Abstract
Objective
This study examined whether stressful life events were associated with weight loss, central adiposity, and health behavior changes of African American breast cancer survivors (AABCS) participating in a weight loss intervention.
Methods
We conducted a secondary-data analyses of Moving Forward, a weight loss efficacy trial for AABCS conducted in 2011–2014. Two-hundred forty-six eligible women were randomized to a 6-month interventionist-guided (IG) or self-guided (SG) weight loss intervention. Data was collected on height, weight, self-reported diet, and self-reported physical activity. Stress (e.g., financial, legal, employment, relationships, safety, prejudice) was measured using an abbreviated version of the Crisis in Family Systems (CRISYS) urban life stress measure. Generalized linear models stratified by group examined the degree to which stress was associated with weight loss or changes in central adiposity, physical activity, and diet during the intervention (Months 1–6) or maintenance (Months 7 to 12) phases.
Results
Participants reported a median of 3.0 life stressors (range 0 to 22) mostly relating to relationships, safety concerns, and financial problems. In the IG group during the intervention phase, exposure to life stressors was not associated with weight loss (p = 0.15) or change in central adiposity (p = 0.69), physical activity (p = 0.15), or diet (p = 0.26). We found similar associations for the maintenance phase and in the SG group.
Conclusion/Implications
Despite facing stress across a myriad of domains (e.g., relationships, safety, finances), AABCS were successful at initiating and maintaining behaviors to achieve weight loss, reductions in central adiposity, and behavioral changes. Future randomized controlled trials are warranted that include more strategies to address the challenges that AABCS face, to determine whether AABCS in particular might benefit from interventions that address barriers (e.g., stress management) to weight loss. Such strategies are critical for improving quality of life and lowering the risk of cancer recurrence.
Keywords: Weight loss, African American breast cancer survivors, Stressful life events
Introduction
African American women have disproportionately higher rates of obesity at breast cancer diagnosis and gain twice as much weight as white women in the years after diagnosis [1]. Racial inequities in weight gain and obesity, among other complex factors (e.g., racial segregation, poverty, food and health-care access), contribute to higher rates of all-cause and cancer mortality among African Americans [1]. Addressing inequities in weight gain may help to reduce cancer mortality rates among African American women [2]. Though lifestyle interventions can often result in more balanced diets, an increase in physical activity, and intentional weight loss leading to improved health outcomes and wellness of African American breast cancer survivors (AABCS) [3–8], behavioral changes and weight loss among African American women are often modest [8]. Intervention trials have documented weight losses ranging from 0.5 to 3.6% in 6 months among AABCS—lower than those reported in studies with predominately white cancer survivor samples (1.5 to 13.9%) with durations ranging from less than 6 to 12 months or more [3, 5–7, 9–12]. Limitations of previous studies with AABCS include small samples and minimal exploration of factors influencing outcomes [8].
Studies show that AABCS experience many challenges that may diminish their efforts to engage in physical activity [8]. These challenges include personal barriers (e.g., time), social support, and environmental characteristics (e.g., access to facilities). Less is known about how these challenges may impact their response to weight loss interventions. Furthermore, AABCS may face unique social factors that impact behavioral and physiological changes [13].
Stress is one challenge shown to affect weight loss, health outcomes, and behavioral change in the general population [14, 15]. Oman and King examined the effect of life events (i.e., stress) on subsequent exercise adherence in a 2-year randomized clinical trial of exercise training and found that life events did not influence the adoption of exercise behavior but did disrupt maintenance of that behavior [16]. Furthermore, in a 1-year randomized controlled weight loss trial, Gavin and colleagues found that experiencing at least one stressful life event was associated with weight gain (after controlling for physical activity) [17]. These studies consistently showed how stressful life events are inversely associated with weight loss and behavior change—but nearly all of these studies lacked racial diversity in their sampling, limiting generalizability. The underrepresentation of African Americans in these studies means the key life events contributing to stress experienced more often by African Americans, such as discrimination and racism, may not have been captured. More research is needed to elucidate the relationship between stress, behavior change, and weight loss among African Americans, particularly during times of increased stress, such as after a cancer diagnosis.
This study aims to broaden the literature on weight loss studies among medically at-risk populations (e.g., Diabetes Prevention Program (DPP), Weight Loss Maintenance Trial (WLM)) by examining challenges to health-centered behavioral change among AABCS. Though previous studies were not specific to cancer survivors, these programs have helped other at-risk participants identify and overcome the challenges they face in adhering to lifestyle interventions through intensive programming that includes health education and telephone counseling [18–20]. These efforts have resulted in high percentages of weight loss (e.g., >5%) [18]. In a similar fashion, this study seeks to understand the challenges—namely the stressful life experiences—experienced by AABCS in particular as they participate in intensive weight loss interventions to inform future programs.
