | 19 (1 study) | ⊕⊕⊝⊝ low1,2 | Stated specific scales were to be used ‐ but no direct data reported form these measures. Criteria for 'deterioration' not explicit |
| Mental state: Deteriorated ‐ switching to haloperidol decanoate from fluphenazine decanoate Follow‐up: 12 months | 222 per 1000 | 40 per 1000 (2 to 744) | RR 0.18 (0.01 to 3.35) | 19 (1 study) | ⊕⊕⊝⊝ low1,2 | Stated specific scales were to be used ‐ but no direct data reported form these measures. Criteria for 'deterioration' not explicit |
| Adverse effects: serious | See comment | See comment | Not estimable | 0 (03) | See comment | Outcome of interest ‐ but no study reported this outcome. |
| Satisfaction with care: Loss to follow up ‐ switching to haloperidol decanoate from fluphenazine decanoate Follow‐up: 12 months | 0 per 1000 | 0 per 1000 (0 to 0) | RR 4.55 (0.25 to 83.7) | 19 (1 study) | ⊕⊝⊝⊝ very low1,2,4,5 | Small trial. Unclear if loss to follow up really reflects 'satisfaction'. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described 2 Small study, likely that others are not identified 3 No study reported this finding 4 Loss to follow up not simply measure of satisfaction 5 Wide confidence intervals
Summary of findings 2. SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN compared to CONTINUATION ON PREVIOUS REGIMEN: 1b. NEW ATYPICAL from OLANZAPINE ‐ medium term (3‐12 months) for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems.
| SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN compared to CONTINUATION ON PREVIOUS REGIMEN: 1b. NEW ATYPICAL from OLANZAPINE ‐ medium term (3‐12 months) for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems | ||||||
| Patient or population: patients with people with schizophrenia who have neuroleptic‐induced weight or metabolic problems Settings: community Intervention: SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN Comparison: CONTINUATION ON PREVIOUS REGIMEN: 1b. NEW ATYPICAL from OLANZAPINE ‐ medium term (3‐12 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| CONTINUATION ON PREVIOUS REGIMEN: 1b. NEW ATYPICAL from OLANZAPINE ‐ medium term (3‐12 months) | SWITCHING ‐ NEW ANTIPSYCHOTIC REGIMEN | |||||
| Weight: 1. Body weight (kg) | The mean Weight: 1. Body weight (kg) in the intervention groups was 1.94 lower (3.97 lower to 0.08 higher) | 287 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | |||
| Weight: 1. Body weight (kg) ‐ switching to aripiprazole | The mean Weight: 1. Body weight (kg) ‐ switching to aripiprazole in the intervention groups was 3.21 lower (9.03 lower to 2.61 higher) | 158 (1 study) | ⊕⊕⊝⊝ low1,3 | |||
| Weight: 2a. BMI ‐ Increase | Study population | RR 0.28 (0.13 to 0.57) | 173 (1 study) | ⊕⊝⊝⊝ very low1,4 | ||
| 329 per 1000 | 92 per 1000 (43 to 188) | |||||
| Medium risk population | ||||||
| 329 per 1000 | 92 per 1000 (43 to 188) | |||||
| Physiological measures: 1c. Average fasting glucose change ‐ switching to aripiprazole and quetiapine | The mean Physiological measures: 1c. Average fasting glucose change ‐ switching to aripiprazole and quetiapine in the intervention groups was 2.53 lower (2.94 to 2.11 lower) | 280 (2 studies) | ⊕⊕⊝⊝ low1,3 | |||
| Global state: Relapse | Study population | RR 1.31 (0.55 to 3.11) | 133 (1 study) | ⊕⊕⊝⊝ low1,3 | ||
| 118 per 1000 | 155 per 1000 (65 to 367) | |||||
| Medium risk population | ||||||
| 118 per 1000 | 155 per 1000 (65 to 367) | |||||
| Adverse events: 1a. Serious adverse event ‐ switching to aripiprazole | Study population | RR 0.64 (0.24 to 1.71) | 172 (1 study) | ⊕⊕⊝⊝ low1,3 | ||
| 107 per 1000 | 68 per 1000 (26 to 183) | |||||
| Medium risk population | ||||||
| 107 per 1000 | 68 per 1000 (26 to 183) | |||||
| Satisfaction with care: Loss to follow up ‐ switching to aripiprazole | Study population | RR 1.4 (0.89 to 2.21) | 173 (1 study) | ⊕⊝⊝⊝ very low1,3,5 | ||
| 259 per 1000 | 363 per 1000 (231 to 572) | |||||
| Medium risk population | ||||||
| 259 per 1000 | 363 per 1000 (231 to 572) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation process not described. 2 In one study there were no results from the various scales mentioned in the methods section . This study was terminated early as only 33% of the enrolment target was achieved. 3 Sponsored by interested industry. 4 The study was not published but found in the drug industry's trial register so likely that there are other similar trials which may have been missed. 5 Satisfaction with care alone may not account for all loss to follow up.
Summary of findings 3. SWITCHING ‐ TECHNIQUES: TO ARIPIPRAZOLE from PREVIOUS REGIMEN ‐ THREE DIFFERENT TECHNIQUES ‐short term (up to 12 months) for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems.
| SWITCHING ‐ TECHNIQUES: TO ARIPIPRAZOLE from PREVIOUS REGIMEN ‐ THREE DIFFERENT TECHNIQUES ‐short term (up to 12 months) for people with schizophrenia who have neuroleptic‐induced weight or metabolic problems | ||||||
| Patient or population: patients with people with schizophrenia who have neuroleptic‐induced weight or metabolic problems Settings: Intervention: SWITCHING ‐ TECHNIQUES: TO ARIPIPRAZOLE from PREVIOUS REGIMEN ‐ THREE DIFFERENT TECHNIQUES ‐short term (up to 12 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SWITCHING ‐ TECHNIQUES: TO ARIPIPRAZOLE from PREVIOUS REGIMEN ‐ THREE DIFFERENT TECHNIQUES ‐short term (up to 12 months) | |||||
| Weight: Gain‐more than or equal to 7% from baseline | Study population | RR 0.61 (0.15 to 2.47) | 207 (1 study) | ⊕⊕⊕⊝ moderate1 | Switching of treatment in this study may not have been for weight control therefore this client group may differ from those in the other trials in this review | |
| 48 per 1000 | 29 per 1000 (7 to 119) | |||||
| Medium risk population | ||||||
| 48 per 1000 | 29 per 1000 (7 to 119) | |||||
| Weight: BMI not improved | See comment | See comment | Not estimable | 0 (0) | See comment | Outcome of interest‐but the study did not report this outcome |
| Physiological measure: Metabolic syndrome | See comment | See comment | Not estimable | 0 (0) | See comment | Outcome of interest‐but the study did not report this outcome |
| Global state: Average change (CGI‐I, high=good) | The mean Global state: Average change (CGI‐I, high=good) in the intervention groups was 0.14 lower (0.49 lower to 0.21 higher) | 203 (1 study) | ⊕⊕⊕⊝ moderate1 | |||
| Mental state: Average change (PANSS, high decline=good) | The mean Mental state: Average change (PANSS, high decline=good) in the intervention groups was 2.52 higher (2.39 lower to 7.43 higher) | 198 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Adverse events: Any event | Study population | RR 1 (0.91 to 1.1) | 207 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 894 per 1000 | 894 per 1000 (814 to 983) | |||||
| Medium risk population | ||||||
| 894 per 1000 | 894 per 1000 (814 to 983) | |||||
| Satisfaction with care: Loss to follow up ‐ non‐compliance | Study population | RR 4 (0.45 to 35.19) | 208 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 10 per 1000 | 40 per 1000 (4 to 352) | |||||
| Medium risk population | ||||||
| 10 per 1000 | 40 per 1000 (4 to 352) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sponsored by interested industry. 2 Wide confidence intervals. 3 Satisfaction with care alone may not account for all loss to follow ups.
Background
Description of the condition
Obesity is a common problem in people with schizophrenia. Weight gain and metabolic disturbance are recognised side effects of many second‐generation antipsychotic drugs, which is the mainstay of treatment for schizophrenia (Allison 1999; Casey 2004; Homel 2002). The prevalence of obesity in people with schizophrenia has been reported to be 1.5 to 4 times higher than the general population (ADA/APA 2004; Coodin 2001; Silverstone 1988). People with schizophrenia have a marked increase in standardised mortality ratio for both natural and unnatural causes of death .The rise in mortality may be attributed to the increased risk of coronary heart disease (Cohn 2004; Goff 2005; Henderson 2005; Mackin 2005; Saari 2005), and increased prevalence of obesity in this population (Coodin 2001; Daumit 2003; Susce 2005). Obesity doubles the risk of all‐cause mortality, coronary heart disease, stroke and Type II diabetes. It also increases the risk of some cancers, musculoskeletal problems and loss of function, and carries negative psychological consequences (DoH 2004).
Many factors such as genetics, adverse effects of medications, poor diet and inactive lifestyle may contribute to the prevalence of obesity in schizophrenia. In a meta‐analysis, every antipsychotic medication except ziprasidone and molindone was found to be associated with some degree of weight gain after just 10 weeks of treatment (Allison 1999). The effects were greatest with olanzapine and clozapine, which swiftly increased body weight by 4‐4.5 kilograms. There is no consensus on exactly how the newer antipsychotic drugs cause weight gain.
Quality of life is further reduced for people with schizophrenia with high body mass index (Kurzthaler 2001; Strassnig 2003) and those gaining weight (Allison 2003). Furthermore, Weiden 2004 reported a significant positive association between obesity, subjective distress from weight gain and medication non‐compliance in a sample of people with schizophrenia. People suffering from schizophrenia also face the combined challenges of living with schizophrenia, and for many, obesity and related illnesses. This combination is a major public health problem (Wirshing 2004) and carries considerable human cost.
Second‐generation antipsychotic drugs, in spite of their benefits, have been associated with the so‐called metabolic syndrome, the triad of weight gain, diabetes and dyslipidaemia. The International Diabetes Federation published a consensus statement on the definition of metabolic syndrome in 2006 (IDF). The criteria for the syndrome includes central obesity (defined as waist circumference of more than 94cm for Europoid* men and 80 cm for Europoid women with ethnic specific values for other groups), as well as any two from the following four factors: ‐ raised triglyceride levels or specific treatment for this lipid abnormality; ‐ reduced HDL cholesterol; ‐ raised blood pressure or treatment of previously diagnosed hypertension; ‐ raised plasma glucose or previously diagnosed Type II diabetes. (* Europoid: a technical term used to describe a physical type like one of the phenotypes, or genotypes types found today in Europe (Wikipedia 2007)).
Description of the intervention
Recognition of the metabolic syndromes in people who are on second‐generation antipsychotic medication and the resulting increase in morbidity and mortality has led to discussions on effective intervention strategies (Birt 2003; Catapana 2004; Green 2000; Le Fevre 2001; Osborn 2001) and consensus statements on its management (e.g., ADA/APA 2004; De Nayer 2005). Treatment of obesity in this population involves non‐pharmacological and pharmacological interventions. Existing evidence suggests that even effective treatments for adult obesity only produce modest weight loss (approximately 2 kg to 5 kg) compared to no treatment or usual care (Faulkner 2007). Switching to a drug with less potential for weight gain and metabolic problems can also lead to weight loss and metabolic improvement.
How the intervention might work
Studies have shown that when one atypical antipsychotic is switched to another, changes in weight occur. In Ried 2003, when people were switched from olanzapine to risperidone and risperidone to olanzapine, the group switched to olanzapine gained weight while those changed to risperidone lost. Hester 2005 found that for people who had gained weight on olanzapine, switching to another antipsychotic drug was an effective option to reduce weight in some patients. The consensus statement on the management of metabolic syndrome associated with atypical antipsychotic use also recommends that if a stable patient on a second‐generation antipsychotic gains 5% of his or her initial weight at any time during therapy, one should consider switching the medication (ADA/APA 2004).
Four different switch strategies can be used: ‐ the cross‐taper strategy (gradually titrating up the new medication while tapering off the older medication); ‐ starting new medication at the therapeutic dose while tapering off the older one; ‐ stopping older antipsychotic drugs abruptly while gradually increasing the new one; or ‐ starting the new drug at therapeutic dose and stopping the old one abruptly. The cross‐taper strategy is considered to be the safest approach.
A recent review looked at four randomised controlled trials on switching strategies and suggested that available evidence does not yet support the clinical superiority of switching antipsychotic drugs on various outcome measures, including weight gain (Remington 2005).
Why it is important to do this review
Anyone might have difficulties undergoing available pharmacological and non‐pharmacological interventions for weight gain due to lack of motivation or other lifestyle factors. For people with schizophrenia these problems may be particularly acute; hence switching to an antipsychotic medication which is less likely to cause weight gain seems to be a practical alternative. Using an antipsychotic which is less likely to cause weight gain initially may also be critical for preventing or reducing weight gain. A recent Cochrane review (Faulkner 2007) looked at pharmacological (anti‐obesity agents) and non‐pharmacological (diet/exercise) interventions for reducing or preventing weight gain in people with schizophrenia but specifically excluded medication switching as an intervention strategy. This sister review extends the investigation by focusing on randomised trials in which switching antipsychotic medication is used as an intervention for people with schizophrenia who have antipsychotic induced weight or metabolic problems.
Objectives
To determine the effects of antipsychotic medication switching as a strategy for reducing or preventing weight gain in people with schizophrenia who have neuroleptic induced weight or metabolic problems.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. Where a trial was described as 'double‐blind', but it was only implied that the study was randomised, we have included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these 'implied randomisation' studies were added, then we included those in the final analysis. If there was a substantive difference, we used only clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
People diagnosed with schizophrenia or schizophrenia‐like illnesses, using any criteria, with weight or metabolic problems. We included trials where it was implied that the majority (more than 50%) of participants had a severe mental illness likely to be schizophrenia.
For purposes of this review we use two definitions of weight or metabolic problems ‐ a grade 'A' definition and a less stringent grade 'B'. A ‐ Everyone starting had weight or metabolic problems as defined by the trialists and there was, at least, a suggestion that this problem had been caused by use of antipsychotic medication. B ‐ Everyone participating had measures relevant to weight or metabolic problems recorded, whether or not they fell into the category of obese or metabolic syndrome.
We did not exclude participants on the basis of age, nationality, sex or setting of treatment.
Types of interventions
A. COMPARISON 01: Absolute effect of switching. 1. Any antipsychotic medication switching; versus 2. continuation of medication as before.
B. COMPARISON 02: Comparative effect of switching versus other weight management techniques. 1. Any antipsychotic medication switching; versus 2. continuation of antipsychotic plus adjunctive pharmacological interventions (used for weight or metabolic benefit such as orlistat, nizatidine); or 3. continuation of antipsychotic plus non‐pharmacological interventions (e.g. diet, exercise, counselling); or 4. discontinuation of antipsychotic plus pharmacological interventions (used for weight or metabolic benefit such as orlistat, nizatidine); or 5. discontinuation of antipsychotic plus non‐pharmacological interventions (e.g. diet, exercise, counselling).
C. COMPARISON 03: Comparative effect of different techniques of switching. 1. Abrupt discontinuation + abrupt replacement; versus 2. abrupt discontinuation + gradual replacement; or 3. gradual discontinuation + abrupt replacement; or 4. gradual discontinuation + gradual replacement.
Types of outcome measures
Primary outcomes
1. Weight and physiological measures 1.1 No clinically important change in body weight (as defined by individual studies) 1.2 Presence of metabolic syndrome
2. Global state 2.1 Relapse
3. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 3.1 No clinically important change in general mental state
Secondary outcomes
1. Weight and physiological measures 1.1 Total body weight (lbs/kg) 1.2 Change in body weight 1.3 No clinically important change in body mass index (BMI) (as defined by individual studies) 1.4 Total BMI 1.5 Change in BMI 1.6 No clinically important change in waist circumference (as defined by individual studies) 1.7 Total waist circumference 1.8 Change in waist circumference 1.9 No clinically important change in waist‐to‐hip circumference ratio (as defined by individual studies) 1.10 Total waist‐to‐hip circumference ratio 1.11 Change in waist‐to‐hip circumference ratio 1.12 No clinically important change in total percent body fat (as defined by individual studies) 1.13 Total percent body fat 1.14 Change in percent body fat 1.15 Serum cholesterol, HDL, LDL, triglyceride profile
2. Global state 2.1 No clinically important change in global state (as defined by individual studies) 2.2 Average endpoint global state score 2.3 Average change in global state scores
3. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 3.1 Average endpoint general mental state score 3.2 Average change in general mental state scores 3.3 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 3.4 Average endpoint specific symptom score 3.5 Average change in specific symptom scores
4. Service outcomes 4.1 Hospitalisation 4.2 Time to hospitalisation
5. General functioning 5.1 No clinically important change in general functioning 5.2 Average endpoint general functioning score 5.3 Average change in general functioning scores 5.4 No clinically important change in specific aspects of functioning, such as social or life skills 5.5 Average endpoint specific aspects of functioning, such as social or life skills 5.6 Average change in specific aspects of functioning, such as social or life skills
6. Behaviour 6.1 No clinically important change in general behaviour 6.2 Average endpoint general behaviour score 6.3 Average change in general behaviour scores 6.4 No clinically important change in specific aspects of behaviour 6.5 Average endpoint specific aspects of behaviour 6.6 Average change in specific aspects of behaviour
7. Adverse effects ‐ general and specific 7.1 Clinically important general adverse effects 7.2 Average endpoint general adverse effect score 7.3 Average change in general adverse effect scores 7.4 Clinically important specific adverse effects 7.5 Average endpoint specific adverse effects 7.6 Average change in specific adverse effects 7.7 Death ‐ suicide and natural causes
8. Engagement with services
9. Satisfaction with treatment 9.1 Leaving the studies early 9.2 Recipient of care not satisfied with treatment 9.3 Recipient of care average satisfaction score 9.4 Recipient of care average change in satisfaction scores 9.5 Carer not satisfied with treatment 9.6 Carer average satisfaction score 9.7 Carer average change in satisfaction scores
10. Quality of life 10.1 No clinically important change in quality of life 10.2 Average endpoint quality of life score 10.3 Average change in quality of life scores 10.4 No clinically important change in specific aspects of quality of life 10.5 Average endpoint specific aspects of quality of life 10.6 Average change in specific aspects of quality of life
11. Economic outcomes 11.1 Direct costs 11.2 Indirect costs
We planned to divide outcome periods into short‐term (less than three months), medium‐term (3‐12 months) and long‐term follow‐up (longer than one year).
Search methods for identification of studies
We followed recommendations of the Schizophrenia Review Group search strategy and the Cochrane Reviewers' Handbook in developing our search strategy (Higgins 2005).
Electronic searches
The studies included in this review are from three separate searches: (a) all the references that came up in the 2005 search for the review looking at the interventions other than switching antipsychotics for reducing or preventing weight gain (Faulkner 2007); (b) additional references that came up in the update search in 2007 and (c) other relevant articles identified by the authors as the review progressed through searching registers of ongoing clinical trials, inspecting reference list of all identified studies, including existing reviews for relevant citations and asking representatives from major pharmaceutical companies (Eli Lilly, Astra Zeneca, and Bristol‐Myers Squibb) if they have conducted or were currently undertaking any weight‐related interventions in relation to schizophrenia. This was done along with the update search.
1. Update search (2007)
The Cochrane Schizophrenia Group Trials Register was searched (June 2007) using the phrase:
[ (weight* or body mass* or bmi* or diet* or * eat* or waist* or obes* or * fat* or metaboli* in title, abstract, index terms of REFERENCE) or ( (switch* in intervention field) and (weight* or body mass* or diet* or eat* or waist* or obes* or metaboli* in outcome field) of study)].
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
2. Cochrane Schizophrenia Group Trials Register register (November 2012)
The Trials Search Co‐ordinator, Samantha Roberts, searched the Cochrane Schizophrenia Group Trials Register register (November 2012) using the phrase:
[ (weight* or body mass* or bmi* or diet* or * eat* or waist* or obes* or * fat* or metaboli* in title, abstract, index terms of REFERENCE) or ( (switch* in intervention field) and (weight* or body mass* or diet* or eat* or waist* or obes* or metaboli* in health care conditions field of study)].
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of relevant journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
2. Previous search strategy (2005)
See Appendix 1
Searching other resources
1. Reference searching
We inspected the reference lists of all identified studies, including existing reviews, for relevant citations.
2. Personal contact
We contacted the first author of each relevant study for information on unpublished trials. We also consulted experts in the area of schizophrenia and weight gain. We contacted authors and experts by email or post to establish missing details in the methods and results sections of the written reports and to determine their knowledge of or involvement in any current work in the area. We also asked contacts at major pharmaceutical companies if they have conducted or were currently undertaking any weight‐related interventions in relation to schizophrenia (including representatives from Janssen Pharmaceuticals, Pfizer Inc, Eli Lilly and Company, Astra Zeneca, and Bristol‐Myers Squibb).
Data collection and analysis
Selection of studies
Four review authors (AM, GF, TC, GR) independently assessed the abstracts of the studies returned by the searches for relevance. When the authors disagreed or the abstract was unclear, we obtained the full report, repeated the assessment process and reached a decision on inclusion by consensus.
Data extraction and management
AM independently extracted data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
1. Extraction
AM independently extracted data from included studies. Again, we discussed any disagreement, documented decisions and, if necessary, contacted authors of studies for clarification. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
2. Management
We extracted the data onto standard, simple forms. Where possible, we entered data into Review Manager (RevMan) (RevMan 2008) in such a way that the area to the left of the 'line of no effect' indicates a 'favourable' outcome for the experimental treatment. Where this was not possible (e.g. scales that calculate higher scores = improvement) we have labelled the graphs in RevMan analyses accordingly so that the direction of effects was clear.