Cancer survivors also face numerous unique stressful life experiences, including ongoing symptom burden, financial challenges, and relationship strain, among others [21–24]. Past weight loss studies have not included measurements of these stressful life experiences and typically use a generalized measure of stress (e.g., perceived stress scale) that captures feelings or thoughts without describing specific life events [25–27]. A multidimensional measure capturing stressful life events across several domains (e.g., CRISYS) can target not only these experiences unique to survivorship, but also those frequently faced by communities of color in urban environments. This can provide a greater understanding of the stress AABCS face on multiple axes—a key component to understanding just how intersecting factors affect intentional weight loss.
Stress is an important measure of study to understand obstacles to behavioral changes, but also due to the effects of stress of on the body. Stress can trigger behavioral dysregulation, leading to increased food intake and decreased physical activity [28], but physiological stress responses also affect weight through neuroendocrine pathways, such as the hypothalamic-pituitary-adrenal axis (HPA axis). These effects can lead to increased central obesity and lipid production [14]. A better understanding of stressful life experiences and their relation to weight management is needed, particularly in AABCS, to inform future intervention efforts.
In this study, we examine whether there is an association between stressful life events and weight loss, waist reduction, and behavior change among AABCS.
Methods
Moving Forward was a community-based, randomized, weight loss intervention trial with 246 overweight/obese AABCS. Survivors were recruited between September 2011 and September 2014 through direct contact by letter and phone using hospital cancer registry contact information from three Chicago-area academic cancer centers and community-based efforts, including referrals from oncologists, flyers, social media, and presentations [29]. The study was delivered in eight Chicago Park District facilities located within predominately African American communities.
Eligible participants were female AABCS (stages I–III), ≥ 18 years of age, with a body mass index (BMI) of ≥ 25 kg/m2 at time of recruitment, at least 6 months post-treatment at time of recruitment (hormonal therapy allowed), physically able to participate in a moderate physical activity program per healthcare provider approval, and agreeable to study procedures. Women were excluded if they were pregnant or planning to become pregnant during the study, taking prescription weight loss medication, or planning weight loss surgery in the coming year. Survivors were randomly assigned to either a 6-month Moving Forward Interventionist-Guided program (IG) or the Moving Forward Self-Guided program (SG) using a random digit generator following the baseline interview.
Goals at 6-month data collection for both programs were 5% weight loss, decreased caloric intake (~500 kcal), increased fruit and vegetable consumption, and increased physical activity(minimum ≥150 min per week) compared to baseline measures in accordance with the American Cancer Society cancer survivor guidelines [30]. The intervention was described in detail previously [4]. Briefly, IG met as a group twice-weekly for in-person classes with supervised exercise and twice-weekly text messaging targeting enhanced self-efficacy, social support, and access to health promotion resources. Participants in the IG and SG received a detailed program binder developed in collaboration with AABCS that addressed weekly topics central to cognitive behavioral approaches to weight loss including goal setting, stimulus control, mindful eating, and identifying and addressing barriers to behavior change. Weekly topics were grounded in principles of culturally competent interventions. Resources such as a guide to a resistance training exercise routine, recipes, and motivational materials were also included. SG participants did not attend classes or receive text messages. At 6 months, both groups received monthly newsletters with reinforcing information from the curriculum, news of local healthy eating and exercise resources, and participant testimonials.
Ethics
The study procedures were reviewed and approved for ethical treatment of human subjects by the University of Illinois at Chicago Institutional Review/Ethics Board (IRB# 2011-0614). Each participant provided written informed consent.
Measures
Demographic data
Demographics included age, education (highest year completed), and self-reported annual household income.
Breast cancer diagnosis and treatment information
Breast cancer diagnosis was self-reported by participants interested in the study during the eligibility screening. Diagnosis and stage of cancer were confirmed by their primary care physician who submitted written approval for respondents prior to participation.
Attendance
We calculated attendance for IG participants only by counting the number of sessions participants attended out of 48 total.
Weight and Central adiposity
Body mass index (BMI)
Height (baseline only) was measured to the nearest 0.1 cm using a portable stadiometer (Seca). Weight was measured to the nearest 0.1 kg using a digital scale (Tanita), with participants wearing light clothes without shoes. Two measurements for height and weight were taken. If there was a discrepancy of more than 0.5 cm for height or 0.2 kg for weight between the first and second measurements, a third measurement was taken. The mean of the two most closely aligned measurements was used to calculate BMI (weight (kg)/height (m)2.
Central adiposity
Central adiposity was measured with a measuring tape to the nearest 0.1 cm at the level midway between the lower rib margin and the iliac crest, with the participant breathing out gently. Two measurements were taken, unless there was a discrepancy of more than 1 cm; then, a third measurement was taken.
Behavioral outcomes
Modified activity questionnaire Kriska and Caspersen [31]
The modified activity questionnaire assessed self-reported leisure activity and television viewing. For leisure activity, respondents reviewed a list of 17 popular activities (e.g., walking, gardening) and selected those that they performed on at least 10 occasions in the last year. This activity questionnaire has been used in many large studies with diverse samples, including cancer survivors [32], and has well-established reliability and validity (Cronbach’s α ranged from 0.67 to 0.71) [31]. Participants were also given an opportunity to report leisure activities that were not on the list. Respondents then provided information on average frequency and duration for each activity. Responses were used to calculate the number of hours/week the participant engaged in moderate and vigorous physical activity (MVPA), along with total MET-hours per week. The questionnaire also asked how many hours per day the participant usually spends watching television.
Dietary intake
A Healthy Eating Index (HEI) was calculated by NutritionQuest using the interviewer administered Block 2005 Food Frequency Questionnaire, a measure well validated with diverse populations (Cronbach’s α ranged from 0.58 to 0.70) [33, 34]. HEI was developed by the US Department of Agriculture to assess diet quality [35]. The HEI scores a set of foods based on the amount of variety in the diet and compliance with specific dietary guidelines and recommendations. Overall score for the HEI ranges from 0 to 100 and is composed of scores from the 13 components that reflect the key recommendations in the Dietary Guidelines for Americans. A higher score represents higher adherence to guidelines.
Contemporary life stress
Crisis in Family Systems (CRISYS) is a self-reported measure validated for use to quantify contemporary sources of life stress in urban and low-income populations (Cronbach’s α = 0.78). While some studies have used generalized measures of stress, it was important for us to capture a broader range of stressful life events faced by our study population. The original measure included 64 life events or stressors [36], but an abbreviated version 41 items was used based on recommendations from our study’s Community Advisory Board (CAB) [37] who contributed meaningfully to intervention development and measurement selection. Related to the CRYSIS, the CAB identified items they saw as inappropriate or irrelevant for our study population given the expected age and life stage of most. For example, several items query about pregnancy (being pregnant, miscarriage, abortion), being in school and having teachers, and dealing with young children (teachers, illnesses). Other items left out referred to community conditions (i.e., dealing with mice, rats, insects in your home). The CAB believed these conditions would not be common and could be seen as offputting. The remaining list of stressful life events covered the following categories: financial, legal, employment, relationships, safety at home and in the community, medical issues pertaining to participants or others, home issues, difficulty with authority, and prejudice. Events reflected those occurring over the course of the 6-month intervention period, completed at the 6-month assessment (post-intervention) or the 6-month maintenance period (12-month assessment). Participants indicated whether they experienced each event by responding “yes” or “no.” A total score reflected the simple sum of stressful life events endorsed by the participant. The stressful life events reported were all weighted the same.
Weight, central adiposity, and each behavioral outcome were measured at baseline, post-intervention (6 months), and maintenance (12 months) unless noted otherwise.
Statistical Analyses
Descriptive statistics were calculated for all variables of interest at three time points: (1) baseline, (2) post-intervention (6 months), and (3) maintenance (12 months) including anthropometric and behavioral outcomes. Generalized linear models were stratified by group (IG or SG). Outcomes for IG and SG groups at baseline, post-intervention, and maintenance were assessed using generalized linear models. The primary outcomes were weight loss, central adiposity, physical activity, and diet. Model 1 adjusted for the outcome at baseline (weight, waist, MVPA, or diet) and percent classes attended (IG only). Model 2 additionally adjusts for age, income, education, and stage of diagnosis at baseline. Adjusted differences in the mean using the linear model are reported. Estimated standard errors for the adjusted differences for model 2 in the results below are reported given that the fit of model 2 was better than model 1 for the majority of the outcomes. The difference in outcomes (weight, waist, MVPA, or diet) between the IG and SG groups was assessed using t test and chi-square for continuous and categorical variables. p values were two-sided and significant at p ≤ 0.05. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). Only women who were not missing the outcome (i.e., weight (n = 210 at post-intervention and n = 189 at maintenance), waist (n = 210 at post-intervention and n = 189 at maintenance), physical activity (n = 210 at post-intervention and n = 204 at maintenance), or diet (n = 212 at post-intervention and n = 204 at maintenance)) were included in the analyses.
Results
We analyzed data from 246 participants (IG n =125, SG n = 121) at baseline, 212 participants post-intervention (IG = 111, SG n = 101), and 207 participants at maintenance (12 months) (IG = 107, SG = 100). Retention was 86% (n = 212) post-intervention and 84% (n = 207) at maintenance (12 months). Groups were comparable at baseline, and demographic characteristics are provided in Table 1. We provide medians of each outcome and our primary independent variable because the ranges were skewed. Median MVPA was 87.1 min/week (range 0–1023.5) at baseline; median HEI was 65.7 (range 38.9–93.9) at baseline. Median number of stressful life events was 3.0 (range 0–22.0) post-intervention (reflecting events over the previous 6 months). The most common stressful life events were related to relationships (e.g., got married, got divorced), safety, and financial (see Fig. 1). Over half of the women (57%) experienced changes in their relationship, such as getting divorced or breaking up with a partner. Nearly half (46%) had something in their neighborhood happen that made them feel unsafe. Over a third (39%) experienced financial changes such as missing a rent or mortgage payment or had their electricity cut off. In addition, over a third (34%) had a family member die or become ill.