3. Scale‐derived data
A wide range of instruments are available to measure outcomes in mental health studies. These instruments vary in quality and many are not validated, or are even ad hoc. It is accepted generally that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure). Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore we included continuous data from rating scales only if the measuring instrument had been described in a peer‐reviewed journal. In addition, we set the following minimum standards for instruments: the instrument should either be (a) a self‐report or (b) completed by an independent rater or relative (not the therapist) and (c) the instrument should be a global assessment of an area of functioning. Whenever possible we took the opportunity to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. Where continuous data were presented from different scales rating the same effect, we presented both sets of data and inspected the general direction of effect.
Assessment of risk of bias in included studies
We assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases. We would not have included studies where sequence generation was at high risk of bias or where allocation was clearly not concealed. The categories are defined below. YES ‐ low risk of bias. NO ‐ high risk of bias. UNCLEAR ‐ uncertain risk of bias.
If disputes arose as to which category a trial has to be allocated, again, we resolved these by discussion, after working with a third reviewer.
In addition, we completed the 'Risk of bias' table for each included trial. In this we recorded our opinions of where each of the studies was vulnerable to bias.
Measures of treatment effect
1. Binary data
We carried out an intention‐to‐treat analysis. On the condition that more than 60% of people completed the study, we counted everyone allocated to the intervention, whether they completed the follow‐up or not. We assumed that those who dropped out had the negative outcome, with the exception of death. Where possible, we made efforts to convert outcome measures to binary data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into "clinically improved" or "not clinically improved". If the authors of a study had used a predefined cut‐off point for determining clinical effectiveness, we used this where appropriate. Otherwise we generally assumed that if there had been a 50% reduction in a scale‐derived score, this could be considered as a clinically significant response. Similarly, we considered a rating of "at least much improved" according to the Clinical Global Impression Scale (Guy 1976) as a clinically significant response.
For binary outcomes we calculated a standard estimation of the fixed‐effect risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios (ORs) and that ORs tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number needed to harm (NNH) as the inverse of the risk difference.
2. Continuous data
2.1 Summary statistic
For continuous outcomes we estimated a mean difference (MD) between groups, based on the fixed‐effect model.
2.2 Endpoint versus change data
Where both final endpoint data and change data were available for the same outcome category, we have presented only final endpoint data. We acknowledge that by doing this much of the published change data may be excluded, but argue that endpoint data are more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. Where studies reported only change data, we contacted authors for endpoint figures, but if endpoint data were unavailable, we reported change data.
2.3 Skewed data
Normally distributed data: continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion:
(a) standard deviations and means were reported in the paper or were obtainable from the authors;
(b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996);
(c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2SD> (S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them. When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), it is difficult to tell whether data are non‐normally distributed (skewed) or not.
We would have entered skewed data from studies of fewer than 200 participants in additional tables rather than into an analysis. Skewed data poses less of a problem when looking at means if the sample size is large and we would have entered them into a synthesis.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999). Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. We have sought statistical advice and have been advised that binary data presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intra‐class correlation co‐efficient (ICC) (Design effect = 1+ (m‐1)*ICC) (Donner 2002). If the ICC was not reported we have assumed it to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account ICC and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we have presented the additional treatment arms in comparisons. Where the additional treatment arms were not relevant, we have not reproduced these data.
Dealing with missing data
We attempted to include all people who had been randomised to either switching of medication or continuing on their previous antipsychotic. Where possible, we gave cases lost to follow‐up at the end of the study the worst outcome. For example, those lost to follow‐up for the outcome of relapse were treated in the analysis as having relapsed. Suicide was treated as relapse. We agreed these rules before knowing the studies included. The effects of inclusion of this assumption were tested with sensitivity analyses for the primary outcome.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies without any comparison to judge clinical heterogeneity.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I2 statistic
This provided an estimate of the percentage of inconsistency thought to be due to chance. We interpreted I2 estimates greater than or equal to 50% as evidence of high levels of heterogeneity (Higgins 2003).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Handbook (Higgins 2009). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects (Egger 1997). We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
Where possible we employed a fixed‐effect model for analyses. We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us; however, random‐effects does put added weight onto the smaller of the studies ‐ those trials that are most vulnerable to bias. For this reason we favour using the fixed‐effect model.
In this review, in the three instances where meta‐analysis was possible, the l2 statistic was substantially less than 50%. When true inter‐trial variability is low as denoted by low values of l2 statistics, the fixed‐effect model may give better precision estimate of a common effect.
Subgroup analysis and investigation of heterogeneity
When we found heterogeneous results , we investigated the reasons for this. Where heterogeneous data substantially altered the results and we identified the reasons for the heterogeneity, we have not summated these studies in the meta‐analysis, but presented them separately and discussed them in the text.
Sensitivity analysis
We wished to investigate the sensitivity of the results of the primary outcomes to grouping drugs into broad families such as 'typical' and 'atypical'. This proved impossible as each comparison compared medications within the same broad families of drugs or did not give details of prior treatment.
Results
Description of studies
For description of the studies see: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The original search identified 814 references from 83 studies. We conducted an update search in 2007 and identified an additional 266 studies and 625 new references. We searched the ISI citation index for each selected trial in order to identify further studies, and inspected the reference sections of selected studies for additional trials. We also identified five additional studies possibly relevant to this review (in addition to the above search methods, we also searched registers of ongoing clinical trials, and contacted representatives from major pharmaceutical companies) from which we have included two in the review. In total we selected 32 relevant trials, but we were able to include only four studies which were directly relevant for the review.
Included studies
We identified four studies which we could include. All were described as randomised. In one study, the participants were randomised to three switching strategies (Casey 2003). Only three were double blind (Cookson 1986; Eli Lilly 2004; Newcomer 2008).
1. Length of trials
One study (Casey 2003) reported data on short‐term follow‐up (up to 12 weeks).Two studies had follow‐up of 13 to 26 weeks (Eli Lilly 2004; Newcomer 2008). Only one study had follow‐up of one year's duration (Cookson 1986) .The ongoing study has an intended duration of one year (Tang 2003).
2. Participants
All but one study used clear operationalised criteria for diagnosis (Cookson 1986). The rest included participants with either schizophrenia or schizoaffective disorder (DSM IV). The majority of participants in all studies except one (Cookson 1986) were male, and had a mean age in their mid to late thirties to early to mid forties.Two studies excluded participants with other axis 1 DSM IV diagnoses, suicide risk and substance dependence (Casey 2003; Newcomer 2008). One study excluded patients who had been on any other antipsychotic except olanzapine, which was the control antipsychotic (Eli Lilly 2004).
Being overweight or obese and/or having metabolic complications were an inclusion criteria in only three studies (Cookson 1986; Eli Lilly 2004; Newcomer 2008). In the other study it was difficult to make out if the participants were obese or overweight at entry, as they had provided only a mean body weight and no BMI (Casey 2003).
All the studies required patients to be stable mentally or be on a stable dose of medication. In the Casey 2003 study, there needed to be a reason for switching, like poor tolerability or inadequate response to previous medication.
3. Setting
Two studies were described as occurring in out‐patient settings (Casey 2003; Eli Lilly 2004). One study was done in a depot injection clinic (Cookson 1986). The setting was not mentioned in Newcomer 2008, but it could be inferred that this was conducted in an out‐patient setting as it had as one of its exclusion criteria an increase in symptoms requiring hospitalisation.
4. Study size
The study by Casey 2003 had the maximum participants (311). Two studies had between 100 and 200 participants (Eli Lilly 2004; Newcomer 2008). In the study by Eli Lilly 2004 the enrolment target was 340 but the study was terminated with only 33% of the original target enrolled. The fewest number of participants were enrolled in Cookson 1986 (19).
5. Interventions
Although all studies looked at the absolute effect of switching, only three studies (Cookson 1986; Eli Lilly 2004; Newcomer 2008) compared the effect of switching of antipsychotic medication with continuation of previous medication. In the study by Casey 2003, which looked at switching from previous antipsychotic medications to another, there was no comparative group continuing on the original medication. In this study baseline measures were compared to before and after switching of medication. For the purposes of this review we have called the different ways of switching 'Technique 1, 2 and 3'.
Technique 1 ‐ immediate initiation + immediate discontinuation of current antipsychotic.
Technique 2 ‐ immediate initiation + tapering of current antipsychotic.
Technique 3 ‐ titrating switch medication upwards + tapering of current antipsychotic .
In all studies except one, oral antipsychotics were used. In one study (Cookson 1986) depot antipsychotics were used.
6. Outcomes
Mean weight changes with measures of dispersion are reported in three studies (Cookson 1986; Eli Lilly 2004; Newcomer 2008). The percentage of people who experienced clinically significant weight gain or weight loss was reported in Casey 2003; mean weight change was also reported but it was not possible to use this as there were no measures of dispersion.
Changes in BMI are reported in Eli Lilly 2004 (mean and standard deviation) and Newcomer 2008 (number of people experiencing clinically relevant BMI increase).
The percentage of people with high waist circumference is reported in Newcomer 2008 but no other studies reported this outcome. Body fat and waist to hip circumference ratio was not an outcome measure in any of the studies.
Laboratory measures for Metabolic Syndrome were measured in two studies. Serum cholesterol, triglycerides HDL, LDL and blood glucose levels were measured in Eli Lilly 2004 and Newcomer 2008.
Time to relapse was the primary outcome measure in the study by Eli Lilly 2004. None of the other studies looked at relapse. Global state was assessed using the CGI I and CGI S scales in 2 studies (Casey 2003; Newcomer 2008).
Hospitalisation as one of the criteria for relapse was looked at in Eli Lilly 2004.
General functioning and behaviour were not an outcome measure in any of the studies.
Number of deaths was an outcome measure in only two studies; no deaths reported (Eli Lilly 2004; Newcomer 2008). Number of people discontinuing treatment was considered in three studies (Cookson 1986; Eli Lilly 2004; Newcomer 2008).
None of the studies looked at quality of life measures, economic outcomes or engagement with services.
All the studies looked at dropouts, but only three studies stated the reasons for discontinuation of treatment (Cookson 1986; Eli Lilly 2004; Newcomer 2008).
6.1 Outcome scales
Details of scales that provided usable data are shown below. Reasons for exclusions of data are given under 'Outcomes' in the 'Included studies' section.
6.1.1 Global state ‐ Clinical Global Impression Scale ‐ CGI Scale (Guy 1976). This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. The review has looked at the two components of the scale, CGI ‐I (clinical improvement) and CGI‐S (severity of illness). A seven‐point scoring system is usually used, with low scores showing decreased severity and/or overall improvement. This scale was used in two studies (Casey 2003; Newcomer 2008).
6.1.2 Mental state ‐ Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986) This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from 1 ‐ absent to 7 ‐ extreme. This scale can be divided into three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity. PANSS was used in two studies (Casey 2003; Eli Lilly 2004).
6.2 Redundant data
We were unable to use some data on a number of outcomes (vital signs, concomitant medications and laboratory measures) used as there were no numerical data (Casey 2003; Newcomer 2008).Though specific scales were used to measure mental state and side effects, the results provided no usable data (Casey 2003; Cookson 1986; Eli Lilly 2004; Newcomer 2008).
6.3 Missing outcome
Primary outcome measures in the review were weight and metabolic measures, global state and mental state. In the four studies which we have included in the review, there were inconsistencies in reporting outcomes. All the primary outcomes were considered in three studies (Casey 2003; Cookson 1986; Eli Lilly 2004). In Casey 2003 and Cookson 1986, other measures of weight like BMI, waist circumference and metabolic syndrome were not considered. In the study by Eli Lilly 2004 relapse was used as an outcome to measure global state, but no other scales were used to measure global state. In this study a number of outcomes (waist circumference, health outcomes and resource utilisation) discussed in the methods section were not reported, probably due to the early termination of the study. Newcomer 2008 did not measure mental state.
Excluded studies
We excluded 28 studies from the review. One was a presentation on guidelines for the prescription of long‐acting atypical depot antipsychotics (Kane 2004). Another study (Ducate 2003) was excluded as there was no comparative group.
We excluded eight studies because there was no switching after randomisation (Alvarez 2006; Chrzanowski 2006; Brar 2005; Emsley 2005; CATIE ‐ Phase I ‐ 2006; Nasrallah 2001; STAR trials 2006; Wang 2006). We excluded six studies as they involved no switching of medications (CATIE ‐ Phase II ‐ 2006; Covell 1999; Kane 2005; Kane 2007; Newcomer 2006; Zhang 2004). Four studies were not randomised (Kim 2007; O'Halloran 2003; Robinson 2000; Su 2005). Weight and metabolic parameters were not measured as outcomes in one study (Irwin 2003,). We excluded another four studies because the primary aim of the switch was not as an intervention for weight gain or metabolic problems. This aim was difficult to consider as inferred as the switch was to olanzapine which is known to cause both weight gain and metabolic problems (Godleski 2003; Kinon 2000; Kinon 2004; Lee 2002). As this review was specifically looking at switching of medication as an intervention, we had to exclude one crossover trial (Kelly 2003). The first half of this study (eight weeks) was a comparison between high dose olanzapine and clozapine on various outcome measures including weight. The second half of the study involved crossover (switching). As data for weight and metabolic problems were not provided after the crossover (switching), we had to exclude the study. One study (Horacek 2004) provided no measure of dispersions for weight (the primary outcome for the review) and so it had to be excluded. We excluded Weiden Daniel 2003 because there were no data as per the original randomised groups (randomised to three switching strategies, from three different antipsychotic groups to ziprasidone). The data given were the pooled data from the three switching strategies for the three groups as per the original antipsychotic they were on prior to the switch to ziprasidone.
2. Awaiting assessment One hundred and sixty seven studies are awaiting assessment.
3. Ongoing studies One study (Tang 2003) is still ongoing. E‐mail (04/03/2008) contact from one of the authors stated that the study is ongoing and they have 50 patients recruited. However, they were planning to terminate the study soon due to funding issues. We have received no reply to further e‐mails requesting updates.
Risk of bias in included studies
For graphical representation of risk of bias please see Figure 1 and Figure 2.
1.

2.

Allocation
All included studies were reported as randomised. Only two studies attempted to describe the method of randomisation. In Casey 2003, randomisation was via a centralised telephone call‐in system, and Cookson 1986 used randomisation sequences (separate for males and females).The rest of the studies did not describe the randomisation or allocation concealment methods used. Concealment of allocation has repeatedly been shown to be of key importance in excluding selection biases (Jüni 2001).
Blinding
Three studies were double blind (Cookson 1986; Eli Lilly 2004; Newcomer 2008). Newcomer 2008 had a two‐week open‐label observation period where participants continued to receive their prior medication. Casey 2003 was open label. None of the double‐blind studies reported any tests for blinding, which is important to test minimisation of observation bias. Lack of blinding for objective outcomes like weight, BMI and metabolic syndrome (major primary objectives in this review) is less likely to cause bias.
Incomplete outcome data
Most studies used last observation carried forward (LOCF) or observed case data set. Reasons for discontinuation were stated in most studies.
In Newcomer 2008 in the LOCF and observed case data set there was 32% dropout.
In Eli Lilly 2004 the protocol was terminated early, with only a fraction of the intended enrolment. The drop‐out percentage was 33% percent. In Casey 2003, the drop‐out rate was 28%. In the Cookson 1986 study, only two people dropped out (11%).
All studies used LOCF and Newcomer 2008 conducted observed case analysis (observed cases, defined as those completing the trial) as well.
The drop‐out rates were different among the three groups in Casey 2003 with one group having more drop‐outs than the other.This was because more people withdrew consent in this group. Also all the randomised participants were not considered in reporting of some data. It is not clear what happened to the rest.
In the Cookson 1986 study, the only drop‐outs were in the intervention group who were weighed before withdrawal; one person gaining weight (2.9 kg) and the other losing it (0.9 kg).
There were more drop‐outs in the intervention group compared to control group in Eli Lilly 2004 and Newcomer 2008. The reasons for discontinuation were different in the two groups. It is therefore likely that these studies could be affected by attrition bias.
There were no numerical data on vital signs and concomitant medications in the Casey 2003 study. In Newcomer 2008 there was no data on vital signs, ECG and routine laboratory tests. In the Cookson 1986 study, even though methodology stated use of scales to measure mental state and side effects, there were no data reported in the results.
Selective reporting
We were unable to identify any intentional underreporting of outcomes in the trials with the possible exception of the Eli Lilly 2004 study. Here a number of outcomes stated in the protocol were not reported or analysed. Authors state that this was because the protocol was terminated early as only 33% of the target enrolment was reached.
Other potential sources of bias
Our judgement on the risk of bias in the different studies is shown in Figure 1
All but one trial (Cookson 1986) were supported by interested drug industries. In the Eli Lilly 2004 trial, protocol was terminated early prior to breaking the blind.This would not cause bias for the primary outcome (time to relapse) but could affect some outcomes of interest like weight, BMI and metabolic syndrome. We estimated the risk of bias to be unclear or high in the studies, and this is likely to overestimate positive effects.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Switching ‐ new antipsychotic regimen versus continuation on previous regimen: 1a. different depot from depot – medium term (1 year)
(Table 1)
There was only one trial included in this group (Cookson 1986, n = 19).
1.1 Weight: body weight
There was no clear difference between groups with an average loss of 2.80 kg by the end of the year in those allocated to haloperidol decanoate but the trial was small (n = 19) and confidence intervals wide (‐7.04 to 1.44).
1.2 Global state
We only found one proxy outcome for global state. Based on dose change (the need for extra medication) there was no clear difference between groups (1 RCT, n = 19, RR 0.18 CI 0.01 to 3.35). This finding was based on only two events.
1.3 Mental state
Two people were reported to have had a deterioration in mental state in the fluphenazine group but no significant difference between the two groups (1 RCT, n = 19, RR 0.18 CI 0.01 to 3.35).
1.4 Loss to follow‐up
Two people left from the group switched to haloperidol decanoate (1 RCT, n = 19, RR 4.55 CI 0.25 to 83.70) and again there was no clear difference between the groups.
2. Switching antipsychotic regimen versus continuation on previous regimen: 2. New atypical from olanzapine
(Table 2)
2.1 Weight: 1. Body weight (kg)
There was a statistically non‐significant mean weight loss of 1.94 kg in people who were switched from olanzapine to other medications compared to those who remained on it (2 RCTs, n = 287, CI ‐3.97 to 0.08). The average weight loss when switched to aripiprazole was 3.21 kg but the confidence intervals were very wide (‐9.03 to 2.61). The weight loss was 1.77 kg when switched to quetiapine (CI ‐3.93 to 0.39).
2.2 Weight: 2a. BMI increase
There was a significant difference in the number of patients who had clinically relevant BMI increase (more than 1 kg/m2) between aripiprazole (8 out of 88) and olanzapine (28 out of 85). This was based on 1 RCT (n = 173, RR 0.28, CI 0.13 to 0.57).
2.3 Weight: 2b. BMI average change
The average BMI was lower in the group switched to quetiapine compared to those who remained on olanzapine (1 RCT, n = 129 MD ‐0.52 CI ‐1.26 to 0.22), though this was not statistically significant.
2.4 Waist circumference increase
Even though fewer people who switched to aripiprazole had an increase in waist circumference compared to those remaining on olanzapine at the end of 16 weeks; the difference between the groups was not significant (1 RCT, n = 173, RR 0.92 CI 0.76 to 1.11).
2.5 Physiological measures: 1a. Average changes
2.5.1 Change in cholesterol
There was no difference in change in cholesterol levels in the two groups (1 RCT, n = 130, MD 0.02 CI ‐0.23 to 0.27).
2.5.2 LDL levels
There was no difference in LDL levels between the two groups. This was based on one RCT (n = 130, MD ‐0.02 CI ‐0.24 to 0.20).
2.5.3 HDL levels
With HDL levels as well there was no difference between the groups. There were no changes to the HDL levels in patients switched to quetiapine but HDL levels increased by 0.03 mmol/l in those remaining on olanzapine (1 RCT, n = 130 CI ‐0.05 to 0.11).
2.5.4 Triglyceride levels
No difference were observed between groups in triglyceride levels (1 RCT, n = 130, MD 0.13 CI‐0.18 to 0.44).
2.6 Physiological measures: 1b. Percentage changes
Only percentage changes were reported in one RCT which looked at switching to aripiprazole and continuing on olanzapine.
2.6.1 Cholesterol
There was a decrease in cholesterol by 9.5 % (n = 80 SE 1.5) in those switched to aripiprazole and 3.3% (n = 76 SE1.6) in those who remained on the olanzapine. The reported P value for this difference was 0.005.
2.6.2 Triglycerides
The triglycerides decreased by 14.46 % in those switched to aripiprazole (n = 54 SE 4.5 which was estimated from a graph) and increased by 5.3% in the group continuing on olanzapine (n = 61 SE 5.6‐ estimate from graph). The P value was 0.002.
2.6.3 LDL levels
The percentage decrease for low density lipoproteins was 11.2% (n = 80 SE 2.5) in the group switched to aripiprazole and it was 4.7% (n = 76 SE 2.7) in the group which stayed on olanzapine.The P value was 0.072.
2.6.4 HDL levels
The high density lipoproteins increased by 1.7% (n = 80 SE 1.8) in the aripiprazole group and decreased by 5.9% (n = 76 SE1.7) in the olanzapine group. The P value was 0.002.
2.7 Physiological measures: 1c. Average changes ‐ sugar
2.7.1 Fasting insulin level
There were no difference between the two groups in the fasting insulin level. A very small decrease of 0.3 μU/ml was noted in the aripiprazole group (1 RCT, n = 155 CI ‐4.04 to 3.44).
2.7.2 Insulin levels
No clear difference between groups in insulin levels. There was an average increase in insulin of 8.63 in the quetiapine group but the confidence intervals were wide (1 RCT, n = 125 CI‐8.82 to 26.08).
2.7.3 c‐peptide levels
There was no difference between the two groups in the c‐peptide levels. The aripiprazole group had a very small increase of 0.36ng/ml (1 RCT, n = 153 CI ‐0.19 to 0.91).
2.7.4 Fasting blood glucose
There was a significant difference between the groups in fasting blood glucose.The glucose levels decreased by an average of 2.53 mg/dL in the groups switched to aripiprazole and quetiapine from olanzapine (2 RCTs, n = 280 CI ‐2.94 to ‐2.11). Both the studies had used different units of measurement for glucose. Millimoles per litre were converted to milligrams per decilitre for analysis.
2.8 Global state: 1a. Relapse
There was no difference in the number of relapses in the group switched to quetiapine and in those continuing on olanzapine (1 RCT, n = 133 RR1.31 CI 0.55 to 3.11).
2.9 Global state: 1b. Average change
There was no difference between groups in the average changes to the CGI S scores at the end point of treatment in those switched to aripiprazole or those who stayed on olanzapine (1 RCT, n = 164, MD 0.29 CI ‐0.01 to 0.59).
2.10 Mental state
There was no difference between the groups in the average PANSS scores. The group switched to quetiapine had decreased by 3.63 in the PANSS score but the confidence intervals were wide (‐10.31 to 3.05). The mean change in the PANSS scores were reported but the standard deviation had to be calculated from the reported P values.
2.11 Loss to follow‐up
Data from the two studies in this group indicate that people are less likely to leave the study early if they are on olanzapine (RR 1.65 CI 1.21‐2.25). In the study by Newcomer 2008, 36% dropped out in the aripiprazole group compared to 25% in the olanzapine group. In the Eli Lilly 2004 study, the drop‐out was 56% in those switched to quetiapine and only 20% in those continuing on olanzapine. Leaving the study early data may be used as a proxy measure for the acceptability of treatment.
2.12 Adverse events: 1. Serious adverse events
There was no difference between the groups which switched medication (aripiprazole, quetiapine) and those who remained on olanzapine (2 RCTs, n = 305 RR 0.99 CI 0.48 to 2.07).
2.13 Adverse events: 2. Treatment emergent adverse events
There was no difference in the treatment‐induced side effects between the groups which switched medication (aripiprazole, quetiapine) and those who remained on olanzapine (2 RCTs, n = 302 RR1.07 CI 0.92 to 1.24).The data were heterogenous (I2 statistic 51%).
3. Switching ‐ techniques: to aripiprazole from previous regimen
(Table 3) Technique 1 = Immediate initiation + immediate discontinuation of current antipsychotic.
Technique 2 = immediate initiation + tapering of current antipsychotic.
Technique 3 = titrating switch medication upwards + tapering of current antipsychotic.
3.1 Weight: Gain (≥7% from baseline)
There was no clear difference between any different technique for antipsychotic switching on the outcome of weight gain (technique 1 versus 2: 1 RCT, n = 207, RR 0.61 CI 0.15 to 2.47; technique 1 versus 3: 1 RCT, n = 205, RR 0.99 CI 0.20 to 4.79; technique 2 versus 3: 1 RCT, n = 206, RR1.63 CI 0.40 to 6.66).
3.2 Global state: 1a. Average change (CGI‐I, high = good)
3.2.1 Technique 1 versus technique 2
There was no significant difference in the CGI I scores between the groups. The CGI I scores showed a decline of 0.14 in the group switched to aripiprazole immediately compared to the group where the dose of previous medication was gradually tapered (1 RCT, n = 203 CI ‐0.49 to 0.21).
3.2.2 Technique 1 versus technique 3
There was no significant difference in the CGI I scores between the groups. The CGI I scores showed a mean difference of 0.01 (1 RCT, n = 203 CI ‐0.34 to 0.36).
3.2.3 Technique 2 versus technique 3
We found no significant difference between groups with the CGI I scores. There was a very small increase of 0.15 in these scores in the first group (1 RCT, n = 204 CI ‐0.20 to 0.50).
3.3 Global state: 1b. Average change (CGI‐S decline = good)
3.3.1 Technique 1 versus technique 2
We found no significant difference between groups with the CGI S scores. The immediate initiation and the immediate discontinuation group had a decrease of 0.06 compared to the second group (1 RCT, n = 203 CI‐0.27 to 0.15).
3.3.2 Technique 1 versus technique 3
We found no significant difference between groups with the CGI S scores. There was a mean decrease of 0.04 in the immediate initiation and immediate discontinuation group (1 RCT, n = 203 CI ‐0.24 to 0.16).
3.3.3 Technique 2 versus technique 3
We found no significant difference between groups with the CGI S scores (mean difference 0.02, 1 RCT, n = 204 CI ‐0.20 to 0.24).
3.4 Mental state: average change (PANSS, high decline = good)
3.4.1 Technique 1 versus technique 2
We found no clear difference between the groups but the PANSS score was higher by 0.59 in the group where aripiprazole was initiated immediately and the current antipsychotic was discontinued without tapering (1 RCT, n = 197 CI ‐4.51 to 5.69).
3.4.2 Technique 1 versus technique 3
We found no difference between groups but the PANSS score showed an increase of 2.52 in the immediate initiation + immediate discontinuation group (1 RCT, n = 198 CI ‐2.39 to 7.43).
3.4.3 Technique 2 versus technique 3
There was no difference between the two groups. The group where the medication was initiated immediately and the current antipsychotic tapered and stopped indicated a small increase in the PANSS score by 1.93 (1 RCT, n = 201 CI ‐2.63 to 6.49).
3.5 Loss to follow‐up: 1a. Any reason
Fewer people were lost to follow‐up in the group comparing technique 2 to technique 3 compared to the other groups (1 RCT, n = 207 RR 0.82 CI 0.70 to 0.97).There was no difference between the other groups for this outcome (technique 1 vs 2 1 RCT, n = 208 RR 1.04 CI 0.87 to 1.26; technique1 vs 3 n = 207 RR 0.86 CI 0.73 to1.01).
3.6 Loss to follow‐up: 1b. Various specific reasons
3.6.1 to 3.6.5: Technique 1 versus technique 2
We found no difference between the groups in the numbers lost to follow‐up for different reasons including adverse events (1 RCT, n = 208, RR 0.60 CI 0.23 to 1.59), worsening of illness (1 RCT, n = 208, RR1.00 CI 0.43 to 2.30), withdrawal of consent (1 RCT, n = 208, RR 0.58 CI 0.24 to 1.42) non‐compliance (1 RCT, n = 208, RR 4.00 CI 0.45 to 35.19) and other causes (1 RCT, n = 208, RR 2.50 CI 0.50 to 12.60).
3.6.6 to 3.6.10: Technique 1 versus technique 3
We found no difference between the groups in the numbers lost to follow‐up for different reasons including adverse events (1 RCT, n = 207, RR 0.99 CI 0.33 to 2.97), worsening of illness (1 RCT, n = 207 RR 1.24 CI 0.51 to 3.01), withdrawal of consent (1 RCT, n = 207 RR 6.93 CI 0.87 to 55.35) non‐compliance (1 RCT, n = 207 RR 3.96 CI 0.45 to 34.85) and other causes (1 RCT, n = 207, RR1.24 CI 0.34 to 4.48).
3.6.11 to 3.6.15: Technique 2 versus technique 3
The number that was lost to follow‐up through withdrawing of consent was more in the immediate initiation + tapering of current antipsychotics group compared to the titrating switch medication upwards and tapering current antipsychotic group favouring the second group (1 RCT, n = 207, RR 11.88 CI 1.57 to 89.74). There was no difference between the groups for other reasons like adverse events (1 RCT, n = 207, RR 1.65 CI 0.62 to 4.38), worsening of illness (1 RCT, n = 207, RR 1.24 CI 0.51 to 3.01), non‐compliance (1 RCT, n = 207, RR 0.99 CI 0.06 to 15.62) and other causes (1 RCT, n = 207, RR 0.50 CI 0.09 to 2.64).
3.7 Adverse events: any event
3.7.1 Technique 1 versus technique 2
There was no difference between groups (1 RCT, n = 207, RR 1.00 CI 0.91 to 1.10).
3.7.2 Technique 1 versus technique 3
There was no clear difference between the groups (1 RCT, n = 205, RR1.10 CI 0.98 to1.23).
3.7.3 Technique 2 versus technique 3
There was no clear difference between the groups (1 RCT, n = 208, RR 1.12 CI 1.00 to 1.26).
Discussion
Summary of main results
1. Switching ‐ new antipsychotic regimen versus continuation on previous regimen: 1a. different depot from depot – medium term (1 year)
Weight gain whilst taking depot medication is a real problem (Silverstone 1988) and we were surprised to only find one very small trial (Cookson 1986, n = 19). From such a tiny trial no clear effects would be expected but this study shows that such studies are possible and, if larger, could be informative.
1.1 Body weight
There was a mean weight loss of 1.3 kg in the switch group (S.D 3.8).The fluphenazine group showed a mean increase in weight by 1.5 kg (S.D 5.4) thereby favouring the intervention group (switch to haloperidol decanoate). Here the authors had not given measures of dispersion for the mean weight change. So the standard deviation had to be calculated using the individual weights from the graph provided.
1.2 Global state
No scales were used to assess global state in this study. Two out of nine patients in the control group (continuation on fluphenazine decanoate) deteriorated and needed extra medication. Using measurable scales would have made this finding more robust.
1.3 Mental state
No valid scales were used to assess mental state, but two people among the fluphenazine group were found to have a slight deterioration in their mental state. This may suggest that patients on haloperidol had a better outcome.
1.4 Loss to follow‐up
Two people left the study early in the group switched to haloperidol decanoate, but there were no dropouts in the group continuing on fluphenazine decanoate. The reasons for drop‐out were unclear. As drop‐outs could be a proxy indicator of tolerability, this may indicate that people tolerate fluphenazine better than haloperidol. However, such a conclusion should be treated with caution given that the study group was very small.
2 Switching antipsychotic regimen versus continuation on previous regimen: 2. new atypical from olanzapine
2.1 Weight: 1. Body weight (kg)
Weight gain is a well‐recognised side effect, particularly with the newer antipsychotics (Allison 1999; Casey 2004; Homel 2002). Two studies in this group showed a mean weight loss of 1.95 kg when switched to aripiprazole and quetiapine from olanzapine. The individual studies showed an average weight loss of 3.21 kg when switched to aripiprazole and 1.77 kg when switched to quetiapine. The sample size was small in both studies and they were medium term in duration.
2.2 Weight: 2a. BMI increase
2.3 Weight: 2b. BMI average change
More individuals who switched to aripiprazole had a decrease in their BMI when compared to olanzapine. In the other study, average changes in BMI were reported, which showed people who switched to quetiapine had a higher mean decrease in BMI compared to those remaining on olanzapine. Overall, switching from olanzapine to either aripiprazole or quetiapine could decrease BMI. A meta‐analysis would have provided more robust results, but this was impossible as changes in BMI were reported differently in the studies.
2.4 Waist circumference increase
Waist circumference did not show a difference between those switched to aripiprazole and those remaining on olanzapine but the graph revealed a trend favouring aripiprazole as fewer people in this group had an increase in their waist circumference. The study duration was only 16 weeks and longer term studies might show a greater effect. No other study in this review looked at waist circumference.
2.5 Physiological measures: 1a. Average changes
2.6 Physiological measures: 1b. Percentage changes
One RCT did not show any significant difference in lipid levels between the groups switched to quetiapine and those remaining on olanzapine. The power of this study was small; the study was terminated early as they were not able to recruit enough participants (only 33% of the enrolment target as per their protocol). Larger and longer term studies may be needed to detect a real treatment effect.
The other study reported a significant percentage decrease in cholesterol and triglycerides and a significant increase in high‐density lipoproteins when switched to aripiprazole, indicating that a switch to aripiprazole can lead to a favourable lipid profile. The change in low‐density lipoproteins were not significant (P = 0.072) at the end of the study. They used percentage changes to report the findings instead of mean changes with measures of dispersion making it difficult to interpret the results.
Although there were two studies in this group, meta‐analysis was not possible as only one study gave a measure of central tendency (Newcomer 2008).
2.7 Physiological measures: 1c. Average changes ‐ sugar
Insulin levels did not show any difference in the groups switched to aripiprazole or quetiapine compared to continuing on olanzapine. The graph favoured a trend towards staying on olanzapine compared to switching to quetiapine.
Meta‐analysis again was not possible as the two studies used different units of measurement. Using international units in studies would make interpreting the results of interventions more uniform and understandable.
Plasma glucose levels were also measured differently in the two studies in this group. Fortunately it was easy to convert millimoles per litre to milligrams per litre as both units are commonly used. Meta‐analysis was then possible, which showed a definite advantage in levels of fasting glucose when switched to aripiprazole or quetiapine compared to remaining on olanzapine
One RCT did not show any difference between switching to aripiprazole and remaining on olanzapine in c‐peptide levels.
2.8 Global state: 1a. Relapse
2.9 Global state: 1b. Average change (CGI‐S high decline = good)
Relapse as an indicator for change in global state was measured in only one study (continuing on olanzapine compared to switching to quetiapine). There was no difference between the groups. In the other study, where participants were switched to aripiprazole from olanzapine, there was no difference between the groups in average changes in the CGI‐S scores, but the graph favoured olanzapine. Longer term studies again may indicate a differential effect.
2.10 Mental state (PANSS, high decline = good)
This was measured using a scale in one study (Eli Lilly 2004). Based on the PANSS scores, there was no difference between the group switched to quetiapine and those remaining on olanzapine, although the graph tended to favour quetiapine. Only average changes in the scores were reported in the study but we were able to calculate the standard deviation needed for analysis from the reported P values. In the other study (Newcomer 2008), mental state was not an outcome so meta‐analysis was not possible for this outcome.
2.11 Loss to follow‐up
This showed a clear advantage for olanzapine as fewer people remaining on it dropped out. This could indicate that olanzapine has a better tolerability profile. Alternatively, the CATIE trials (CATIE 2006) have shown that people continuing on their prior antipsychotics do remain in treatment longer than those switched to a new medication irrespective of the medications involved, so this finding may not be specific for olanzapine.
2.12 Adverse events: 1. Serious adverse events
2.13 Adverse events: 2. Treatment emergent adverse events
We found no difference between the two groups in the serious adverse events or in any treatment induced adverse events.
3. Switching ‐ techniques: to aripiprazole from previous regiment
3.1 Weight gain ( (≥7% from baseline)
Technique 1 vs technique 2, technique 1 vs technique 3 and technique 2 vs technique 3.
There was no difference between the groups in weight gain.
3.2 to 3.3 Global state (CGI Iand CGI S)
Technique 1 versus technique 2, technique 1 versus technique 3 and technique 2 versus technique 3.
There was no difference in the global state as measured by the CGI‐I and CGI‐S scales when all groups were compared to each other showing that the method of switching medication does not affect this outcome.
3.4 Mental state
Technique 1 versus technique 2, technique 1 versus technique 3 and technique 2 versus technique 3.
Mental state as measured by PANSS did not show a difference between groups but the graph tended to favour the group where the switch medication was titrated upwards and current antipsychotic was tapered and stopped.
3.5 Loss to follow‐up: 1a. Any reason
3.6 Loss to follow‐up: 1b. Various specific reasons
Technique 1 versus technique 2, technique 1 versus technique 3, technique 2 versus technique 3.
The number lost to follow‐up for any reason was significantly less in technique 3 compared to technique 2 (1 RCT, n = 207 RR 0.82 CI 0.70 to 0.97). Looking at the breakdown of reasons reported for loss to follow‐up, the number of people who withdrew consent was the least in the group switched using technique 3 and most in the group switched using technique 2. It is difficult to draw any inferences as the study does not give reasons for why consent was withdrawn. Hence it is difficult to attach any significance to this finding.
The loss to follow‐up due to other reasons (adverse events, worsening of illness, non‐compliance and other causes which included protocol violation, meeting withdrawal criteria) were the same in all other comparison groups.
3.7 Adverse events ‐ any event
Technique 1 versus technique 2, technique 1 versus technique 3, technique 2 versus technique 3. Even though there was no clear difference between the groups, the graph favoured the group where aripiprazole was titrated up and prior medication was tapered and stopped. There could be two reasons to explain this. There is a high likelihood of developing adverse side effects on abrupt introduction of an antipsychotic medication compared to gradual introduction and also the potential for a combined effect of two antipsychotics when the first medication is tapered and the switch medication initiated at the full dose.
Overall completeness and applicability of evidence
Weight gain and metabolic problems associated with the use of antipsychotic medications, particularly with atypical antipsychotic medications are a well recognised and disabling side effect. This can, in addition to the impact on health, affect compliance as well. As the potential to cause these problems varies between different medications, switching to one with a lesser potential for causing weight gain and metabolic problems could be a pragmatic approach to deal with the issue of weight gain.
Surprisingly studies looking at this very important clinical issue were scarce. We were able to include only three studies which primarily looked at switching of medications as an intervention to deal with this problem. The other study included in this review looked at other primary outcomes, but the study had some data on body weight before and after the switch.
As the studies reported data using different units of measure, it was difficult to conduct a meta‐analysis which would have increased the applicability of the findings. Some of the relevant outcomes were reported with means only without giving standard deviations ‐ this made some data unusable. Other possibly relevant studies also had to be excluded due the poor reporting of data.
The majority of the included studies were less than a year in duration. As this review was primarily looking at weight gain and metabolic issues which are likely to be affected over a longer period of time, studies of longer duration would be more informative.
Except for one study (Cookson 1986), the studies had a reasonable number of participants. The drop‐out rate was high (around 30%) in all studies except one (Cookson 1986). Although last observation was carried forwards in all the included studies, the large drop‐out rate casts some doubt on applying these findings in routine clinical settings.
Only one study was a multicentre, multinational study (Newcomer 2008). One study was conducted in the United Kingdom and the other in the United States of America. The setting of the other study is described as multicentre, but in one country which is likely to be in the western hemisphere. This should be taken into account when applying these findings in different care settings.
In all the studies, the majority of the participants were males, making the applicability of these findings to females less clear.
Quality of the evidence
The outcomes, if reported using similar units of measurement, would have increased the quality of evidence, particularly as the number of studies that could be included in this review was very limited. One study reported outcomes using percentage changes which made it difficult to include the study for meta‐analysis.
Three of the four included studies were sponsored by interested pharmaceutical industries, which can be a potential source of bias.
Potential biases in the review process
One study was incomplete and the findings were available from the pharmaceutical company’s trial information. The findings may have been different if the study was completed. There may well be other unpublished studies or reports not included in the review which could lead to a reporting and publishing bias.
We do acknowledge that the electronic search for this review was undertaken some time ago and that new trials may have become available since 2007. We are also aware that Eli Lilly 2004 and Newcomer 2008 both became known to us after the electronic search date and that inclusion of these trials does not reflect the results of the systematic search. This is a potential bias in the review. We plan for a swift update search to bring this review fully in line with current trials.
In addition we have stated in the protocol that we would undertake independent data extraction from selected studies. This did not happen because of time constraints so there is a potential for bias to be operating at this juncture.
Agreements and disagreements with other studies or reviews
This review replicated the finding from a previous study (Remington 2005) that using different switching strategies does not have a significant impact on the outcome of weight gain.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
Schizophrenia is a serious mental health problem needing long‐term care and treatment with antipsychotic medications. Drawbacks of the traditional antipsychotics were the extra‐pyramidal side effects, limiting its prescription and affecting compliance. The newer antipsychotic medications were a relief to both prescribers and patients in this regard. It has now become evident that the long‐term use of some of these medications can affect physical health adversely (weight gain and metabolic problems). Although this review could include only a few studies, the results show that switching to a medication which is less likely to cause weight gain and metabolic problems will reduce body weight, BMI and provide other health benefits. Switching medication may be a pragmatic approach to reduce these side effects without affecting compliance and efficacy.
2. For clinicians
Clinicians face a dilemma between efficacy and side effects when prescribing anti‐psychotic medication to patients with schizophrenia, as they are related to a higher risk of obesity and diabetes. A reasonable approach to manage side effects is to consider making appropriate changes to medications. Even though this review was limited by the small number of studies and poor reporting in the studies, the findings indicate that switching medications to one with the least potential for weight gain and metabolic side effects could be effective in addressing these adverse events. Switching should be done cautiously, as the potential for relapse may be higher and also people may be more likely to drop out of treatment after switching, as indicated by the Eli Lilly 2004 study.
3. For policy makers
Obesity and metabolic problems are serious health hazards which have direct and indirect implications on healthcare resources. The newer antipsychotic prescriptions have increased dramatically and people on these medications risk having these side effects. In the current climate of finite resources, appropriate interventions to tackle these side effects have to be a priority for healthcare providers. There may also be medico‐legal implications which can affect individual practitioners and healthcare providers. Switching might be more cost‐effective than pharmacological or behavioral interventions in preventing and treating obesity in this population. However, none of the included studies had any data on service or economic outcomes. Studies reporting these outcomes will make it easier for organisations to review the cost effectiveness of their services.