Table 1.
Characteristics for the African American breast cancer survivors participating in Moving Forward: a behavioral weight loss intervention
| Variable | Group |
|||
|---|---|---|---|---|
| Total (n = 246) n (%) | Interventionist guided (n = 125) n (%) | Self-guided (n = 121) n (%) | p value | |
|
| ||||
| Age, years | 0.308 | |||
| n | 246 | 125 | 121 | |
| Mean ± SD | 57.5 ± 10.1 | 56.8 ± 10.0 | 58.1 ±10.1 | |
| Range | 30.6–82.2 | 33.0–82.0 | 30.6–79.8 | |
| Education | 0.399 | |||
| Some HS, HS grad, GED | 59 (24.0) | 24 (19.2) | 35 (28.9) | |
| Some college, associate’s degree, 2-year certificate | 93 (37.8) | 48 (38.4) | 45 (37.2) | |
| College graduate | 47 (19.1) | 24 (19.2) | 23 (19.0) | |
| Graduate or professional degree | 47 (19.1) | 29 (23.2) | 18 (14.9) | |
| Combined family income, last 12 months, $ | 0.208 | |||
| < 20,000 | 58 (23.6) | 24 (24.0) | 28 (23.1) | |
| 20,000–39,999 | 56 (22.8) | 25 (20.0) | 31 (25.6) | |
| 40,000–59,999 | 48 (19.5) | 20 (16.0) | 28 (23.1) | |
| 60,000–79,999 | 33 (13.4) | 18 (14.4) | 15 (12.4) | |
| ≥80,000 | 50 (20.3) | 32 (25.6) | 18 (14.9) | |
| Missing | 1 (0.4) | 0 (0.0) | 1 (0.8) | |
| Cancer stage* | 0.187 | |||
| I | 85 (38.3) | 51 (44.0) | 34 (32.1) | |
| II | 98 (44.1) | 46 (39.7) | 52 (49.1) | |
| III | 39 (17.6) | 19 (16.4) | 20 (18.9) | |
| Missing | 24 | 9 | 15 | |
| Central adiposity, cm | 0.529 | |||
| Mean ± SD | 113.2 ± 15.2 | 112.6 ± 15.3 | 113.9 ± 15.1 | |
| Missing | 0 | 0 | 0 | |
| Range | 81.5–169.6 | 81.5–169.6 | 84.6–166.3 | |
| 6-month weight change, kg | <0.001 | |||
| n | 210 | 110 | 100 | |
| Mean ± SD | −2.5 ± 4.3 | −3.5 ± 4.7 | −1.4 ± 3.5 | |
| Missing | 36 | 15 | 21 | |
| Range | −15.9–13.4 | −15.9–13.4 | −13.3–6.2 | |
| MVPA at baseline, min/week | 246 | 125 | 121 | 0.913 |
| Mean ± SD | 164.0 ± 207.9 | 162.6 ± 188.5 | 165.5 ± 226.9 | |
| Missing | 0 | 0 | 0 | |
| Range | 0–1023.5 | 0–1015.4 | 0–1023.5 | |
| 6-month MVPA change, min/week | 0.206 | |||
| n | 212 | 111 | 101 | |
| Mean ± SD | 89.1 ± 301.8 | 114.2 ± 248.7 | 61.6 ± 350.2 | |
| Missing | 34 | 14 | 20 | |
| Range | ||||
| 12-month MVPA change, min/week | <0.001 | |||
| n | 204 | 104 | 100 | |
| Mean ± SD | 90.2 ± 274.9 | 108.7 ± 275.7 | 71.0 ± 274.2 | |
| Missing | 42 | 21 | 21 | |
| HEI at baseline | 0.380 | |||
| n | 221 | 112 | 109 | |
| Mean ± SD | 65.1 ± 11.1 | 65.7 ± 11.4 | 64.4 ± 10.8 | |
| Missing | 25 | 13 | 12 | |
| 6-month HEI change | 0.034 | |||
| n | 190 | 100 | 90 | |
| Mean ± SD | 5.0 ± 10.1 | 6.4 ± 10.0 | 3.3 ± 10.1 | |
| Missing | 56 | 25 | 31 | |
| 12-month HEI change | ||||
| n | 184 | 94 | 90 | 0.402 |
| Mean ± SD | 4.4 ± 10.2 | 5.0 ± 9.5 | 3.8 ± 10.8 | |
| Missing | 62 | 31 | 31 | |
| CRISYS, at 6 months, count | 0.209 | |||
| n | 212 | 111 | 101 | |
| Mean ± SD | 4.0 ± 3.5 | 3.7 ± 2.9 | 4.3 ± 4.1 | |
| Missing | 34 | 14 | 20 | |
| Percent of classes attended | ||||
| Interventionist guided | 125 (54.9) | 125 (54.9) | NA | |
BMI body mass index, HEI healthy eating index, HS high school, MVPA moderate-to-vigorous physical activity, SD standard deviation
Percents do not include missing
Fig. 1.
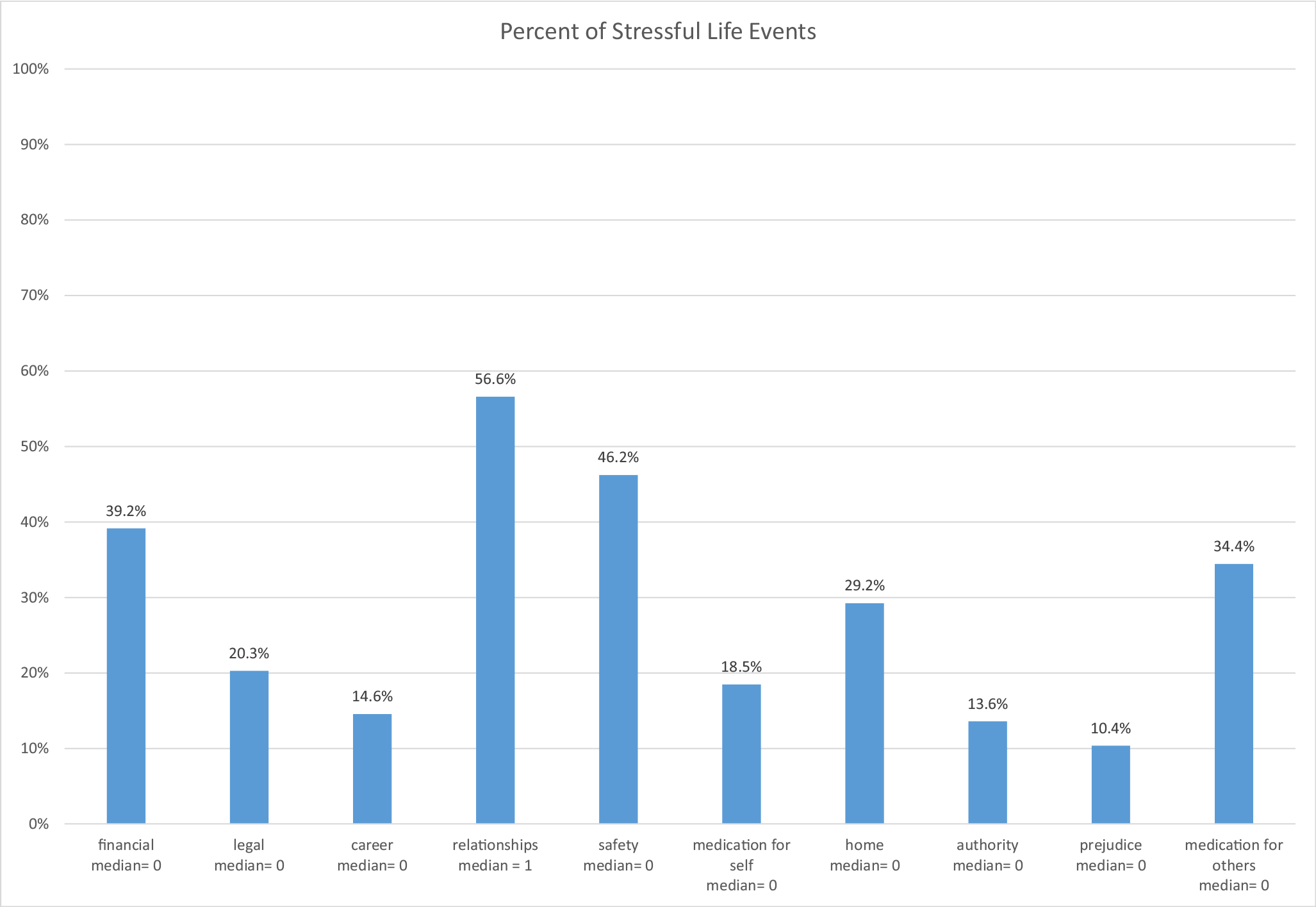
Percent of contemporary life stressors
Are stressful life events associated with lower weight loss?
Table 2 shows the linear model for the association between stress and weight loss stratified by group (IG and SG). During the intervention, stress was not significantly associated with weight loss in the IG group (β = −0.23 p = 0.15, model 2) or the SG group (β = 0.02, p = 0.83, model 2). Similarly, for the maintenance phase (12 months), stress was not associated with weight loss among the IG group (β = −0.19, p = 0.37, model 2) or SG group during maintenance (β = 0.12, p = 0.14, model 2).