4. Note: the 167 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.
Implications for research.
1. General
Though obesity and metabolic problems associated with atypical antipsychotic use are well recognised and many clinicians do attempt to switch medications to minimise these effects, studies looking at this were surprisingly limited. To support clinical practice, more robust evidence is needed. Multicentre trials in different countries, with a comparison group or groups, and a good number of participants over an adequate length of time would provide more applicable evidence. Poor reporting of data made some of the outcome measures unusable and some possible relevant trials had to be excluded. Having internationally recognised standards of measuring outcomes and reporting would help in summing up and analysing the data, which would enhance the strength of evidence.
Including economic analyses in the trials would help clinicians, service providers and policy makers in making cost effective choices.
2. Specific
No studies looked at patient satisfaction or their engagement with the services, which is an important outcome in such trials. There were no studies which compared other methods like lifestyle modifications in controlling weight and metabolic problems in patients on antipsychotic medications.
One suggested outline for a trial is presented in Table 4
1. Suggested design of study.
| Methods | Allocation: randomised, clearly concealed and described. Blinding: double and tested. Duration: one year. CONSORT 2010 guidelines to be incorporated in the protocol and final report. Trial will be prospectively registered in the WHO ICTRP register. Protocol will be published in a peer‐reviewed journal. |
| Partipants | Diagnosis: people with schizophrenia or similar disorders (ICD 10/DSM IV). N = 400‐500.* Age: 18‐65. Sex: both. Weight: obese and/or metabolic problems(internationally accepted criteria for both). On olanzapine or risperidone. Exclusion: diabetes or hypercholesterolaemia requiring pharmacological management. |
| Interventions | 1.Switching to aripiprazole/quetiapine+ routine advice on life style. N = 100. 2.Continuing on olanzapine/risperidone+ routine advice on life style. N = 100. 3. Switching to aripiprazole/quetiapine+ structured lifestyle modifications. N = 100. 4. Continuing on olanzapine/risperidone + structured lifestyle modifications. N = 100. |
| Outcomes | Body Weight** BMI**. Waist circumference. Metabolic measures: lipid profile, HbA1c,blood glucose**. Global state‐relapse, scales**. Mental state: scales**. Adverse events: scales. Physical health: vital signs Patient satisfaction**. Compliance/engagement with the services**. Loss to follow‐up. Economic outcomes. |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. ** These were selected as our primary outcomes. |
CONSORT‐ Consolidated Standards of Reporting Trials HbA1c‐ Haemoglobin A1c ICTRP‐ International Clinical Trials Registry Platform WHO ‐ World Health Organisation
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2012 | Amended | Update search of Cochrane Schizophrenia Group's Trial Register (see Search methods for identification of studies), 167 studies added to awaiting classification. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 26 February 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Judith Wright and Samantha Roberts for their assistance in the literature searches. We would also like to thank Clive Adams from the Cochrane Schizophrenia Group for his help and support throughout this review. We fully acknowledge the use of standard text in the Methods section of this review. The Cochrane Schizophrenia Group uses a template for protocols that are adapted for each review. Guy Faulkner was supported by a publication grant from the Ontario Mental Health Foundation.
Appendices
Appendix 1. Previous search strategy
Search for original interventions for weight gain Cochrane review (Faulkner 2007). Cochrane Schizophrenia Group's Register (January 2005) was searched using a subject/text‐word search strategy with schizophrenia, antipsychotic medication, exercise, intervention, cognitive therapy, behavioural therapy, diet, weight loss, weight gain, weight change, weight, physical therapy as the main search terms.
MEDLINE (1966‐1/2005) CINAHL (1982‐1/2005), UMI ProQuest Digital Dissertations (1861 to 1/2005), HealthSTAR (1990‐1/2005), Sports Discus (1975‐1/2005), EMBASE (1974‐1/2005), PsycINFO (1872 to 1/2005), and registers of ongoing clinical trials were also searched (see Cochrane Schizophrenia Group Module for full details).
Data and analyses
Comparison 1. Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1a. different depot from depot ‐ medium term (3‐12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: Body weight (kg) ‐ switching to haloperidol decanoate from fluphenazine decanoate | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐7.04, 1.44] |
| 2 Global state: Changing dose because of deterioration ‐ switching to haloperidol decanoate from fluphenazine decanoate | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.35] |
| 3 Mental state: Deteriorated ‐ switching to haloperidol decanoate from fluphenazine decanoate | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.35] |
| 4 Loss to follow up ‐ switching to haloperidol decanoate from fluphenazine decanoate | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [0.25, 83.70] |
1.1. Analysis.
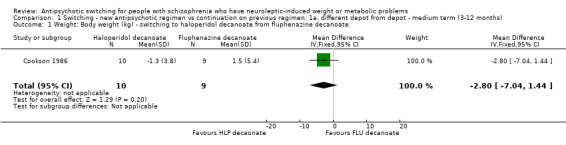
Comparison 1 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1a. different depot from depot ‐ medium term (3‐12 months), Outcome 1 Weight: Body weight (kg) ‐ switching to haloperidol decanoate from fluphenazine decanoate.
1.2. Analysis.

Comparison 1 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1a. different depot from depot ‐ medium term (3‐12 months), Outcome 2 Global state: Changing dose because of deterioration ‐ switching to haloperidol decanoate from fluphenazine decanoate.
1.3. Analysis.

Comparison 1 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1a. different depot from depot ‐ medium term (3‐12 months), Outcome 3 Mental state: Deteriorated ‐ switching to haloperidol decanoate from fluphenazine decanoate.
1.4. Analysis.

Comparison 1 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1a. different depot from depot ‐ medium term (3‐12 months), Outcome 4 Loss to follow up ‐ switching to haloperidol decanoate from fluphenazine decanoate.
Comparison 2. Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: 1. Body weight (kg) | 2 | 287 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐3.97, 0.08] |
| 1.1 switching to aripiprazole | 1 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐3.21 [‐9.03, 2.61] |
| 1.2 switching to quetiapine | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐3.93, 0.39] |
| 2 Weight: 2a. BMI ‐ increase | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.57] |
| 3 Weight: 2b. BMI ‐ average change | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.26, 0.22] |
| 3.1 switching to quetiapine | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.26, 0.22] |
| 4 Waist circumference ‐ increase | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.76, 1.11] |
| 5 Physiological measures: 1a. Average changes ‐ switching to quetiapine | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 lipids ‐ cholesterol levels | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.23, 0.27] |
| 5.2 lipids ‐ low density lipoproteins | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.24, 0.20] |
| 5.3 lipids ‐ high density lipoproteins | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.05, 0.11] |
| 5.4 lipids ‐ triglyceride | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.18, 0.44] |
| 6 Physiological measures: 1b. Percentage changes ‐ switching to aripiprazole | Other data | No numeric data | ||
| 6.1 lipids ‐ cholesterol | Other data | No numeric data | ||
| 6.2 lipids ‐ triglycerides | Other data | No numeric data | ||
| 6.3 lipids ‐ low density lipoproteins | Other data | No numeric data | ||
| 6.4 lipids ‐ high density lipoproteins | Other data | No numeric data | ||
| 7 Physiological measures: 1c. Average changes ‐ sugar | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 fasting insulin level | 1 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.3 [‐4.04, 3.44] |
| 7.2 insulin level | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 8.63 [‐8.82, 26.08] |
| 7.3 c‐peptides | 1 | 153 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.19, 0.91] |
| 7.4 sugar ‐ fasting glucose ‐ switching to aripiprazole and quetiapine | 2 | 280 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐2.94, ‐2.11] |
| 8 Global state: 1a. Relapse | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.55, 3.11] |
| 9 Global state: 1b. Average change (CGI‐S, high decline = good) ‐ switching to aripiprazole | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.01, 0.59] |
| 10 Mental state: Average change (PANSS, high decline = good) ‐ switching to quetiapine | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | ‐3.63 [‐10.31, 3.05] |
| 11 Loss to follow‐up | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.22, 2.28] |
| 11.1 switching to aripiprazole | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.89, 2.21] |
| 11.2 switching to quetiapine | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.27, 2.96] |
| 12 Adverse events: 1. Serious adverse event | 2 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.07] |
| 12.1 switching to aripiprazole | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.24, 1.71] |
| 12.2 switching to quetiapine | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.56, 5.96] |
| 13 Adverse events: 2. Treatment emergent adverse events | 2 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 13.1 switching to aripiprazole | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.92, 1.53] |
| 13.2 switching to quetiapine | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.14] |
2.1. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 1 Weight: 1. Body weight (kg).
2.2. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 2 Weight: 2a. BMI ‐ increase.
2.3. Analysis.
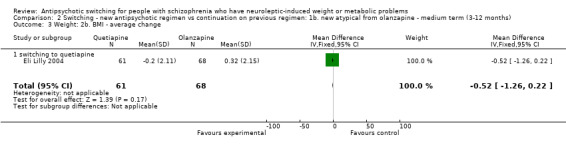
Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 3 Weight: 2b. BMI ‐ average change.
2.4. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 4 Waist circumference ‐ increase.
2.5. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 5 Physiological measures: 1a. Average changes ‐ switching to quetiapine.
2.6. Analysis.
Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 6 Physiological measures: 1b. Percentage changes ‐ switching to aripiprazole.
| Physiological measures: 1b. Percentage changes ‐ switching to aripiprazole | |||||
|---|---|---|---|---|---|
| Study | Group | N | % change | SE | stated p |
| lipids ‐ cholesterol | |||||
| Newcomer 2008 | Switch to aripiprazole | 80 | ‐9.5 | 1.5 | ˜0.005 |
| Newcomer 2008 | Stay on olanzapine | 76 | ‐3.3 | 1.6 | |
| lipids ‐ triglycerides | |||||
| Newcomer 2008 | Switch to aripiprazole | 54 | ‐14.46 | 4.5 (estimate from graph) | ˜0.002 |
| Newcomer 2008 | Stay on olanzapine | 61 | + 5.3 | 5.6 (estimate from graph) | |
| lipids ‐ low density lipoproteins | |||||
| Newcomer 2008 | Switch to aripiprazole | 80 | ‐11.2 | 2.5 | ˜0.072 |
| Newcomer 2008 | Stay on olanzapine | 76 | ‐4.7 | 2.7 | |
| lipids ‐ high density lipoproteins | |||||
| Newcomer 2008 | Switch to aripiprazole | 80 | +1.7 | 1.8 | ˜0.002 |
| Newcomer 2008 | Stay on olanzapine | 76 | ‐5.9 | 1.7 | |
2.7. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 7 Physiological measures: 1c. Average changes ‐ sugar.
2.8. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 8 Global state: 1a. Relapse.
2.9. Analysis.
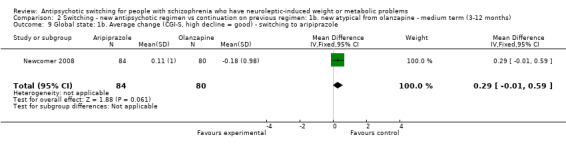
Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 9 Global state: 1b. Average change (CGI‐S, high decline = good) ‐ switching to aripiprazole.
2.10. Analysis.
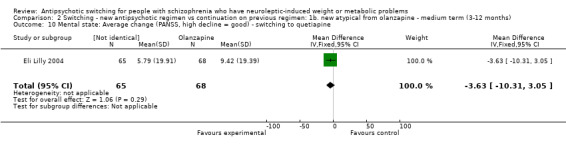
Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 10 Mental state: Average change (PANSS, high decline = good) ‐ switching to quetiapine.
2.11. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 11 Loss to follow‐up.
2.12. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 12 Adverse events: 1. Serious adverse event.
2.13. Analysis.

Comparison 2 Switching ‐ new antipsychotic regimen vs continuation on previous regimen: 1b. new atypical from olanzapine ‐ medium term (3‐12 months), Outcome 13 Adverse events: 2. Treatment emergent adverse events.
Comparison 3. Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight: gain (≥ 7% from baseline) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 technique 1 vs technique 2 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.47] |
| 1.2 technique 1 vs technique 3 | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.20, 4.79] |
| 1.3 technique 2 vs technique 3 | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.40, 6.66] |
| 2 Global state: 1a. Average change (CGI‐I, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 technique 1 vs technique 2 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.49, 0.21] |
| 2.2 technique 1 vs technique 3 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.34, 0.36] |
| 2.3 technique 2 vs technique 3 | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.20, 0.50] |
| 3 Global state: 1b. Average change (CGI‐S, decline = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 technique 1 vs technique 2 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.27, 0.15] |
| 3.2 technique 1 vs technique 3 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.24, 0.16] |
| 3.3 technique 2 vs technique 3 | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.20, 0.24] |
| 4 Mental state: Average change (PANSS, high decline = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 technique 1 vs technique 2 | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐4.51, 5.69] |
| 4.2 technique 1 vs technique 3 | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [‐2.39, 7.43] |
| 4.3 technique 2 vs technique 3 | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [‐2.63, 6.49] |
| 5 Loss to follow up: 1a. Any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 technique 1 vs technique 2 | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.26] |
| 5.2 technique 1 vs technique 3 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 5.3 technique 2 vs technique 3 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.97] |
| 6 Loss to follow‐up: 1b. Various specific reasons | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 technique 1 vs technique 2 ‐ adverse events | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.23, 1.59] |
| 6.2 technique 1 vs technique 2 ‐ worsening illness | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.30] |
| 6.3 technique 1 vs technique 2 ‐ withdrew consent | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.24, 1.42] |
| 6.4 technique 1 vs technique 2 ‐ non‐compliance | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.45, 35.19] |
| 6.5 technique 1 vs technique 2 ‐ other (lost to follow‐up, protocol violation and patient met withdrawal criteria) | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.60] |
| 6.6 technique 1 vs technique 3 ‐ adverse events | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.33, 2.97] |
| 6.7 technique 1 vs technique 3 ‐ worsening illness | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.51, 3.01] |
| 6.8 technique 1 vs technique 3 ‐ withdrew consent | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.93 [0.87, 55.35] |
| 6.9 technique 1 vs technique 3 ‐ non‐compliance | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [0.45, 34.85] |
| 6.10 technique 1 vs technique 3 ‐ other (lost to follow‐up, protocol violation, patient met withdrawal criteria) | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.48] |
| 6.11 technique 2 vs technique 3 ‐ adverse events | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.62, 4.38] |
| 6.12 technique 2 vs technique 3 ‐ worsening illness | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.51, 3.01] |
| 6.13 technique 2 vs technique 3 ‐ withdrew consent | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.88 [1.57, 89.74] |
| 6.14 technique 2 vs technique 3 ‐ non‐compliance | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.62] |
| 6.15 technique 2 vs technique 3 ‐ other (lost to follow‐up, protocol violation, patient met withdrawal criteria) | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.64] |
| 7 Adverse events: Any event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 technique 1 vs technique 2 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 7.2 technique 1 vs technique 3 | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.23] |
| 7.3 technique 2 vs technique 3 | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.00, 1.26] |
3.1. Analysis.
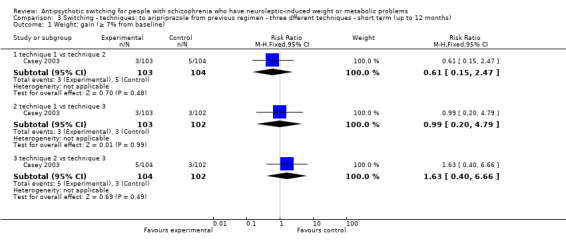
Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 1 Weight: gain (≥ 7% from baseline).
3.2. Analysis.

Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 2 Global state: 1a. Average change (CGI‐I, high = good).
3.3. Analysis.

Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 3 Global state: 1b. Average change (CGI‐S, decline = good).
3.4. Analysis.

Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 4 Mental state: Average change (PANSS, high decline = good).
3.5. Analysis.

Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 5 Loss to follow up: 1a. Any reason.
3.6. Analysis.

Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 6 Loss to follow‐up: 1b. Various specific reasons.
3.7. Analysis.

Comparison 3 Switching ‐ techniques: to aripriprazole from previous regimen ‐ three dfferent techniques ‐ short term (up to 12 months), Outcome 7 Adverse events: Any event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Casey 2003.
| Methods | Allocation: randomised. Blindness: open. Duration: 8 weeks. Setting: outpatients, multicentre, United States. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. N = 311. Age: 18 to 65 years; mean ˜39±10. Weight: ˜ mean 90 kg (SD 20). Sex: 218 men, 93 women. History: chronic and stable illness, on single oral typical (haloperidol or thioridazine) or atypical (olanzapine or risperidone) antipsychotic, adequate clinical reason for switch, no hospitalisation for 2 months prior to study, no risk of suicide, not pregnant, no neurological diagnosis, no acute or unstable diagnosis, no alcohol or psychoactive substance dependence. | |
| Interventions | 1. Switch to aripiprazole 30mg: immediate initiation with immediate discontinuation of current antipsychotic. N = 104. 2. Switch to aripiprazole 30mg: immediate initiation and tapering of current antipsychotics over two weeks. N = 104. 3. Switch to aripiprazole 30mg: titrating aripiprazole upwards and tapering of current antipsychotic over two weeks. N = 103. |
|
| Outcomes | Body weight. Global state: CGI‐S, CGI‐I Mental state: PANSS. Loss to follow‐up. Adverse events. Unable to use ‐ vital signs and laboratory measures (no numerical data). Adverse events: AIMS, Barnes Akathesia scale, SAS. prolactin, QTc interval.* Concomitant medication: (no numerical data reported).** |
|
| Notes | * safety data set total N = 309. ** may cause weight gain. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"were randomised to one of the three aripiprazole treatment groups". Comment: method not stated but authors report this was done via a "centralised telephone system". We feel this indicates the likely use of a random sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: randomised "via a centralised telephone call‐in system". Comment: this would adequately conceal allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Open label". Comment: lack of blinding is less prone to cause bias for outcomes like weight. Lack of blinding may introduce bias for subjective outcomes like adverse events. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was a difference in drop‐outs between 3 groups.The reason for the difference was due to administrative reasons (between group differences in those who withdrew consent). The discontinuation was similar across groups for other reasons. For 4%, reason for discontinuation stated as "other which included lost to follow up, protocol violation and meeting withdrawal criteria. No further details. There were no numerical data reported on vital signs and concomitant medication. The total number randomised was 311 but safety data set had 309 people only. Unsure what happened to the rest. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | Sponsored by interested industry. |
Cookson 1986.
| Methods | Allocation: randomised, stratified by sex, no further details. Blindness: double. Duration: 1 year. Setting: depot injection clinic, London, United Kingdom. | |
| Participants | Diagnosis: unclear, likely schizophrenia. N = 19. Age: average ˜43 years, range 26‐60. BMI > 25. Sex: 9 male, 10 female. History: regular attenders, stable. | |
| Interventions | 1. Haloperidol decanoate: dose (four times the dose and the same frequency as previously prescribed fluphenazine decanoate) N = 10. 2. Fluphenazine decanoate: same dose and frequency as had previously been on. N = 9. | |
| Outcomes | Weight: final average change.
Global state: dose change, extra medication.
Mental state: overall impression. Loss to follow‐up. Unable to use ‐ Mental state: Krawiecka‐Goldberg scale, Schizophrenia Change Scale and Comprehensive Psychopathological Rating Scale (no numerical data). Adverse effects: Simpson and Angus Scale and Abnormal Involuntary Movement Scale (no usable data). |
|
| Notes | Weight data: mean change reported but SD calculated by reading off graph. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"separate randomisation sequences were used for male and female patients". Comment: we feel there is adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No mention about concealment of allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:" double blind" Comment: the methods used if any to ensure participants and trialists were blind to the interventions used are not described. We feel as the primary outcome is objective (weight) inadequate blinding is unlikely to cause bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Both the 2 drop‐outs were in the intervention group. One had lost weight which was the primary outcome measure but the other person had gained weight. Missing data not inputted into the final analysis. Scales were used for assessment of mental state and side effects, but no data available from these. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Eli Lilly 2004.
| Methods | Allocation: randomised. Blindness: double. Duration: 24 weeks. Setting: 26 outpatient study centres in one country. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. N = 133. Age = 45.4 (olanzapine) 42.5 (quetiapine). BMI =/>25. Sex: 81 men, 52 women. History: obese or overweight patients with metabolic complications, on stable dose of olanzapine and compliant for the last 15 days. Excluded: people who received any antipsychotic except olanzapine in the past 30 days. | |
| Interventions | 1.Continue on the same olanzapine dose (mean modal dose of 16.9 mg per day). N = 68. 2.Switch to quetiapine (mean dose = 439.7mg). N = 65. |
|
| Outcomes | Weight, BMI. Laboratory: cholesterol, triglycerides, LDL, HDL, fasting glucose, insulin relapse. Mental state: PANSS. Loss to follow‐up. Adverse events, serious adverse events, treatment emergent adverse events. Unable to use: no numerical data. Waist circumference. Barnes Akathesia scale, Simpson Angus rating scale. Health outcome: quality of life SF36, GAF, DAI 10 eating behaviour. Resource utilisation questionnaire. | |
| Notes | Protocol was terminated early prior to breaking the blind as only 33% of the enrolment target as per protocol enrolled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned". Comment: as the method is not stated it is not clear whether there was adequate sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No data in the report about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind". Comment: the methods used if any to ensure participants and trialists were blind to the interventions used are not reported, but both the intervention medication and the control were in capsule forms. We feel inadequate blinding is not likely to introduce bias for objective outcomes like weight BMI and metabolic syndrome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20/68 missing from the control group compared to 37/65 in the intervention group. Reasons for discontinuation differ across both groups particularly in lack of efficacy (3/65 in intervention group and 1/68 in control group), clinical decision/lost to follow‐up 3/65 intervention group vs 0/68 in control group and patient decision (3/65 vs 1/68). |
| Selective reporting (reporting bias) | Unclear risk | No reports from the various scales mentioned in the methods section. Authors state as the protocol was terminated early "some planned secondary analysis unfeasible or irrelevant". |
| Other bias | High risk | Protocol was terminated early prior to breaking the blind. Sponsored by interested industry. |
Newcomer 2008.
| Methods | Allocation: randomised. Blindness: double. Duration: 16 weeks. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. N = 173.
Age: ˜39 years (SD 10).
BMI: > 27kg/m2.
Sex: 111 male and 62 females.
History: 10‐20 mg olanzapine 1‐24 month prior to screening, CGI score ≦ 4, weight gain prior to olanzapine verified. Excluded: other axis 1 disorder, Type I or II diabetes mellitus, any clinically significant neurological abnormality, stroke, TIA, increased risk of suicide, substance dependence except caffeine and nicotine, increase in symptoms requiring hospitalisation or change in antipsychotic therapy, weight loss 3 months before screening, vital sign ECG or laboratory test abnormalities etc. |
|
| Interventions | 1. Switch to aripiprazole monotherapy: mean dose 16 mg per day. N = 88. 2. Continue olanzapine monotherapy: mean dose 15.9mg per day. N = 85. |
|
| Outcomes | Weight: body weight, BMI, waist circumference. Physiological: triglyceride, fasting plasma glucose, fasting insulin, fasting c‐peptide, fasting lipids Global state: CGI‐ I, CGI‐S. Loss to follow‐up. Adverse events: serious adverse events, treatment emergent adverse events. Unable to use ‐ Physiological measures: vital signs, ECG, routine laboratory tests (no usable data). Adverse effects: AIMS, Simpson‐Angus scale (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "evenly randomly assigned". Comment: we do not feel this is an adequate random sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double blind study". Comment: methods taken to ensure blindness not reported. For outcomes like weight, BMI, waist circumference and metabolic syndrome, inadequate blinding is unlikely to cause bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32/88 missing from the intervention group compared to 22/85 in the control group. Reasons for discontinuation were roughly the same in both groups except in lack of efficacy 7/56 versus 0/63. No numerical data on vital signs, ECG and routine laboratory tests. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Some authors are employed by an interested drug industry. |
AIMS BMI CGI‐I CGI‐S DAI ECG GAF HDL LDL PANSS QTc SAS SD SF10 TIA
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2006 | Allocation: randomised. Participants: outpatients with schizophrenia with negative symptoms. Interventions: olanzapine versus risperidone, previously on conventional antipsychotics, no switching after randomisation. |
| Brar 2005 | Allocation: randomised. Participants: people with schizophrenia, formally on olanzapine already switched to risperidone remaining overweight. Interventions: behavioural therapy versus usual care, no switching after randomisation. |
| CATIE ‐ Phase I ‐ 2006 | Allocation: randomised. Participants: people with schizophrenia. Interventions: olanzapine, quetiapine, risperidone and ziprasidone versus perphenazine ‐ no switching after randomisation. |
| CATIE ‐ Phase II ‐ 2006 | Allocation: randomised. Partcipants: subjects with schizophrenia who had discontinued randomly assigned atypical antipsychotic during the phase 1 CATIE trials. Interventions: randomly assigned to a different antipsychotic. No switching after randomisation (Phase 2 CATIE trials). |
| Chrzanowski 2006 | Allocation: randomised. Participants: patients with acutely relapsing or chronic stable schizophrenia. Interventions: aripiprazole versus olanzapine, no switching after randomisation. |
| Covell 1999 | Allocation: randomised. Participants: unsure ‐ extract from conference proceedings, unable to find full text version. Interventions: clozapine versus conventional antipsychotics, no switching. |
| Ducate 2003 | Allocation: not randomised, case series of randomly selected people. |
| Emsley 2005 | Allocation: randomised. Participants: patients with schizophrenia. Interventions: quetiapine versus haloperidol, no switching after randomisation. |
| Godleski 2003 | Allocation: randomised. Participants: stable people with schizophrenia. Interventions: continue on current depot or switch to oral olanzapine; aim of switch was not specifically as an intervention for weight gain or metabolic problems and as the switch was to a medication known to cause weight gain and metabolic problems this was difficult to be inferred. |
| Horacek 2004 | Allocation: randomised. Participants: people with schizophrenia receiving typical antipsychotics, not clearly over weight. Interventions: continue on typical antipsychotic versus switching to flexibly dosed quetiapine. Outcome: weight change, mental state, cognitive functions, adverse events, vital signs ‐ no usable data (means, no measures of dispersion). |
| Irwin 2003 | Allocation: randomised, crossover. Participants: people with schizophrenia. Interventions: olanzapine versus risperidone. Outcome: no weight or physiological measure data for metabolic syndrome. |
| Kane 2004 | Allocation: not randomised, presentation on guidelines on use of long‐acting injectable atypical antipsychotics. |
| Kane 2005 | Allocation: unclear. Participants: people with treatment refractory schizophrenia on ziprasidone. Interventions: chlorpromazine versus ziprasidone, no switching. |
| Kane 2007 | Allocation: randomised. Participants: people with schizophrenia with history of antipsychotic resistance. Interventions: aripiprazole versus perphenazine, no switching. |
| Kelly 2003 | Allocation: randomised, crossover. Participants: patients with treatment resistant schizophrenia. Interventions: clozapine versus high dose olanzapine (50mg). Outcome: weight, metabolic parameters, side effects, blood pressure (no usable data ‐ data only pre‐switch). |
| Kim 2007 | Allocation: not randomised. |
| Kinon 2000 | Allocation: randomised. Participants: people with schizophrenia or schizoaffective disorder. Interventions: switching from conventional antipsychotics to olanzapine through four switching strategies ‐ aim of switch was not specifically as an intervention for weight gain or metabolic problems and as the switch was to a medication known to cause weight gain and metabolic problems this was difficult to infer. |
| Kinon 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: remain on baseline antipsychotic therapy vs switch to olanzapine ‐ aim of switch was not specifically as an intervention for weight gain or metabolic problems and as the switch was to a medication known to cause weight gain and metabolic problems this was difficult to infer. |
| Lee 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: switch from typical antipsychotics to olanzapine using 2 switching techniques ‐ aim of switch was not specifically as an intervention for weight gain or metabolic problems and as the switch was to a medication known to cause weight gain and metabolic problems this was difficult to infer. |
| Nasrallah 2001 | Allocation: randomised. Participants: patients with schizophrenia on oral fluphenazine and showing partial response. Interventions: randomised to quetiapine or haloperidol, no switching after randomisation. |
| Newcomer 2006 | Allocaton: randomised. Participants: patients with schizophrenia with abnormal values for non HDL cholesterol. Interventions: aripiprazole versus olanzapine, no switching. |
| O'Halloran 2003 | Allocation: not randomised, observational study. |
| Robinson 2000 | Allocation: not randomised, continuation of a trial 5 people continued on double blind olanzapine or risperidone. |
| STAR trials 2006 | Allocation: randomised. Participants: people with schizophrenia in the community. Interventions: STAR study‐randomised to aripiprazole or standard of care (olanzapine/risperidone/quetiapine), no switching after randomisation. |
| Su 2005 | Allocation: not randomised. |
| Wang 2006 | Allocation: randomised. Participants: people with schizophrenia. Interventions: risperidone versus olanzapine, no switching. |
| Weiden Daniel 2003 | Allocation: randomised. Participants: people with schizophrenia or schizoaffective disorder. Interventions: switched from conventional antipsychotics, olanzapine or risperidone to ziprasidone through 3 switching strategies. Outcome: weight change, BMI, non‐fasting triglycerides, total cholesterol, prolactin levels, CGI‐I and CGI‐S, PANSS, BPRS, Simpson‐Angus scale, BAS, AIMS, Vital signs, ECG, routine laboratory tests, exacerbation of pre existing illnesses, treatment emergent illnesses, adverse drug reactions ‐ no usable data (reported pooled data which was not from the original randomised group). |
| Zhang 2004 | Allocation: unclear. Participants: inpatient men with schizophrenia. Interventions: chlorpromazine versus clozapine and sulpiride, no switching. |
AIMS ‐ Abnormal Involuntary Movement Scale
BAS BMI ‐ Body Mass Index
BPRS ‐ Brief Psychiatric Rating Scale
CATIE ‐ Clinical Antipsychotic Trials in Intervention Effectiveness
CGI ‐ Clinical Global Improvement
DAI‐10 ‐ Drug Attitude Inventory
ECG ‐ Electrocardiogram
GAF ‐ Global Assessment of Functioning
HDL ‐ High Density Lipoprotein
LDL ‐ Low Density Lipoprotein
PANSS ‐ Positive and Negative Syndrome Scale
RCT ‐ Randomised Control Trial
SAS ‐ Simpson Angus Scale
SD ‐ Standard Deviation
SF36 ‐ Short Form (36)
STAR ‐ Schizophrenia Trial of Aripiprazole
TIA ‐Transient Ischaemic Attack
Characteristics of studies awaiting assessment [ordered by study ID]
Ader 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Alvarez‐Jimenez 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Arman 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Attux 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ballon 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Baptista 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Baptista 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Barak 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Biedermann 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Bobo 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Borba 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Brown 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Buchanan 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Bustillo 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Capra 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Carina 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Citrome 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Czobor 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Day‐Poulsen 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
De Lima 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Deberdt 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Deberdt 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Detke 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Eli 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Faries 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Firestone 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Fleischhacker 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Fleischhacker 2008a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Fleischhacker 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ganguli 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ganguli 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ginsberg 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Henderson 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Henderson 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Henderson 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Hoffmann 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Iglesias‐Garcia 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
IRCT201009112181N5.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
IRCT201107187049N1.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Joffe 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Karagianis 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kelly 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kelly 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kelly 2011a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kent 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kim 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kim 2007a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kim 2007b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kim 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Klein 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Klein 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kluge 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ko 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Krakowski 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kuang 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kwon 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Lambert 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Lan 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Lencz 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Li 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Lieberman 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Linne 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Mauri 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
McElroy 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Meyer 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Meyer 2009a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Millar 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Millar 2008a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Moore 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00095524.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00303602.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00423878.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00645099.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00690235.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00709202.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00734435.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00759460.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00759993.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00793780.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00806234.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00816907.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00845507.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00857818.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00902694.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00934908.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00990925.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01052714.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01075295.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01167348.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01272752.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01272765.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01295372.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01300637.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01324973.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01491490.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01567124.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT01593774.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Newcomer 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Newcomer 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Nicol 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Oliveira 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Pae 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Perez‐Iglesias 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Perez‐Iglesias 2008a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Perez‐Iglesias 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Peuskens 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Poulin 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Poyurovsky 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Raposo 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ratliff 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Saddichha 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Safa 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Schneiderhan 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Smith 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Smith 2010a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Spivak 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Stroup 2011a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Stroup 2011b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Sun 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Takeuchi 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Tao 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Tek 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Tian 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Tiwari 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Uzcategui 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Vaz‐Leal 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wang 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wang 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Warren 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Weiden.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Weiden 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wirshing 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wu 2008a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wu 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Yoon 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Yoon 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Zhang 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
但雪姣 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
刘建金 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
刘朝军 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
刘根 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
刘献标 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
哈秀英 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
崔开艳, 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张东卫 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张华坤 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张峰 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
彭永红 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
掌永莉 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
李晓一 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
李梁 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
李轶琛 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨云秀 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨春强 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨诚 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨雀屏 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨雀屏 2009a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
汪卫华 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
汪卫华 2009a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
王秀芳 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
白锦波 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
罗亨全 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
耿寒松 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
钟潇琦 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
陈俊雄 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
陶世武 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
陶世武 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Characteristics of ongoing studies [ordered by study ID]
Tang 2003.
| Trial name or title | Olanzapine causes greater increases in serum lipids than risperidone. |
| Methods | Allocation: randomised. Duration: 1 year. Site: multicenter. |
| Participants | Diagnosis: schizophrenia or related disorders. N = currently 50. |
| Interventions | Switching from other psychotropic drugs to: 1. olanzapine 2. risperidone. |
| Outcomes | Weight: body weight, BMI. Physiological: triglycerides, cholesterol, glucose, HBA1c, high density and low density lipoproteins. |
| Starting date | Unclear. |
| Contact information | herbert.meltzer@Vanderbilt.Edu |
| Notes | E‐mail contact 2008 ‐ planning to terminate study soon due to funding issues. Not ready to publish. |
HbA1c‐Haemoglobin A1c BMI‐Body Mass Index
Differences between protocol and review
a. We did not calculate NNT as per protocol, as largely this has been superseded by the use of the Summary of Findings table.
Also, this review has been ongoing for some time. During this period Summary of Findings tables became possible. We did not pre‐state outcomes that should be the focus of this table, but have chosen these post‐hoc: These outcomes were chosen after the data were known.
1. Weight ‐ no clinically important change in body weight (as defined by individual studies)
2. Change in BMI
3. Presence of metabolic syndrome
4. Global state ‐ no clinically important change in global state (as defined by individual studies)
5. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) ‐ no clinically important change in general mental state
6. Averse effects ‐ serious
7. Satisfaction with treatment ‐ recipient of care not satisfied with treatment
b. In the protocol, a random‐effects model was planned to be used for measuring treatment effect but the review has used a fixed‐effect model and the reasons for this have been detailed under data synthesis. The Methods section also had to be modified slightly to accommodate the requirements of RevMan 5 (published protocol used RevMan 4).
c. The original protocol states that we were to work independently in extraction of data. Time constraints precluded this so these data were only extracted by AM ‐ but the review has been checked by GF, TC and GR.
Contributions of authors
Anitha Mukundan ‐ Instigated the review, participated in literature searches, selected studies, extracted data and wrote report.
Guy Faulkner ‐ participated in literature searches, selected studies, and contributed to the report.
Tony Cohn ‐ participated in literature searches, selected studies.
Gary Remington ‐ participated in literature searches, selected studies.
Sources of support
Internal sources
Yorkshire Deanery, UK.
University of Toronto, Canada.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Casey 2003 {published data only}
- Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D, Ingenito GG. Switching patients to aripiprazole from other antipsychotic agents: A multicenter randomized study. Psychopharmacology 2003;166(4):391‐9. [DOI] [PubMed] [Google Scholar]
- Medori R, Gharbia N, Saha A, Ali M, Stock E, Ingenito G. Switching to aripiprazole monotherapy. Journal of the European College of Neuropsychopharmacology 2002;12(Suppl 3):S292. [Google Scholar]
Cookson 1986 {published data only}
- Cookson JC, Kennedy NM, Gribbon D. Weight gain and prolactin levels in patients on long‐term antipsychotic medication: a double‐blind comparative trial of haloperidol decanoate and fluphenazine decanoate. International Clinical Psychopharmacology 1986;1(Suppl 1):41‐51. [PUBMED: 3549879] [PubMed] [Google Scholar]
Eli Lilly 2004 {published data only}
- Eli Lilly, Company. Comparison of continuing olanzapine to switching to quetiapine in overweight or obese patients with schizophrenia and schizoaffective disorder. Eli Lilly and Company Clinical Trial Registry 2004. [8894]
Newcomer 2008 {published data only}
- Newcomer JW, Campos JA, Marcus RN, Breder C, Berman RM, Kerselaers W, L'Italien GJ, Nys M, Carson WH, McQuade RD. A multicenter, randomized, double‐blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. Journal of Clinical Psychiatry 2008;69(7):1046‐56. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2006 {published data only}
- Alvarez E, Ciudad A, Olivares JM, Bousono M, Gomez JC. A randomized, 1‐year follow‐up study of olanzapine and risperidone in the treatment of negative symptoms in outpatients with schizophrenia. Journal of Clinical Psychopharmacology 2006;26(3):238‐49. [PUBMED: 16702888] [DOI] [PubMed] [Google Scholar]
- Ciudad A, Olivares JM, Bouson˜o M, Gomez JC, Alvarez E. Improvement in social functioning in outpatients with schizophrenia with prominent negative symptoms treated with olanzapine or risperidone in a 1 year randomized, open‐label trial. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2006;30(8):1515‐22. [PUBMED: 16820255] [DOI] [PubMed] [Google Scholar]
Brar 2005 {published data only}
- Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry 2005;66(2):205‐12. [PUBMED: 15705006] [DOI] [PubMed] [Google Scholar]
CATIE ‐ Phase I ‐ 2006 {published data only}
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Keefe R, Perkins DO, Davis S, Davis CE, Lebowitz B, Hsiao J. CATIE trial results. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S184. [Google Scholar]
- Stroup TS. Comparison of ziprasidone versus other atypical drugs in prospectively defined, unresponsive patients. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
CATIE ‐ Phase II ‐ 2006 {published data only}
- Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. American Journal of Psychiatry 2006;163(4):611‐22. [PUBMED: 16585435] [DOI] [PubMed] [Google Scholar]
Chrzanowski 2006 {published data only}
- Chrzanowski WK, Marcus RN, Torbeyns A, Nyilas M, McQuade RD. Effectiveness of long‐term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52‐week, open‐label comparison with olanzapine. Psychopharmacology 2006;189(2):259‐66. [PUBMED: 17058105] [DOI] [PubMed] [Google Scholar]
Covell 1999 {published data only}
- Covell NH, Essock SM. Weight with clozapine as compared to conventional antipsychotic medications. Schizophrenia Research 1999;1, 2 & 3:354. [Google Scholar]
Ducate 2003 {published data only}
- Ducate S, Pondrom M, Thivierge S, Patel H. Switching to ziprasidone in correctional inpatients: Efficacy and safety. Proceedings of the 156th Annual meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California USA. 2003.
Emsley 2005 {published data only}
- Emsley R, Turner HJ, Schronen J, Botha K, Smit R, Oosthuizen PP. Effects of quetiapine and haloperidol on body mass index and glycaemic control: a long‐term, randomized, controlled trial. International Journal of Neuropsychopharmacology 2005;8(2):175‐82. [DOI] [PubMed] [Google Scholar]
Godleski 2003 {published data only}
- Godleski LS, Goldsmith LJ, Vieweg WV, Zettwoch NC, Stikovac DM, Lewis SJ. Switching from depot antipsychotic drugs to olanzapine in patients with chronic schizophrenia. Journal of Clinical Psychiatry 2003;64(2):119‐22. [DOI] [PubMed] [Google Scholar]
Horacek 2004 {published data only}
- Horacek J, Janu L, Seifertova D. The effect of quetiapine on different domains of cognition and negative symptoms in schizophrenia. Schizophrenia Research 2004;67(1):168. [Google Scholar]
- Horacek J, Seifertova D, Janu L, Petranova T. The effect of quetiapine on different domains of cognition and negative symptoms in schizophrenia. Proceedings of the 12th Biennial Winter Workshop on Schizophrenia; 2004 Feb 7‐13; Davos, Switzerland. 2004.
Irwin 2003 {published data only}
- Irwin J, Moses SN, Edgar JC, Torres F, Thoma RJ, Hanlon FM, Anderson L, Weisend MP, Miller GA, Tuason VB, Canive JM. Olanzapine and risperidone in schizophrenia: a randomized double‐blind crossover study. Schizophrenia Research 2003;60(1):286. [Google Scholar]
Kane 2004 {published data only}
- Kane JM, Conley RR, Keith SJ, Nasrallah HA, Turner M. Guidelines for the use of long‐acting injectable atypical antipsychotics. Journal of Clinical Psychiatry 2004;65(1):120‐31. [DOI] [PubMed] [Google Scholar]
Kane 2005 {published data only}
- Kane JM. Ziprasidone’s long‐term efficacy in treatment‐ refractory schizophrenia. Proceedings of the 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta, Georgia, USA. 2005.
Kane 2007 {published data only}
- Kane JM, Meltzer HY, Carson WH Jr, McQuade RD, Marcus RN, Sanchez R, Aripiprazole Study Group. Aripiprazole for treatment‐resistant schizophrenia: results of a multicenter, randomized, double‐blind, comparison study versus perphenazine. Journal of Clinical Psychiatry 2007;68(2):213‐23. [PubMed] [Google Scholar]
Kelly 2003 {published data only}
- Kelly DL, Conley RR, Richardson CM, Tamminga CA, Carpenter Jr WT. Adverse effects and laboratory parameters of high‐dose olanzapine vs. clozapine in treatment‐resistant schizophrenia. Annals of Clinical Psychiatry 2003;15(3‐4):181‐6. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim SH, Ivanova O, Abbasi FA, Lamendola CA, Reaven GM. Metabolic impact of switching antipsychotic therapy to aripiprazole after weight gain. Journal of Clinical Psychopharmacology 2007;27(4):365‐68. [DOI] [PubMed] [Google Scholar]
Kinon 2000 {published data only}
- Kinon B, Basson B, Malcolm S, Breier A. Strategies for switching from conventional antipsychotic drugs or risperidone to olanzapine. Proceedings of the 39th Annual Meeting of the New Clinical Drug Evaluation Unit; 1999 Jun 1‐4; Boca Raton, Florida, USA. 1999.
- Kinon B, Breier A, Malcolm S, Basson B. Strategies for switching from conventional antipsychotic drugs or risperidone to olanzapine. 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999. [DOI] [PubMed]
- Kinon BJ, Basson B, Wang J, Malcolm SK, Stauffer VL. Rapid reduction in hyperprolactinemia upon switching treatment to olanzapine from conventional antipsychotic drugs or risperidone. Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; 2000 May 30 ‐ Jun 2; Boca Raton, Florida, USA. 2000.