Table 2.
Association between stressful life events and weight loss stratified by group
| Interventionist guided | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Intervention: Baseline to 6 months | Maintenance: 6 to 12 months | ||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| β | S.E. | β | p | S.E. | β | p | S.E. | β | p | S.E. | β | |
| Intercept | 10.40 | 2.30 | <0.001 | 17.28 | 4.87 | 0.001 | 9.51 | 2.96 | 0.002 | 10.99 | 6.49 | 0.094 |
| Stressful life events | −0.11 | 0.13 | 0.396 | −0.23 | 0.16 | 0.147 | −0.07 | 0.17 | 0.693 | −0.19 | 0.21 | 0.368 |
| Adj R-sq | 0.195 | 0.119 | 0.195 | 0.119 | ||||||||
| AICC (smaller is better) | 426.465 | 427.558 | 426.465 | 427.558 | ||||||||
| SBC (smaller is better) | 333.294 | 364.198 | 333.294 | 364.198 | ||||||||
| Self-guided | ||||||||||||
| Parameter | Model 1 | Model 2 | Model 1 | Model 2 | ||||||||
| β | S.E. | p | β | S.E. | p | β | S.E. | p | β | S.E. | p | |
| Intercept | 0.23 | 1.97 | 0.909 | 2.54 | 4.51 | 0.574 | 1.26 | 1.52 | 0.410 | 1.11 | 3.47 | 0.751 |
| Stressful life events | 0.04 | 0.09 | 0.625 | 0.02 | 0.10 | 0.830 | 0.12 | 0.07 | 0.082 | 0.12 | 0.08 | 0.143 |
| Adj R-sq | −0.010 | −0.073 | 0.027 | −0.046 | ||||||||
| AICC (smaller is better) | 354.170 | 336.134 | 273.964 | 261.320 | ||||||||
| SBC (smaller is better) | 259.565 | 277.028 | 186.108 | 205.737 | ||||||||
Note. Model 1 adjusts for baseline weight, percent classes attended (IG only). Model 2 additionally adjusts for age, income, education, and breast cancer stage
Note. Model 1 adjusts for baseline weight. Model 2 additionally adjusts for age, income, education, and breast cancer stage
Are stressful life events associated with physical activity?
Table 3 shows the linear model for the association between stress and change in physical activity stratified by group (IG and SG). Stress was not associated with change in physical activity in the IG or SG group during the intervention phase, respectively: (β = −1.10, p = 0.15, model 2) or (β = 0.98, p = 0.11, model 2). Similarly, during the maintenance phase, higher stress was not associated with physical activity change in the IG group (β = 1.52, p = 0.11, model 2). In contrast, among the SG group, the association between stress and change in physical activity during the maintenance phase was not conclusive (β = −1.42, p = 0.06).
Table 3.
Association between stressful life events and physical activity stratified by group from 6 to 12 months
| Interventionist guided | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Intervention: Baseline to 6 months | Maintenance: 6 to 12 months | ||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| β | S.E. | p | β | S.E. | p | β | S.E. | p | β | S.E. | p | |
| Intercept | 9.08 | 6.01 | 0.134 | 42.44 | 16.07 | 0.010 | 1.31 | 7.26 | 0.857 | −31.73 | 20.44 | 0.124 |
| Stressful life events | −0.86 | 0.65 | 0.192 | −1.10 | 0.74 | 0.145 | 1.11 | 0.80 | 0.168 | 1.52 | 0.93 | 0.105 |
| Adj R-sq | 0.277 | 0.275 | 0.039 | 0.044 | ||||||||
| AICC (smaller is better) | 781.983 | 731.635 | 755.604 | 719.213 | ||||||||
| SBC (smaller is better) | 679.249 | 662.349 | 660.525 | 654.396 | ||||||||
| Self-guided | ||||||||||||
| Parameter | Model 1 | Model 2 | Model 1 | Model 2 | ||||||||
| β | S.E. | p | β | S.E. | p | β | S.E. | p | β | S.E. | p | |
| Intercept | 6.64 | 3.27 | 0.045 | 9.62 | 18.47 | 0.604 | 3.93 | 3.72 | 0.293 | 17.35 | 21.93 | 0.432 |
| Stressful life events | 0.79 | 0.53 | 0.137 | 0.98 | 0.61 | 0.114 | −1.08 | 0.60 | 0.077 | −1.42 | 0.73 | 0.055 |
| Adj R-sq | 0.037 | 0.111 | 0.045 | 0.061 | ||||||||
| AICC (smaller is better) | 722.446 | 646.521 | 724.741 | 652.674 | ||||||||
| SBC (smaller is better) | 626.875 | 587.415 | 632.066 | 595.623 | ||||||||
Note. Model 1 adjusts for baseline physical activity, percent classes attended (IG only). Model 2 adjusts for age, income, education, and breast cancer stage
Note. Model 1 adjusts for baseline physical activity. Model 2 adjusts for age, income, education, and breast cancer stage
Are stressful life events associated with central adiposity?