- Kinon BJ, Basson BR, Gilmore JA, Malcolm S, Stauffer VL. Strategies for switching from conventional antipsychotic drugs or risperidone to olanzapine. Journal of Clinical Psychiatry 2000;61(11):833‐40. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Basson BR, Wang J, Malcolm SK, Stauffer VL. Rapid reduction in hyperprolactinemia. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, Illinois, USA. 2000.
Kinon 2004 {published data only}
- Kinon BJ, Ahl J, Liu‐Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive co‐morbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology 2006;31(5):577‐88. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Liu‐Seifert H, Ahl J, Ahmed S, Baker RW. Longitudinal effect of olanzapine on fasting serum lipids: a randomized, prospective, 4‐month study. Annals of the New York Academy of Sciences 2004;1032:295‐6. [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee CT, Conde BJ, Mazlan M, Visanuyothin T, Wang A, Wong MM, Walker DJ, Roychowdhury SM, Wang H, Tran PV. Switching to olanzapine from previous antipsychotics: a regional collaborative multicenter trial assessing 2 switching techniques in Asia Pacific. Journal of Clinical Psychiatry 2002;63(7):569‐76. [DOI] [PubMed] [Google Scholar]
Nasrallah 2001 {published data only}
- Emsley RA, Bailey P, Jones AM, Raniwalla J. Efficacy of quetiapine fumarate in partial responders. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20; Washington DC, USA. 1999.
- Nasrallah HA, Rennie P, Altman C. Patterns of response in patients with schizophrenia partially responsive to fluphenazine and switched to quetiapine or haloperidol. European Neuropsychopharmacology 2001;11(3):262. [Google Scholar]
- Weiden PJ, Ashworth P. Switching from fluphenazine to quetiapine ameliorates extrapyramidal symptoms and neuroendocrine side effects in patients with schizophrenia. European Neuropsychopharmacology 2001;11(3):262. [Google Scholar]
Newcomer 2006 {published data only}
- Newcomer JW, L'Italien G, Vester‐Blokland, McQuade RD, Carson WH, Marcus RN. Improvement of non‐hdl cholesterol levels among patients randomized to aripiprazole versus olanzapine. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
O'Halloran 2003 {published data only}
- O'Halloran RA, Hodge A, Kim K‐S, Habil MHH, Barahona A, Vargas MT, Woodbury MA. Comparison of olanzapine and quetiapine treatments for schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
Robinson 2000 {published data only}
- Robinson G, Wheeler A, Byrd J, Visser S. Longer‐term effects of switching from typical to atypical antipsychotics in patients with stable schizophrenia. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl 3):S291. [Google Scholar]
STAR trials 2006 {published data only}
- Corey‐Lisle P, Kolotkin RL, Crosby RD, L'Italien G. Changes in weight and weight‐related quality of life in aripiprazole versus standard‐of‐care treatment. Proceedings of the 14th Congress of the Association of European Psychiatrists; 2006 March 4‐8; Nice, France. 2006.
- Hanssens L, L'Italien G, Marcus RN, McQuade RD, Carson WH, Beuzen J‐N. Reasons for switching among community‐treated schizophrenic patients in a naturalistic setting: schizophrenia trial of aripiprazole: STAR study. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
- L'Italien G, Hanssens L, Marcus RN, McQuade RD, Carson WH, Beuzen J‐N. Metabolic effects of aripiprazole versus standard of care (the STAR trial). Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
- McQuade R, Radhakrishnan M, Stock E, Lam S, Iwamoto T, Pans M, Carson W. Investigator assessment of clinical parameters after initiating aripiprazole therapy. Proceedings of the 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26, Atlanta, GA, USA 2005.
- Riera L, Hu R, Stock E, Nyilas M, Torbeyns a, Borian F, Gentile K, Carson W, Iwamoto T. Broad effectiveness trial with aripiprazole. Schizophrenia Research 2004;67(1):157. [Google Scholar]
- Riera L, Tandon R, Stock EG, Kujawa MJ, Torbeyns AF, Borian FE, McQuade RD, IwamotoT. Broad Effectiveness Trial with Aripiprazole. Proceedings of the Twelfth Biennial Winter Workshop on Schizophrenia; 2004 Feb 7‐13; Davos Switzerland. 2004.
Su 2005 {published data only}
- Su K‐P, Wu P‐L, Pariante CM. A crossover study on lipid and weight changes associated with olanzapine and risperidone. Psychopharmacology 2005;183:383‐86. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang X, Savage R, Borisov A, Rosenberg J, Woolwine B, Tucker M, May R, Feldman J, Nemeroff CB, Miller AH. Efficacy of risperidone versus olanzapine in patients with schizophrenia previously on chronic conventional antipsychotic therapy: a switch study. Journal of Psychiatric Research 2006;40(7):669‐76. [DOI] [PubMed] [Google Scholar]
Weiden Daniel 2003 {published data only}
- Cutler NR, Simpson G, Weiden PJ, Romanoa SJ. Effects of oral ziprasidone on weight and serum lipids in patients with schizophrenia. Schizophrenia Research 2002;53(3 Suppl 1):160. [Google Scholar]
- Daniel D, Lieberman J, Birnbaum R. Improvement in markers of health status 6 weeks after switching from olanzapine to ziprasidone. Journal of the European College of Neuropsychopharmacology 1999;9:S265. [Google Scholar]
- Daniel D, Stern R, Kramer T. Switching from olanzapine to ziprasidone: an interim analysis of a 6‐week study. Journal of the European College of Neuropsychopharmacology 1999;9:S265. [Google Scholar]
- Daniel DG, Lieberman JA, Birnbaum RJ. Improvement in markers of health status six weeks after switching from olanzapine to ziprasidone. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20; Washington DC, USA. 1999.
- Daniel DG, Weiden P, O'Sullivan RL. Improvement in indices of health status in outpatients with schizophrenia following a switch to ziprasidone from conventional antipsychotics, olanzapine or risperidone. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, Illinois, USA. 2000.
- Daniel DG, Weiden P, O'Sullivan RL. Improvement in indices of health status in outpatients with schizophrenia following a switch to ziprasidone from conventional antipsychotics, olanzapine or risperidone. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Romano SJ, Cutler NR, Weiden PJ, Simpson GM. Ziprasidone's effects on weight and lipids in patients with schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Weiden P, Simpson G, Kramer T, Harvey P. Switching from conventional antipsychotics to ziprasidone: an interim analysis of a 6‐week study. Journal of the European College of Neuropsychopharmacology 1999;9:S264. [Google Scholar]
- Weiden PJ, Daniel DD, Simpson G, Romano SJ. Improvement in indices of health status In outpatients with schizophrenia switched to ziprasidone. Journal of Clinical Psychopharmacology 2003;23(6):595‐600. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Kramer TAM, Harvey PD, Powchik P, Simpson GM, Switch Study Group. Switching from conventional antipsychotics to ziprasidone: an interim analysis of a six‐week study. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20; Washington DC, USA. 1999.
- Weiden PJ, Newcomer JW, Loebel AD, Yang R, Lebovitz HE. Long‐term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33(5):985‐94. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Simpson GM, Potkin SG, O'Sullivan RL. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. Journal of Clinical Psychiatry 2003;64:580‐8. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Weiden PJ, Daniel DG, Simpson G, Romano SJ. Health status in stable patients switched to ziprasidone. Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama, Japan. 2002.
Zhang 2004 {published data only}
- Kim SH, Ivanova O, Abbasi FA, Lamendola CA, Reaven GM, Glick ID. Metabolic impact of switching antipsychotic therapy to aripiprazole after weight gain. Journal of Clinical Psychopharmacology 2007;27(4):365‐68 . [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang G, Tang Q. A control study of weight gain caused by chlorpromazine, clozapine and sulpiride in schizophrenics. Journal of Clinical Psychosomatic Diseases 2004;10(3):182‐84. [Google Scholar]
References to studies awaiting assessment
Ader 2008 {published data only}
- Ader M, Garvey WT, Phillips LS, Nemeroff CB, Gharabawi G, Mahmoud R, et al. Ethnic heterogeneity in glucoregulatory function during treatment with atypical antipsychotics in patients with schizophrenia. Journal of Psychiatric Research 2008;42(13):1076‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez‐Jimenez 2010 {published data only}
- Alvarez‐Jimenez M, Martinez‐Garcia O, Perez‐Iglesias R, Ramirez ML, Vazquez‐Barquero JL, Crespo‐Facorro B. Prevention of antipsychotic‐induced weight gain with early behavioural intervention in first‐episode psychosis: 2‐year results of a randomized controlled trial. Schizophrenia Research 2010;116(1):16‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arman 2008 {published data only}
- Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double‐blind, placebo‐controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Medical Journal 2008;29(8):1130‐4. [PubMed] [Google Scholar]
Attux 2011 {published data only}
- Attux C, Martini LC, Reis AF, Bressan RA. A non‐pharmacological intervention for weight gain management for patients with schizophrenia: Multicentric, randomized controlled clinical trial‐are modest effects important for challenging outcomes. Schizophrenia Bulletin 2011;1:294. [Google Scholar]
Ballon 2011 {published data only}
- Ballon JS, Hamer RM, Catellier DJ, Stewart D, Lavange L, Golden L, et al. Metformin and impaired glucose tolerance in overweight persons with schizophrenia. Schizophrenia Bulletin 2011;1:24. [Google Scholar]
Baptista 2008 {published data only}
- Baptista T, Uzcategui E, Rangel N, Fakih Y, Galeazzi T, Beaulieu S, et al. Metformin plus sibutramine for olanzapine‐associated weight gain and metabolic dysfunction in schizophrenia: a 12‐week double‐blind, placebo‐controlled pilot study. Psychiatry Research 2008;159(1‐2):250‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baptista 2009 {published data only}
- Baptista T, Rangel N, Fakih Y, Uzcategui E, Galeazzi T, Beaulieu S, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double‐blind, placebo‐controlled, 12‐week trial. Pharmacopsychiatry 2009;42(1):14‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barak 2010 {published data only}
- Barak N. Betahistine safely mitigates olanzapine adverse: Role of histamine receptors in weight gain and somnolence. Proceedings of the 163rd Annual Meeting of the American Psychiatric Association; 2010 May 22‐26; New Orleans, LA. 2010.
Biedermann 2010 {published data only}
- Biedermann F, Lechleitner M, Hofer A, Huber R, Futhmu NE‐, Ebenbichler C, et al. Sibutramine in the treatment of antipsychotic‐induces weight gain: a randomized, placebo‐controlled double‐blind study. Schizophrenia Research 2010;117(2‐3):260‐1. [Google Scholar]
Bobo 2011 {published data only}
- Bobo WV, Bonaccorso S, Jayathilake K, Meltzer HY. Prediction of long‐term metabolic effects of olanzapine and risperidone treatment from baseline body mass index in schizophrenia and bipolar disorder. Psychiatry Research 2011;189(2):200‐7. [DOI] [PubMed] [Google Scholar]
Borba 2011 {published data only}
- Borba CPC, Fan X, Copeland PM, Paiva A, Freudenreich O, Henderson DC. placebo‐controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. Journal of Clinical Psychopharmacology 2011;31(5):653‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2011 {published data only}
- Brown C, Goetz J, Hamera E. Weight loss intervention for people with serious mental illness: A randomized controlled trial of the renew program. Psychiatric Services 2011;62(7):800‐2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchanan 2011 {published data only}
- Buchanan RW, Boggs DL, Conley RR, Gorelick DA, McMahon RP, Gold JM, et al. A pilot study evaluating the cognitive effects of rimonabant in people with schizophrenia. Proceedings of the 50th Annual Meeting of the American College of Neuropsychopharmacology; 2011 Dec 4‐8; Waikoloa, Hawaii. 2011.
Bustillo 2003 {published data only}
- Bustillo JR, Lauriello J, Parker K, Hammond R, Rowland L, Bogenschutz M, et al. Treatment of weight gain with fluoxetine in olanzapine‐treated schizophrenic outpatients. Neuropsychopharmacology 2003;28(3):527‐9. [DOI] [PubMed] [Google Scholar]
Capra 2009 {published data only}
- Capra C. Effects of a nutritional supplement on weight gain after initiation of atypical antipsychotic medication in early psychosis patients. Australian New Zealand Clinical Trials Registry 2009.
Carina 2009 {published data only}
- Carina C. Effects of a nutritional supplement on weight gain after initiation of atypical antipsychotic medication in early psychosis patients. Australian New Zealand Clinical Trials Registry 2009.
Citrome 2009 {published data only}
- Citrome L, Stauffer VL, Chen L, Kinon BJ, Kurtz DL, Jacobson JG, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. Journal of Clinical Psychopharmacology 2009;29(3):278‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Czobor 2002 {published data only}
- Czobor P, Volavka J, Sheitman B, Lindenmayer J‐P, Citrome L, McEnvoy J, et al. Antipsychotic‐induced weight gain and therapeutic response: a differential association. Journal of Clinical Psychopharmacology 2002;22(3):244‐51. [DOI] [PubMed] [Google Scholar]
Day‐Poulsen 2009 {published data only}
- Day‐Poulsen H, Thomas P, Loze JY, Fortan L, Kerselaers W, Owen R. A 16‐week, multicentre, randomized, open‐label study assessing effects of aripiprazole versus other atypicals for schizophrenia patients with metabolic syndrome. Proceedings of the 17th European Psychiatric Association, EPA Congress; 2009 Jan 24‐28; Lisbon Portugal 2009;24:S1181. [Google Scholar]
Deberdt 2004 {published data only}
- Deberdt W, Trzaskoma Q, Carlson C, Bymaster F, Winokur A, Floris M. Amantadine for weight gain in olanzapine‐treated patients. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
Deberdt 2008 {published data only}
- Deberdt W, Lipkovich I, Heinloth AN, Liu L, Kollack‐Walker S, Edwards SE, et al. double‐blind, randomized trial comparing efficacy and safety of continuing olanzapine versus switching to quetiapine in overweight or obese patients with schizophrenia or schizoaffective disorder. Therapeutics and Clinical Risk Management 2008;4(4):713‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Lima 2007 {published data only}
- Lima MS, Oliveira AV, Dossenbach M, Mari JJ. Weight gain associated with olanzapine and first generation antipsychotics: results from a pragmatic randomized controlled trial. European Neuropsychopharmacology 2007;17(Suppl 4):S467. [Google Scholar]
Detke 2008 {published data only}
- Detke H, McDonnell D, Andersen S, Watson S. Olanzapine long‐acting injection in patients with schizophrenia at risk of relapse: 12‐week switching data. European Neuropsychopharmacology 2008;18:S435. [Google Scholar]
Eli 2007 {published data only}
- Eli LandC. The comparison of efficacy and safety of continuing olanzapine to switching to quetiapine in overweight or obese patients with schizophrenia or schizoaffective disorder. Eli Lilly and Company Clinical Trial Registry 2007.
Faries 2008 {published data only}
- Faries DE, Ascher‐Svanum H, Nyhuis AW, Kinon BJ. Switching from risperidone to olanzapine in a one‐year, randomized, open‐label effectiveness study of schizophrenia. Current Medical Research and Opinion 2008;24(5):1399‐405. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Firestone 2011 {published data only}
- Firestone L, Ames D, Aragaki DLR, Erickson Z, Kim H, Lee C, et al. Efficacy and safety of a lifestyle balance program for antipsychotic medication associated obesity. PM and R 2011;3(10):S258‐9. [Google Scholar]
Fleischhacker 2008 {published data only}
- Fleischhacker W, Heikkinen ME, Olie JP, Dewaele P, McQuade R, Hennicken D, et al. Long‐term effect on weight of aripiprazole‐clozapine in schizophrenia patients with suboptimal response on clozapine. European Neuropsychopharmacology 2008;18:S447. [Google Scholar]
Fleischhacker 2008a {published data only}
- Fleischhacker WW, Heikkinen ME, Olie JP, Landsberg W, Dewaele P, McQuade R, et al. Weight change on aripiprazole‐clozapine combination in schizophrenic patients with weight gain and suboptimal response on clozapine: 16‐week double‐blind study. European Psychiatry 2008;23:S114‐S5. [Google Scholar]
Fleischhacker 2010 {published data only}
- Fleischhacker WW, Heikkinen ME, Olie J‐P, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: A randomized, double‐blind, placebo‐controlled trial. International Journal of Neuropsychopharmacology 2010;13(8):1115‐25. [DOI] [PubMed] [Google Scholar]
Ganguli 2008 {published data only}
- Ganguli R, Brar JS, Mahmoud R, Berry SA, Pandina GJ. Assessment of strategies for switching patients from olanzapine to risperidone: a randomized, open‐label, rater‐blinded study. BMC Medicine 2008;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganguli 2011 {published data only}
- Ganguli R, Brar JS. Behavioural intervention for weight loss in schizophrenia: An rct with active controls. Indian Journal of Psychiatry 2011;5(Suppl 1):S44. [Google Scholar]
- Ganguli R, Brar JS. Weight reduction in schizophrenia, by lifestyle change: A randomized clinical trial. Canadian Journal of Diabetes 2011;2:153. [Google Scholar]
Ginsberg 2004 {published data only}
- Ginsberg DL. Add‐on sibutramine for olanzapine‐induced weight gain. Primary Psychiatry 2004;11(7):24. [Google Scholar]
Henderson 2004 {published data only}
- Henderson DC, Daley TB, Louie P. A placebo‐controlled trial of sibutramine added to patients with olanzapine‐induced weight gain. World Journal of Biological Psychiatry 2004;5(suppl 1):152. [Google Scholar]
Henderson 2009 {published data only}
- Henderson DC, Fan X, Copeland PM, Sharma B, Borba CP, Boxill R, et al. Aripiprazole added to overweight and obese olanzapine‐treated schizophrenia patients. Journal of Clinical Psychopharmacology 2009;29(2):165‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2011 {published data only}
- Henderson DC, Freudenreich O, Borba CPC, Wang X, Copeland PM, Macklin E, et al. Effects of modafinil on weight, glucose and lipid metabolism in clozapine‐treated patients with schizophrenia. Schizophrenia Research 2011;130(1‐3):53‐6. [DOI] [PubMed] [Google Scholar]
Hoffmann 2009 {published data only}
- Hoffmann V, Case M, Jacobson J. Algorithms including amantadine, metformin and zonisamide for mitigation of weight gain during olanzapine treatment in outpatients with schizophrenia. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA 2009.
Iglesias‐Garcia 2010 {published data only}
- Iglesias‐Garcia C, Toimil‐Iglesias A, Alonso‐Villa MJ. Pilot study of the efficacy of an educational programme to reduce weight, on overweight and obese patients with chronic stable schizophrenia. Journal of Psychiatric and Mental Health Nursing 2010;17(9):849‐51. [DOI] [PubMed] [Google Scholar]
IRCT201009112181N5 {published data only}
- IRCT201009112181N5. The role of ranitidine in the prevention of olanzapine induced weight gain. http://www.irct.ir/ 2010.
IRCT201107187049N1 {published data only}
- IRCT201107187049N1. An assessment of effects of melatonin on metabolic effects of olanzapine in patients with schizophrenia. http://www.irct.ir/searchresult.php?id=7049&number=1 2011.
Joffe 2008 {published data only}
- Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, et al. Orlistat in clozapine‐ or olanzapine‐treated patients with overweight or obesity: a 16‐week randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2008;69(5):706‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karagianis 2010 {published data only}
- Karagianis J, Landry J, Hoffmann VP, Grossman L, Haan L, Maguire G, et al. An exploratory analysis of factors associated with weight change in a 16‐week trial of oral vs. Orally disintegrating olanzapine: The platypus study. International Journal of Clinical Practice 2010;64(11):1520‐9. [DOI] [PubMed] [Google Scholar]
Kelly 2008 {published data only}
- Kelly DL, Conley RR, Love RC, Morrison JA, McMahon RP. Metabolic risk with second‐generation antipsychotic treatment: a double‐blind randomized 8‐week trial of risperidone and olanzapine. Annals of Clinical Psychiatry 2008;20(2):71‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kelly 2011 {published data only}
- Kelly DL, Gorelick DA, Conley RR, Boggs DL, Linthicum J, Liu F, et al. Effects of the cannabinoid‐1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia. A randomized, double‐blind, pilot study. Journal of Clinical Psychopharmacology 2011;31(1):86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2011a {published data only}
- Kelly DL, Gorelick D, Conley R, Boggs DL, Linthicum J, Liu F, et al. Effects of the cannabinoid‐1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: A randomized, doubleblind pilot study. Schizophrenia Bulletin 2011;1:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kent 2011 {published data only}
- Kent JM, Daly EJ, Janssens L, Newcomer JW, Osselaer N, Husken G, et al. Metabolic and body mass parameters observed with jnj‐37822681, a novel fast‐dissociating d receptor antagonist, versus olanzapine. Biological Psychiatry 2011;69(9 Suppl):228. [MEDLINE: ] [Google Scholar]
Kim 2006 {published data only}
- Kim J, Yim S, Nam J. A 12‐week, randomized, open‐label, parallel‐group trial of topiramate in limiting weight gain during olanzapine treatment in patients with schizophrenia. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia Research; 2006 Feb 4‐10; Davos, Switzerland. Davos, SWITZERLAND: Elsevier Science Bv, 2006:86. [DOI] [PubMed]
Kim 2007a {published data only}
- Kim SW, Yoon JS, Lee SH, Yang DS, Lee JH, Kim WJ, et al. Effectiveness of switching from risperidone to amisulpride in stabilized schizophrenic patients with depression. European Neuropsychopharmacology 2007;17(Suppl 4):S440. [Google Scholar]
Kim 2007b {published data only}
- Kim SI, Sung YM, Paik KW, Yun KW, Kim YC, Lim W. The efficacy of topiramate for weight loss and psychiatric symptom severity in overweight or obese patients maintained on atypical antipsychotics. Proceedings of the 160th Annual Meeting of the American Psychiatric Association; 2007 May 19‐24; San Diego, CA 2007.