Supplementary Table 4 shows the linear model for the association between stress and change in central adiposity (i.e., central adiposity) stratified by group. Stress was not associated with change in central adiposity in the IG or SG group during the intervention phase, respectively: (β = −0.10, p = 0.69, model 2), (β = −0.11, p = 0.58, model 2) in fully adjusted models. Similarly, in the maintenance phase stress was not associated with change in central adiposity among the IG during the intervention or SG group (β = 0.07, p = 0.72, model 2), (β = 0.05, p = 0.78, model 2), respectively.
Are stressful life events associated with diet/Healthy Eating Index?
Supplementary Table 5 shows the linear model for the association between stress and changes in Healthy Eating Index score stratified by group. During the intervention phase, stress was not associated with dietary changes in the IG group (β = 0.42, p = 0.26, model 2) or SG group (β = 0.25, p = 0.33, model 2). Similarly, stress was not associated with maintenance of dietary changes among the IG (β = 0.05, p = 0.92, model 2) or the SG (β = −0.21, p = 0.44, model 2).
Discussion
We examined the relationship between stressful life events and behavior change, central adiposity, and weight loss in a weight loss intervention trial targeting AABCS. The most commonly reported sources of stress for this study included relationships, lack of safety, and finances. Unexpectedly, analyses revealed no associations between these events and changes in weight, central adiposity, diet, or physical activity for either group (IG vs SG) during the intervention or maintenance phases.
To our knowledge, no other study has examined the association between stressful life events, behavior change, weight loss, and maintenance among cancer survivors. However, our results are partially consistent with previous studies examining the relationship between life events and behavior change, weight loss, and maintenance among overweight/obese adults [16] and adults with metabolic syndrome [17]. Similar to our findings, these studies report no association between stress and behavior change during the intervention phase [16]. Different from our results, these studies report an inverse association between stress and weight loss and behavior change during the maintenance phase [16, 17]. These inconsistencies may be due to several reasons, including different racial composition of the sample, survivor population, measure of stressful life events, and intervention components.
Unhealthy behavioral patterns like overeating and not exercising are common responses during times of stress [28]. Our participants may have controlled these common responses to stress post-intervention through learning how to manage stressful life events in more positive ways in Moving Forward. Participants may have learned specific ways to manage stress, including techniques for mindful eating, stimulus control, problem solving, and exercise offered in the program binder received by both the IG and SG groups. Skills related to paying attention to hunger and fullness cues also could have helped to better manage stress response to engage in problem eating behavior. Furthermore, Moving Forward encouraged increased exercise, which is known to reduce stress [38]. Past studies with women in the general population show women who practiced strategies taught during lifestyle interventions including problem solving skills, confrontive ways of coping with life’s demands, relaxation techniques and others were more likely to succeed with weight loss maintenance [39–41].
Group affiliation may have also contributed to a lack of significant findings. The IG women’s affiliation with Moving Forward likely helped the women reduce their stress by associating with other survivors in the group [42–44]. Taylor and colleagues showed that an effective coping mechanism for stress among females is to “tend and befriend,” which includes engaging in protective behaviors and affiliating with social groups that may reduce their stressful exposure [44]. Further, the social support literature shows that befriending others has substantial mental and physical health benefits [42, 43]. The IG participants were included in group sessions where they bonded with other women, which may have led to mental and physical health benefits. This may explain why the IG group experienced a higher weight loss than the SG group. Perhaps, group affiliation led to attendance to intervention sessions (which included twice weekly supervised exercise) and greater adherence to the recommended behavioral changes.
Taken together, a weight loss curriculum that includes strategies to manage stress and group affiliation may have contributed to our lack of finding any association between stress, behavior change, central adiposity, and weight loss. Unfortunately, we did not measure group affiliation or coping/stress management techniques. Ideally, future studies will consider these constructs providing greater understanding of their influence on behavioral and weight loss outcomes in AABCS.
Study limitations
Our study is not without limitations. Although we collected measures over time and saw significant changes, we cannot suggest causality between factors, and thus can only interpret our findings as associations. The CRYSIS measure includes questions related to severity and assignment as positive, negative, or neutral. To reduce participant burden, we eliminated these qualifiers (severity, positive and negative) in our data collection, which may have contributed to a null association. Although our validated measures are widely used in cancer survivor studies [31, 34], MVPA and dietary intake were self-reported, thus potentially leading to underreporting or overreporting activity and intake. Our participants reported high baseline levels of physical activity, which also may have contributed to null findings.
Meanwhile, the strengths of this study are the randomized design, the focus on an underserved population, a multidimensional measure of stress that includes discrimination, analyses of challenges experienced by AABCS (i.e., stress), and the use of validated measures. Though a causal link was not established between stress and behavior outcomes, our findings open future avenues of research in several related fields.