Kim 2012 {published data only}
- Kim SW, Chung YC, Lee YH, Lee JH, Kim SY, Bae KY, et al. Paliperidone er versus risperidone for neurocognitive function in patients with schizophrenia: A randomized, open‐label, controlled trial. International Clinical Psychopharmacology 2012;27(5):267‐74. [DOI] [PubMed] [Google Scholar]
Klein 2006 {published data only}
- Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double‐blind, placebo‐controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. American Journal of Psychiatry 2006;163(12):2072‐9. [DOI] [PubMed] [Google Scholar]
Klein 2008 {published data only}
- Klein DJ. Metformin treatment of weight gain associated with antipsychotic drug therapy. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):32. [Google Scholar]
Kluge 2007 {published data only}
- Kluge M, Schuld A, Himmerich H, Dalal M, Schacht A, Wehmeier PM, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double‐blind study. Journal of Clinical Psychopharmacology 2007;27(6):662‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kluge M, Schuld A, Schacht A, Himmerich H, Dalal MA, Wehmeier PM, et al. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug‐induced fever. Psychoneuroendocrinology 2009;34(1):118‐28. [DOI] [PubMed] [Google Scholar]
Ko 2005 {published data only}
- Ko YH, Joe SH, Jung IK, Kim SH. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clinical Neuropharmacology 2005;28(4):169‐75. [DOI] [PubMed] [Google Scholar]
Krakowski 2009 {published data only}
- Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double‐blind clozapine, olanzapine or haloperidol. Schizophrenia Research 2009;110(1‐3):95‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuang 2007 {published data only}
- Kuang Y‐H, Zhao L. A comparative study on the efficacy and weight gain between quetiapine and clozapine in the treatment of schizophrenia [喹硫平与氯氮平对精神分裂症患者疗效及体重的影响]. Shandong Archives of Psychiatry [山东精神医学] 2007;20(4):217‐8. [Google Scholar]
Kwon 2006 {published data only}
- Kwon JS, Choi J‐S, Bahk W‐M, Kim CY, Kim CH, Shin YC, et al. Weight management program for treatment‐emergent weight gain in olanzapine‐treated patients with schizophrenia or schizoaffective disorder: a 12‐week randomized controlled clinical trial. Journal of Clinical Psychiatry 2006;67(4):547‐53. [DOI] [PubMed] [Google Scholar]
Lambert 2009 {published data only}
- Lambert TJ. Switching to aripiprazole from olanzapine leads to weight loss in overweight people with schizophrenia or schizoaffective disorder. Evidence‐Based Mental Health 2009;12(2):50. [DOI] [PubMed] [Google Scholar]
Lan 2008 {published data only}
- Lan T, Chiu H, Hu T, Huang H, Wu B, Tuan Y. Prolactin level as an indicator to clinical psychopathological severity in aripiprazole treated schizophrenia. European Neuropsychopharmacology 2008;18:S440. [MEDLINE: ] [Google Scholar]
Lencz 2010 {published data only}
- Lencz T, Robinson DG, Napolitano B, Sevy S, Kane JM, Goldman D, et al. Drd2 promoter region variation predicts antipsychotic‐induced weight gain in first episode schizophrenia. Pharmacogenetics and Genomics 2010;20(9):569‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li J, Kong L‐S, Kong L‐X. Aripiprazole in the treatment of schizophrenia with diabetes caused by antipsychotic drugs of the safety study [阿立哌唑在治疗抗精神病药物所致糖尿病13例的安全性研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(23):2784‐5. [Google Scholar]
Lieberman 2010 {published data only}
- Lieberman J, Jarskog LF, Hamer R, Catellier D, Stewart D, LaVange L, et al. Metformin for obesity and metabolic abnormalities in schizophrenia. Proceedings of the 49th Annual meeting of the Americam College of Neuropsychopharmacology; 2010 Dec 5‐9; Miami, Florida. 2010.
Linne 2008 {published data only}
- Linne Y, Rossner S, Neovius M. Body composition and metabolic changes during antipsychotic treatment. A randomized trial comparing sertindole and olanzapine study design presentation. Proceedings of the 14th Biennial Winter Workshop on Schizophrenia and Bipolar Disorders; 2008 Feb 3‐7; Montreux, Switzerland. 2008.
Mauri 2008 {published data only}
- Mauri M, Simoncini M, Castrogiovanni S, Iovieno N, Cecconi D, Dell'Agnello G, et al. A psychoeducational program for weight loss in patients who have experienced weight gain during antipsychotic treatment with olanzapine. Pharmacopsychiatry 2008;41:17‐23. [DOI] [PubMed] [Google Scholar]
McElroy 2012 {published data only}
- McElroy SL, Winstanley E, Mori N, Martens B, McCoy J, Moeller D, et al. A randomized, placebo‐controlled study of zonisamide to prevent olanzapine‐associated weight gain. Journal of Clinical Psychopharmacology 2012;32(2):165‐72. [DOI] [PubMed] [Google Scholar]
Meyer 2009 {published data only}
- Meyer JM, Rosenblatt LC, Kim E, Baker RA, Whitehead R. The moderating impact of ethnicity on metabolic outcomes during treatment with olanzapine and aripiprazole in patients with schizophrenia. Journal of Clinical Psychiatry 2009;70(3):318‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meyer 2009a {published data only}
- Meyer JM, Henry RR, Braff DL. Antipsychotic stay vs. switch for metabolic benefit: psychiatric cost. Schizophrenia Bulletin 2009;35(Suppl 1):26. [Google Scholar]
Millar 2008 {published data only}
- Millar H, Felter C, Landsberg W. The effects of aripiprazole in combination with clozapine: patient functioning results from a double‐blind, 16‐week study in patients with schizophrenia (CN138‐170). Journal of Psychopharmacology 2008;22(5):A17. [Google Scholar]
Millar 2008a {published data only}
- Millar H, Felter C, Dudley E, Sullivan G. Weight outcomes in an open‐label comparision of switching strategies from risperidone to aripiprazole in patients with schizophrenia (CN138‐169). Journal of Psychopharmacology 2008;22(5):A17. [Google Scholar]
Moore 2006 {published data only}
- Moore N. Switching to aripiprazole from other second‐generation antipsychotics. Australian New Zealand Clinical Trials Registry 2006.
NCT00095524 {published data only}
- L'ltalien GJ, Newcomer JW, Berman R, Marcus RN, Kerselaers W, McQuade RD. Rates of metabolic syndrome and non‐high‐density lippprotein‐cholesterol among overweight patients switched from olanzapine to aripiprazole (Study CN138‐122). Proceedings of the 160th Annual Meeting of the American Psychiatric Association; 2007 May 19‐24; San Diego, CA 2007.
- Newcomer JW, Campos A, Marcus RN, Breder C, Berman R, Kerselaers W, et al. A multicenter, randomized, double‐blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. Journal of Clinical Psychiatry 2008;69(7):1046‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00303602 {published data only}
- Karagianis J, Grossman L, Landry J, Reed VA, Haan L, Maguire GA, et al. A randomized controlled trial of the effect of sublingual orally disintegrating olanzapine versus oral olanzapine on body mass index: the PLATYPUS Study. Schizophrenia Research 2009;113(1):41‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karagianis J, Landry J, Grossman L, Hoffmann V, Maguire GA, Milev R, et al. Factors associated with weight change in a 16‐week randomized double‐blind, double‐dummy trial of sublingual orally disintegrating olanzapine vs. oral olanzapine in patients who gained weight during olanzapine treatment: the PLATYPUS Study. Biological Psychiatry 2009;1:302. [Google Scholar]
NCT00423878 {published data only}
- Stroup T. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, orrisperidone to aripiprazole to reduce metabolic risk. Proceedings of the 164th annual general meeting of the American Psychiatric Association; 2011 May 14‐18; Honolulu, Hawaii. 2011.
- Stroup TS, McEvoy JP, Swartz MS, Hamer RM, Perkins DO, Lieberman JA. Comparison of Antipsychotics for Metabolic Problems (CAMP): a NIMH schizophrenia trials network study. Clinical Schizophrenia and Related Psychoses 2007;1(1):69‐72. [Google Scholar]
NCT00645099 {published data only}
- NCT00645099. A 6 month study to compare the metabolic effects of paliperidone er and olanzapine in patients with schizophrenia. http://www.clinicaltrials.gov 2008.
NCT00690235 {published data only}
- NCT00690235. Demonstrate the effects of pramlintide on weight reduction in schizophrenia. http://www.clinicaltrials.gov 2008.
NCT00709202 {published data only}
- NCT00709202. To examine the effect of betahistine on antipsychotic induced weight gain in adolescents. http://www.clinicaltrials.gov 2008.
NCT00734435 {published data only}
- NCT00734435. Olanzapine given in combination with zonisamide sr to prevent weight gain in schizophrenic subjects. http://www.clinicaltrials.gov 2008.
NCT00759460 {published data only}
- NCT00759460. Study on metabolic parameters of sertindole in patients with schizophrenia. http://www.clinicaltrials.gov 2008.
NCT00759993 {published data only}
- NCT00759993. Chromium piccolinate in the prevention of weight gain induced by serotonergic medications initiated on psychiatric inpatient units. http://www.clinicaltrials.gov 2008.
NCT00793780 {published data only}
- NCT00793780. Pilot trial of naltrexone for obesity in women with schizophrenia. http://www.clinicaltrials.gov 2008.
NCT00806234 {published data only}
- NCT00806234. Reducing weight gain and improving metabolic function in children being treated with antipsychotics. http://www.clinicaltrials.gov 2008.
NCT00816907 {published data only}
- NCT00816907. The use of metformin in the treatment of antipsychotic‐induced weight gain in schizophrenia (The METS Study). http://www.clinicaltrials.gov 2009.
NCT00845507 {published data only}
- NCT00845507. Exenatide for the treatment of weight gain associated with olanzapine in obese adults. http://ClinicalTrials.gov/show/NCT00845507 2008.
NCT00857818 {published data only}
- NCT00857818. Metabolic abnormalities ‐ OPT. http://www.clinicaltrials.gov 2009.
NCT00902694 {published data only}
- NCT00902694. Examining an exercise and nutrition program for reducing weight in people with severe mental illnesses (the achieve study). http://ClinicalTrials.gov/show/NCT00902694 2009.
NCT00934908 {published data only}
- NCT00934908. Prevention of weight gain and dyslipidemia by green tea in patients initiating therapy with olanzapine. http://www.clinicaltrials.gov 2009.
NCT00990925 {published data only}
- NCT00990925. Lifestyle modification for weight loss in schizophrenia. http://www.clinicaltrials.gov 2009.
NCT01052714 {published data only}
- NCT01052714. Healthy lifestyles for mentally ill people who have experienced weight gain from their antipsychotic medications ‐ 2. http://www.clinicaltrials.gov 2010.
NCT01075295 {published data only}
- NCT01075295. Prevention of weight gain in early schizophrenia and schizoaffective disorder. http://www.clinicaltrials.gov 2010.
NCT01167348 {published data only}
- NCT01167348. The effectiveness of auricular acupressure on bodybweight parameters on patients with schizophrenia. http://ClinicalTrials.gov/show/NCT01167348 2010.
NCT01272752 {published data only}
- NCT01272752. Anti‐psychotic medication (new use) weight loss study. http://ClinicalTrials.gov/show/NCT01272752 2011.
NCT01272765 {published data only}
- NCT01272765. Anti‐psychotic medication (stable dose) weight loss study [her: placebo|Dietary Supplement: IHBG‐10]. http://ClinicalTrials.gov/show/NCT01272765 2011.
NCT01295372 {published data only}
- NCT01295372. Safety and efficacy of zicronapine in patients with schizophrenia. http://ClinicalTrials.gov/show/NCT01295372 2011.
NCT01300637 {published data only}
- NCT01300637. Effects of adjunctive metformin on metabolic profiles in clozapine‐treated schizophrenic patients. http://ClinicalTrials.gov/show/NCT01300637 2011.
NCT01324973 {published data only}
- NCT01324973. Web‐based weight management for individuals with mental illness. http://ClinicalTrials.gov/show/NCT01324973 2011.
NCT01491490 {published data only}
- NCT01491490. Treatment on iatrogenic weight gain and dyslipidaemia associated with olanzapine. http://ClinicalTrials.gov/show/NCT01491490 2011.
NCT01567124 {published data only}
- NCT01567124. Alleviating the metabolic side effects of antipsychotic medications. http://ClinicalTrials.gov/show/NCT01567124 2012.
NCT01593774 {published data only}
- NCT01593774. Melatonin for prevention of metabolic side effects of olanzapine. http://ClinicalTrials.gov/show/NCT01593774 2012.
Newcomer 2009 {published data only}
- Newcomer JW, Haupt DW, Fahnestock PA, Flavin KS, Nicol GE, Schweiger JA, et al. Changes in adiposity, insulin sensitivity and lipid metabolism during randomized antipsychotic treatment in schizophrenia. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA 2009.
Newcomer 2010 {published data only}
- Newcomer JW, Nicol GE, Haupt DW, Flavin KS, Schweiger JA, Yingling MD. Changes in adiposity, insulin sensitivity and related metabolic measures during randomized medication switches to aripiprazole from other atypical antipsychotics. Diabetes 2010;59(Suppl 1):488. [Google Scholar]
Nicol 2009 {published data only}
- Nicol G, Haupt DW, Yingling MD, Fahnestock PA, Flavin KS, Schweiger JA, et al. Changes in adiposity and metabolic measures during medication switches to aripiprazole from other atypical antipsychotics. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA 2009.
Oliveira 2005 {published data only}
- Oliveira AVG. Ganho de peso em pacientes em uso de olanzapina e antipsicóticos típicos: um ensaio clínico randomizado. PhD thesis. São Paulo, 2005.
Pae 2007 {published data only}
- Pae C‐U, Lim H‐K, Patkar AA, Masand P, Lee C, Paik I‐H. Switching strategies to aripiprazole from other antipsychotics in stabilized schizophrenia: a randomized, 12 week, open label, parallel group study. Proceedings of the 160th Annual Meeting of the American Psychiatric Association; 2007 May 19‐24; San Diego, CA 2007.
Perez‐Iglesias 2008 {published data only}
- Perez‐Iglesias R, Crespo‐Facorro B, Martinez‐Garcia O, Ramirez‐Bonilla ML, Alvarez‐Jimenez M, Pelayo‐Teran JM, et al. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug‐naive population. Schizophrenia Research 2008;99(1‐3):13‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perez‐Iglesias 2008a {published data only}
- Perez‐Iglesias R, Vazquez‐Barquero JL, Amado JA, Berja A, Garcia‐Unzueta MT, Pelayo‐Teran JM, et al. Effect of antipsychotics on peptides involved in energy balance in drug‐naive psychotic patients after 1 year of treatment. Journal of Clinical Psychopharmacology 2008;28(3):289‐95. [DOI] [PubMed] [Google Scholar]
Perez‐Iglesias 2010 {published data only}
- Perez‐Iglesias R, Martinez‐Garcia O, Mata I, Pelayo‐Teran JM, Pardo‐Garcia G, Vazquez‐Barquero JL, et al. Predictors of antipsychotic‐induced weight gain after the first 3 years of treatment. Early Intervention in Psychiatry 2010;4(Suppl 1):141. [Google Scholar]
Peuskens 2003 {published data only}
- Peuskens J. Less weight gain with amisulpride: results from double‐blind studies vs. risperidone and olanzapine. Proceedings of the 16th European College of Neuropsychopharmacology Congress; 2003 Sep 20‐24; Prague, Czech Republic. 2003.
Poulin 2007 {published data only}
- Poulin M‐J, Chaput J‐P, Simard V, Vincent P, Bernier J, Gauthier Y, et al. Management of antipsychotic‐induced weight gain: prospective naturalistic study of the effectiveness of a supervised exercise programme. Australian and New Zealand Journal of Psychiatry 2007;41(12):980‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poyurovsky 2007 {published data only}
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Faragian S, Maayan R, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine‐treated schizophrenia patients: a double‐blind placebo‐controlled study. Proceedings of the 62nd Annual Scientific Meeting of the Society of Biological Psychiatry. San Diego, CA: Elsevier Science Inc, 2007:129. [DOI] [PubMed]
Raposo 2011 {published data only}
- Raposo NRB, Ferreira AS, Gattaz WF. Body mass index increase, serum leptin, adiponectin, neuropeptide y and lipid levels during treatment with olanzapine and haloperidol. Pharmacopsychiatry 2011;44(5):169‐72. [DOI] [PubMed] [Google Scholar]
Ratliff 2011 {published data only}
- Ratliff JC, Palmese LB, Tonizzo KM, Reutenauer EL, Tek C. Pilot trial of contingency management for the treatment of antipsychotic‐induced weight gain. Obesity 2011;1:S99‐S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saddichha 2010 {published data only}
- Saddichha S, Manjunatha N, Akhtar S. Obesity, diabetes and hypertension associated with antipsychotic use in drug nave schizophrenia. Australian and New Zealand Journal of Psychiatry 2010;1:12‐3. [Google Scholar]
Safa 2008 {published data only}
- Safa M, Sadr S, Delfan B, Saki M, Javad Tarrahi M. Metabolic effects of olanzapine and risperidone in patients with psychotic disorders. International Journal of Psychiatry in Clinical Practice 2008;12(4):299‐302. [DOI] [PubMed] [Google Scholar]
Schneiderhan 2011 {published data only}
- Schneiderhan ME, Tomaszewski D, Fike J, Madden S, Schweiss S, Davey CS. Preliminary data of metabolic risk at 6 months in subjects on antipsychotic agents: A 12‐month randomized multi‐centered trial. Journal of Pharmacy Practice 2011;2:276. [Google Scholar]
Smith 2010 {published data only}
- Smith RC, Kanellopoulou I, Rachakonda S, Lindenmayer J‐P, Davis JM. Olanzapine vs. Risperidone effects on appetite and ghrelin in chronic schizophrenic patients. Proceedings of the 49th Annual meeting of the Americam College of Neuropsychopharmacology; 2010 Dec 5‐9; Miami, Florida. 2010.
Smith 2010a {published data only}
- Smith RC, Kanellopoulou I, Rachakonda S, Lindenmayer JP, Davis JM. Olanzapine vs. Risperidone effects on appetite and ghrelin in chronic schizophrenic patients. Neuropsychopharmacology 2010;35:S315‐S6. [Google Scholar]
Spivak 2009 {published data only}
- Spivak B. Sibutramine for schizophrenia. Stanley Foundation Research Programs 2009.
Stroup 2011a {published data only}
- Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: Comparison of antipsychotics for metabolic problems (camp). American Journal of Psychiatry 2011;168(9):947‐56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroup 2011b {published data only}
- Stroup TS, Hamer RM, Ray N, Esscok SM, Lieberman JA. The effect of switching from olanzapine, quetiapine, or risperidone to aripiprazole on risk of cardiovascular disease: Results from the comparison of antipsychotics for metabolic problems (camp) study. Proceedings of the 50th Annual Meeting of the American College of Neuropsychopharmacology; 2011 Dec 4‐8;a, Waikoloa, Hawaii. 2011:S101.
Sun 2007 {published data only}
- Sun J. Ranitidine prevention and treatment of olanzapine‐induced weight gain and glucose metabolism disorders [雷尼替丁防治奥氮平所致体重增加及糖代谢障碍的研究]. Chinese Journal of Nervous and Mental Disorders [中国神经精神疾病杂志] 2007; Vol. 33, issue 9:560‐2.
Takeuchi 2008 {published data only}
- Takeuchi H, Suzuki T, Uchida H, Nakajima S, Nomura K, Kikuchi T, et al. A randomized, open‐label comparison of 2 switching strategies to aripiprazole treatment in patients with schizophrenia: add‐on, wait, and tapering of previous antipsychotics versus add‐on and simultaneous tapering. Journal of Clinical Psychopharmacology 2008;28(5):540‐3. [DOI] [PubMed] [Google Scholar]
Tao 2008 {published data only}
- Tao S‐W, Chen Q, Yang C, Pan R‐D, Pan T‐W, Li L, et al. A comparison of the effects on lipid metabolic among four different atypical antipsychotic agents [4种非典型抗精神病药物对精神分裂症患者脂代谢的影响]. Journal of the Youjiang Medical College for Nationalities 2008;6:927‐30. [Google Scholar]
Tek 2011 {published data only}
- Tek C, Ratliff JC, Reutenauer EL, Palmese LB, Tonizzo KM, O'Malley SS. Low‐dose naltrexone for antipsychotic‐induced weight gain in women with schizophrenia. Obesity 2011;1:S181. [Google Scholar]
Tian 2008 {published data only}
- Tian H, Zhu Q. A comparative study of new antipsychopathic drugs lead to gain weight [三种非典型抗精神病药物所致体重增加的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(18):2119, 2122. [Google Scholar]
Tiwari 2010 {published data only}
- Tiwari AK, Zai CC, Meltzer HY, Lieberman JA, Muller DJ, Kennedy JL. Association study of polymorphisms in insulin induced gene 2 (insig2) with antipsychotic‐induced weight gain in european and african‐american schizophrenia patients. Human Psychopharmacology 2010;25(3):253‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uzcategui 2007 {published data only}
- Uzcategui E, EIFakih Y, Rangel N, Galeazzi T, Baptista T. Metformin plus sibutramine in the treatment of weight gain and metabolic dysfunction during olanzapine administration: a double‐blind, placebo controlled pilot study. Proceedings of the 160th Annual Meeting of the American Psychiatric Association; 2007 May 19‐24; San Diego, CA 2007.
Vaz‐Leal 2008 {published data only}
- Vaz‐Leal FJ. Metformin plus a lifestyle intervention was more effective than either alone for antipsychotic‐induced weight gain: commentary. Evidence‐Based Medicine 2008;13(5):146. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang BH, Du GP. Behavioural intervention for antipsychotic caused weight gain [行为干预对抗精神病药物所致体质量增加的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2008;20(18):2117‐8. [Google Scholar]
Wang 2012 {published data only}
- Wang M, Tong J‐h, Zhu G, Liang G‐m, Yan H‐f, Wang X‐z. Metformin for treatment of antipsychotic‐induced weight gain: A randomized, placebo‐controlled study. Schizophrenia Research 2012;138(1):54‐7. [DOI] [PubMed] [Google Scholar]
Warren 2011 {published data only}
- Warren KR, Buchanan RW, Feldman S, Conley R, Linthicum J, Patricia Ball M, et al. Effects of the cannabinoid‐1 receptor antagonist rimonabant on satiety signaling in overweight people with schizophrenia: A randomized, double‐blind pilot study. Schizophrenia Bulletin 2011;1:29. [Google Scholar]
Weiden {published data only}
- Weiden P, Radulovic L, Wang C, Allison D. Sibutramine for the treatment of obesity in schizophrenia: randomized, placebo controlled pilot study. Unpublished.
Weiden 2008 {published data only}
- Harvey PD, Meltzer H, Simpson GM, Potkin SG, Loebel A, Siu C, et al. Improvement in cognitive function following a switch to ziprasidone from conventional antipsychotics, olanzapine, or risperidone in outpatients with schizophrenia. Schizophrenia Research 2004;66(2‐3):101‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Newcomer JW, Loebel AD, Yang R, Lebovitz HE. Long‐term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33(5):985‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wirshing 2009 {published data only}
- Wirshing DA, Erickson ZD, Mena SJ, Guzik LH, Vesterman E, Tran K. Management of obesity associated with antipsychotic medication. Proceedings of the World Psychiatric Association International congress; 2009 April 1‐4th; Florence Italy. 2009.
Wu 2008a {published data only}
- Wu R‐R, Zhao J‐P, Guo X‐F, He Y‐Q, Fang M‐S, Guo W‐B, et al. Metformin addition attenuates olanzapine‐induced weight gain in drug‐naive first‐episode schizophrenia patients: a double‐blind, placebo‐controlled study. American Journal of Psychiatry 2008;165(3):352‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wu R‐R, Zhao J‐P, Guo X‐F, He Y‐Q, Fang M‐S, Guo W‐B, et al. Metformin addition attenuates olanzapine‐induced weight gain in drug‐naive first‐episode schizophrenia patients: a double‐blind, placebo‐controlled study: correction. American Journal of Psychiatry 2008;165(4):540. [DOI] [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu RR, Jin H, Gao K, Twamley EW, Ou JJ, Shao P, et al. Metformin for treatment of antipsychotic‐induced amenorrhea and weight gain in women with first‐episode schizophrenia: A double‐blind, randomized, placebo‐controlled study. American Journal of Psychiatry 2012;169(8):813‐21. [DOI] [PubMed] [Google Scholar]
Yoon 2008 {published data only}
- Yoon B‐H, Bahk W‐M, Bae A, Kim M‐K, Jon D‐I, Min KJ. Effects of 3‐months trial of zonisamide and weight management program in obese schizophrenic inpatients. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):150. [Google Scholar]
Yoon 2009 {published data only}
- Yoon B‐H, Bahk W‐M, Bae A, Min KJ, Shin Y‐C, Jon D‐I, et al. The effect of weight loss on obesity‐related quality of life and body image in obese schizophrenic patients. Biological Psychiatry 2009;1:288. [Google Scholar]
Zhang 2009 {published data only}
- Zhang P. antipsychotic drugs on body mass of patients with schizophrenia and intervention] Google Translate [抗精神病药物对精神分裂症患者体质量的影响及干预 []. Chinese Journal of Pharmaco Epidemiology [药物流行病学杂志] 2009;18(6):396‐8. [Google Scholar]
但雪姣 2010 {published data only}
- 但雪姣. Quetiapine and clozapine on blood lipids, body weight control study [Google Translate] [奎硫平与氯氮平对血脂、体重影响对照研究]. Journal of Chinese Rural Physician [中国社区医师:综合版] 2010; Vol. 1:30.
刘建金 2011 {published data only}
- 刘建金. Topiramate combined olanzapine Prevention second‐generation antipsychotic‐induced body weight and metabolic dysfunction [托吡酯联合奥氮平防治第二代抗精神病药所致体质量增加及代谢功能障碍的研究]. 现代实用医学 2011;23(06):665‐7. [Google Scholar]
刘朝军 2009 {published data only}
- 刘朝军, 田素英, 张传芝, 卢世臣, 韩鹏. Influences on serum prolactin, blood glucose and body we ight for patients with first‐episode schizophrenia treated with aripiprazole [阿立哌唑对首发精神分裂症患者代谢的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(4):244. [Google Scholar]
刘根 2010 {published data only}
- 刘根凤, 陈姬, 徐萍, 王冬云. Systematic health education of diabetic patients with schizophrenia [系统化健康教育对精神分裂症伴糖尿病患者的影响]. 齐鲁护理杂志 2010;16(25):9‐10. [Google Scholar]
刘献标 2010 {published data only}
- 刘献标, 吴春香, 肖永珍. Quetiapine and sulpiride on serum prolactin in schizophrenic patients and the impact of body weight [Google Translate] [喹硫平与舒必利对精神分裂症患者体重及血清催乳素的影响]. Hebei Medicine [河北医学] 2010;16(6):683‐6. [Google Scholar]
哈秀英 2011 {published data only}
- 哈秀英, 桂梦荔, 王淑莲. Atypical antipsychotics caused weight gain in clinical research [非典型抗精神病药物所致体重增加的临床研究]. 宁夏医学杂志 2011; Vol. 33, issue 08:775‐6.
崔开艳, 2010 {published data only}
- 崔开艳, 刘兰芬, 杨丽敏. Ziprasidone and risperidone on prolactin in patients with schizophrenia and body mass, blood glucose, blood lipid [Google Translate] [齐拉西酮与利培酮对精神分裂症患者泌乳素及体质量、血糖、血脂的影响]. Shandong Archives of Psychiatry [山东精神医学] 2010; Vol. 23, issue 1:7‐9.
张东卫 2009 {published data only}
- 张东卫, 甘景梨, 高存友, 段惠峰. Aripiprazole and risperidone on glucose and lipid [Google Translate] [阿立哌唑与利培酮对血糖、血脂的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(5):343‐5. [Google Scholar]
张华坤 2010 {published data only}
- 张华坤, 蔡欣, 谢金香, 孙玲, 曲志君. Antipsychotics due to the intervention of the metabolic syndrome [抗精神病药所致代谢综合征的干预]. 临床精神医学杂志 2010;20(06):380‐2. [Google Scholar]
张峰 2010 {published data only}
- 张峰, 钱秀丽, 贺启元. Change‐control study of ziprasidone and risperidone‐induced weight and metabolic indicators [齐拉西酮与利培酮所致体重及代谢指标变化对照研究]. 中国民康医学 2010;22(22):2840‐75. [Google Scholar]
彭永红 2008 {published data only}
- 彭永红, 吴辉颜, 吕明维. Agengts treatment antipsychotics due to obesity [防风通圣丸治疗抗精神病药所致肥胖]. Journal of Traditional Chinese Medicine [Chung i tsa chih ying wen pan][中醫雜志] 2008;23(11):1718‐9. [Google Scholar]
掌永莉 2010 {published data only}
- 掌永莉, 马丽波, 王旸, 李宪伟. Aripiprazole male schizophrenia patients with prolactin and blood sugar, weight [阿立哌唑对男性精神分裂症患者泌乳素及血糖、体重的影响]. 精神医学杂志 2010;23(04):277‐9. [Google Scholar]
李晓一 2011 {published data only}
- 李晓一, 邢葆平, 章迎春, 吴皓. Controlled study of aripiprazole and olanzapine in the treatment of metabolic effects in patients with schizophrenia [阿立哌唑与奥氮平治疗对精神分裂症患者代谢影响的对照研究]. 全科医学临床与教育 2011;9(04):384‐6. [Google Scholar]
李梁 2009 {published data only}
- 李梁, 黄海燕, 陶世武, 陈强, 杨诚, 潘天伟. Three kinds of antipsychotic drugs on glucose metabolism in patients with schizophrenia [Google Translate] [3种抗精神病药物对精神分裂症患者糖代谢的影响]. 当代医学 2009;15(24):1‐2. [Google Scholar]
李轶琛 2009 {published data only}
- 李轶琛, 钟宝亮, 马筠, 龚传鹏, 徐杨, 周晓亮, et al. Effects of 6‐month aripiprazle, risperidone or clozapine treatment [阿立哌唑、利培酮和氯氮平对精神分裂症患者糖脂代谢及体质量影响的6个月临床观察]. Chinese Mental Health Journal [中国心理卫生杂志] 2009;23(8):569‐74. [Google Scholar]
杨云秀 2011 {published data only}
- 杨云秀. The effect of nursing intervention in schizophrenia in patients with diabetes mellitus [护理干预在精神分裂症合并糖尿病患者中应用的效果观察]. 现代中西医结合杂志 2011; Vol. 20, issue 23:2963‐4.
杨春强 2009 {published data only}
- 杨春强, 李宝珠. Aripiprazole and olanzapine on body weight, blood glucose control study effects [Google Translate] [阿立哌唑与奥氮平对体质量、血糖影响的对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009; Vol. 19, issue 5:339‐40.
杨诚 2010 {published data only}
- 杨诚, 陶世武, 陈强, 潘润德, 潘天伟, 李梁, et al. Association study of four kinds of antipsychotics and glucose metabolism in patients with schizophrenia and glycosylated hemoglobin [4种抗精神病药物与精神分裂症患者糖代谢及糖化血红蛋白的关联性研究]. 中国现代医学杂志 2010; Vol. 20, issue 18:2816‐20.
杨雀屏 2009 {published data only}
- 杨雀屏, 朱培俊. Affect control study of quetiapine and risperidone on serum prolactin [奎硫平与利培酮对血清催乳素影响对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009; Vol. 19, issue 1:57‐8.
杨雀屏 2009a {published data only}
- 杨雀屏, 朱培俊. Quetiapine and risperidone on serum prolactin control study [Google Translate] [奎硫平与利培酮对血清催乳素影响对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(1):57‐8. [Google Scholar]
汪卫华 2009 {published data only}
- 汪卫华, 赵汉清, 王焕林, 崔庶, 汪广剑, 仲爱芳, et al. Impact of antipsychotics on metabolic comparison [抗精神病药对代谢的影响比较]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(1):55‐7. [Google Scholar]
汪卫华 2009a {published data only}
- 汪卫华, 赵汉清, 王焕林, 崔庶, 汪广剑, 仲爱芳, et al. Antipsychotics on metabolism [Google Translate] [抗精神病药对代谢的影响比较]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009;19(1):55‐7. [Google Scholar]
王秀芳 2011 {published data only}
- 王秀芳, 郭红丽, 张洪伟, 吴雪飞, 张建. Aripiprazole and ziprasidone, the prevalence of metabolic syndrome in patients with schizophrenia [阿立哌唑和齐拉西酮对精神分裂症患者代谢综合征患病率的影响]. 医学信息(中旬刊) 2011; Vol. 6:2570‐1.
白锦波 2010 {published data only}
- 白锦波, 黄海燕, 陶世武, 潘天伟, 李梁. Three kinds of atypical antipsychotic drugs on lipid metabolism in patients with schizophrenia [Google Translate] [3种非典型抗精神病药物对精神分裂症患者脂代谢的影响]. 中国医药指南 2010;8(17):8‐10. [Google Scholar]
罗亨全 2010 {published data only}
- 罗亨全. Oral Ou Lanning and risperidone cause significant weight gain in contrast observation [Google Translate] [口服欧兰宁与利培酮引起体重增加的对比观察]. Journal of the Heze Medical College [菏泽医学专科学校学报] 2010; Vol. 22, issue 1:21‐2.
耿寒松 2009 {published data only}
- 耿寒松, 刘荣芹, 边辛丑, 苏秀茹, 崔春青, 蒋龙, et al. Comparative study of the effects of ariplprazole and risperidone on the therapeutic effect and patients’weight in patients with schizophrenia [阿立哌唑与利培酮对精神分裂症疗效与体重的对照研究]. Hebei Medical Journal 2009; Vol. 31, issue 4:407‐9.
钟潇琦 2010 {published data only}
- 钟潇琦, 郑建玲, 黄雄. Three kinds of antipsychotic therapy on glucose and lipid metabolism in female first‐episode schizophrenia and the impact of prolactin [Google Translate] [三种抗精神病药治疗女性首发精神分裂症对糖脂代谢及催乳素的影响]. 中国实用医药 2010;5(24):15‐7. [Google Scholar]
陈俊雄 2010 {published data only}
- 陈俊雄, 吴树跃, 林榕, 陈怀恩. Metformin combined behavioral intervention therapy of clozapine in the treatment of schizophrenia in patients with body weight, blood glucose, blood lipids [二甲双胍合并行为干预疗法对氯氮平治疗精神分裂症患者体重、血糖、血脂的影响]. 四川精神卫生 2010;23(04):198‐202. [Google Scholar]
陶世武 2009 {published data only}
- 陶世武, 杨诚, 陈强, 潘润德, 潘天伟, 李梁, et al. Four kinds of atypical antipsychotics on glucose and lipid metabolism in patients with schizophrenia [Google Translate] [4种非典型抗精神病药物对精神分裂症患者血糖及血脂代谢的影响]. Guangxi Medical Journal 2009;31(9):1238‐41. [Google Scholar]
陶世武 2010 {published data only}
- 陶世武, 杨诚, 潘润德, 潘天伟, 李梁. Four atypical antipsychotics, the prevalence of metabolic syndrome in patients with schizophrenia [四种非典型抗精神病药物对精神分裂症患者代谢综合征患病率的影响]. 精神医学杂志 2010;23(05):365‐7. [Google Scholar]
References to ongoing studies
Tang 2003 {published data only}
- Tang T, Meltzer HY. Olanzapine causes greater increases in serum lipids than risperidone. Schizophrenia Research 2003;60(1):367. [Google Scholar]
Additional references
ADA/APA 2004
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596‐601. [DOI] [PubMed] [Google Scholar]
Allison 1999
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic‐induced weight gain: a comprehensive research synthesis. American Journal of Psychiatry 1999;156(11):1686‐9. [DOI] [PubMed] [Google Scholar]
Allison 2003
- Allison DB, Mackell JA, McDonnell DD. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatric Services 2003;54:565‐7. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecing skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birt 2003
- Birt J. Management of weight gain associated with antipsychotics. Annals of Clinical Psychiatry 2003;15:49‐58. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Casey 2004
- Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, Lindenmayer JP, Manoukian SV, Banerji, MA, Lebovitz HE, Hennekens CH. Antipsychotic‐induced weight gain and metabolic abnormalities: Implications for increased mortality in patients with schizophrenia. Journal of Clinical Psychiatry 2004;65(Suppl 7):4‐18. [PubMed] [Google Scholar]
Catapana 2004
- Catapana L, Castle D. Obesity in schizophrenia: What can be done about it?. Australasian Psychiatry 2004;12:23‐5. [DOI] [PubMed] [Google Scholar]
CATIE 2006
- Essock SM, Covell NH, Davis SM, Stroup TS, Rosenheck RA, Lieberman JA. Effectiveness of switching antipsychotic medications. American Journal of Psychiatry 2006;163:2090‐5. [DOI] [PubMed] [Google Scholar]
Cohn 2004
- Cohn T, Prud'homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: High prevalence of the metabolic syndrome. Canadian Journal of Psychiatry 2004;49:753‐60. [DOI] [PubMed] [Google Scholar]
Coodin 2001
- Coodin S. Body mass index in persons with schizophrenia. Canadian Journal of Psychiatry 2001;46:549‐55. [DOI] [PubMed] [Google Scholar]
Daumit 2003
- Daumit GL, Clark JM, Steinwachs DM, Graham CM, Lehman A, Ford DE. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. Journal of Nervous and Mental Disease 2003;191:799‐805. [DOI] [PubMed] [Google Scholar]
De Nayer 2005
- Nayer A, Hert M, Scheen A, Gaal L, Peuskens J on behalf of the Consensus Group. Belgian consensus on metabolic problems associated with atypical antipsychotics. International Journal of Psychiatry in Clinical Practice 2005;9:130‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
DoH 2004
- Department of Health. At Least Five a Week. A Report From the Chief Medical Officer. HMSO, London, 2004. [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faulkner 2007
- Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia.. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005148.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goff 2005
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah NA, Daumit GL, Lamberti S, D'Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten‐year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Research 2005;80:45‐53. [DOI] [PubMed] [Google Scholar]
Green 2000
- Green A, Patel J, Goisman R. Weight gain from novel antipsychotic drugs: Need for action. General Hospital Psychiatry 2000;22:224‐35. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the health survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy U. ECDEU Assessment Manual for Psychopharmacology. Revised. Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Henderson 2005
- Henderson DC, Nguyen DD, Copeland PM, Hayden DL, Borba CP, Louie PM, Freudenreich O, Eden Evins A, Cather C, Goff DC. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: Results of a 10‐year naturalistic study. Journal of Clinical Psychiatry 2005;66:1116‐21. [DOI] [PubMed] [Google Scholar]
Hester 2005
- Hester EK, Thrower MR. Current options in the management of olanzapine ‐ associated weight gain. Annals of Pharmacotherapy 2005;39(2):302‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library 2005, issue 3.
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Homel 2002
- Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia. Schizophrenia Research 2002;55:277‐84. [DOI] [PubMed] [Google Scholar]
IDF
- International Diabetic Federation. IDF consensus worldwide definition of the metabolic syndrome. http://www.idf.org/webdata/docs/MetS_def_update2006.pdf 2006.
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Kurzthaler 2001
- Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. Journal of Clinical Psychiatry 2001;62(Suppl 7):32‐7. [PubMed] [Google Scholar]
Le Fevre 2001
- Fevre PD. Improving the physical health of patients with schizophrenia: Therapeutic nihilism or realism?. Scottish Medical Journal 2001;46:11‐31. [DOI] [PubMed] [Google Scholar]
Mackin 2005
- Mackin P, Watkinson HM, Young AH. Prevalence of obesity, glucose homeostasis disorders and metabolic syndrome in psychiatric patients taking typical or atypical antipsychotic drugs: A cross‐sectional study. Diabetologia 2005;48:215‐21. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Osborn 2001
- Osborn DPJ. The poor physical health of people with mental illness. Western Journal of Medicine 2001;175:329‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Remington 2005
- Remington G, Chue P, Stip E, Kopala L, Girard T, Christensen B. The crossover approach to switching antipsychotics: What is the evidence?. Schizophrenia Research 2005;76:267‐72. [DOI] [PubMed] [Google Scholar]
Ried 2003
- Ried LD, Renner BT, Bengston MA, Wilcox BM, Acholonu WW. Weight change after an atypical antipsychotic switch. The Annals of Pharmacotherapy October 2003;37:1381‐6. [DOI] [PubMed] [Google Scholar]
Saari 2005
- Saari KM, Lindeman SM, Viilo KM, Isohanni MK, Jarvelin MR, Lauren LH, Savolainen MJ, Koponen HJ. A 4‐fold risk of metabolic syndrome in patients with schizophrenia: The Northern Finland 1966 birth cohort study. Journal of Clinical Psychiatry 2005;66:559‐63. [DOI] [PubMed] [Google Scholar]
Silverstone 1988
- Silverstone T, Smith G, Goodall E. Prevalence of obesity in patients receiving depot antipsychotics. British Journal of Psychiatry 1988;153:214‐7. [DOI] [PubMed] [Google Scholar]
Strassnig 2003
- Strassnig M, Brar JS, Ganguli R. Body mass index and quality of life in community‐dwelling patients with schizophrenia. Schizophrenia Research 2003;62:73‐6. [DOI] [PubMed] [Google Scholar]
Susce 2005
- Susce MT, Villanueva N, Diaz FJ, Leon J. Obesity and associated complications in patients with severe mental illnesses: a cross‐sectional survey. Journal of Clinical Psychiatry 2005;66:167‐73. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Weiden 2004
- Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophrenia Research 2004;66:51‐7. [DOI] [PubMed] [Google Scholar]
Wikipedia 2007
- Wikipedia. Europoid. http: //en.wikipedia.org/wiki/Europoid (accessed 10 January 2007).
Wirshing 2004
- Wirshing DA. Schizophrenia and obesity: impact of antipsychotic medications. Journal of Clinical Psychiatry 2004;65(Suppl 18):13‐26. [PubMed] [Google Scholar]