In conclusion, the relationship between stressful life events and outcomes from a weight loss intervention targeting AABCS remains unclear. We did find that AABCS who experienced stress were generally able to engage in positive health behavior changes that led to weight loss, though the weight loss achieved and maintenance therein was modest. Although this initial study did not demonstrate a clear relationship between stress and outcomes, further research is needed to better understand the complex role of stress as well as other challenges in weight loss attempts (including studies of African American women without cancer) to inform enhanced programming. Future studies may include a more intentional focus on stress management and/or group affiliation to promote greater impact than was achieved in this and other lifestyle interventions with AABCS.
Footnotes
Declarations
Conflict of interest The authors declare no competing interests.
References
- 1.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in obesity prevalence in adults with a history of cancer: results from the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Prev Chronic Dis. 2009;6(1):A22. [PMC free article] [PubMed] [Google Scholar]
- 4.Stolley M, Sheean P, Gerber B, Arroyo C, Schiffer L, Banerjee A, et al. Efficacy of a weight loss intervention for African American breast cancer survivors. J Clin Oncol. 2017;35(24):2820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: the Stepping STONE study. Contemp Clin Trials. 2016;46:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djuric Z, Mirasolo J, Kimbrough LV, Brown DR, Heilbrun LK, Canar L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. J Natl Med Assoc. 2009;101(6):552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado-Cruzata L, Zhang W, McDonald JA, Tsai WY, Valdovinos C, Falci L, et al. Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J Nutr. 2015;145(4):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paxton RJ, Garner W, Dean LT, Logan G, Allen-Watts K. Health behaviors and lifestyle interventions in African American breast cancer survivors: a systematic review. Front Oncol. 2019;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valle CG, Deal AM, Tate DF. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: a randomized controlled pilot study. J Cancer Surviv. 2017;11(1):133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung S, Zhu S, Friedmann E, Kelleher C, Kozlovsky A, Macfarlane KW, et al. Weight loss with mindful eating in African American women following treatment for breast cancer: a longitudinal study. Support Care Cancer. 2016;24(4):1875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang W, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity. 2013;21(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev. 2014;15(9):749–68. [DOI] [PubMed] [Google Scholar]
- 13.Black AR, Woods-Giscombé C. Applying the stress and ‘strength’ hypothesis to Black women’s breast cancer screening delays. Stress Health. 2012;28(5):389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björntorp P Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2(2):73–86. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. [DOI] [PubMed] [Google Scholar]
- 16.Oman RF, King AC. The effect of life events and exercise program format on the adoption and maintenance of exercise behavior. Health Psychol. 2000;19(6):605–12. [DOI] [PubMed] [Google Scholar]
- 17.Gavin KL, Wolfson J, Pereira M, Sherwood N, Linde JA. Life events, physical activity, and weight loss maintenance: decomposing mediating and moderating effects of health behavior. J Phys Act Health. 2019;16(4):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK. Weight loss and African–American women: a systematic review of the behavioural weight loss intervention literature. Obes Rev. 2012;13(3):193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35(2):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson PD, et al. African American women coping with breast cancer: a qualitative analysis. in Oncology nursing forum. 2003. [DOI] [PubMed] [Google Scholar]
- 22.Leak A, Hu J, King CR. Symptom distress, spirituality, and quality of life in African American breast cancer survivors. Cancer Nurs. 2008;31(1):E15–21. [DOI] [PubMed] [Google Scholar]
- 23.Tate JD. The role of spirituality in the breast cancer experiences of African American women. J Holist Nurs. 2011;29(4):249–55. [DOI] [PubMed] [Google Scholar]
- 24.Ashing-Giwa KT, Padilla G, Tejero J, Kraemer J, Wright K, Coscarelli A, et al. Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2004;13(6):408–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.KHC K, et al. Stress, race, and body weight. Health psychology : official journal of the Division of Health Psychology. American Psychological Association. 2009;28(1):131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elder CR, Gullion CM, Funk KL, DeBar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes. 2012;36(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trief PM, Cibula D, Delahanty LM, Weinstock RS. Depression, stress, and weight loss in individuals with metabolic syndrome in SHINE, a DPP translation study. Obesity. 2014;22(12):2532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5): 933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolley MR, Sharp LK, Fantuzzi G, Arroyo C, Sheean P, Schiffer L, et al. Study design and protocol for moving forward: a weight loss intervention trial for African-American breast cancer survivors. BMC Cancer. 2015;15:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4): 242–74. [DOI] [PubMed] [Google Scholar]
- 31.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(6):5–9. [PubMed] [Google Scholar]
- 32.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2003;97(7):1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990:58–64. [DOI] [PubMed] [Google Scholar]
- 34.Block G, Wakimoto P. A revision of the Block Dietary Questionnaire and database, based on NHANES III data. Berkeley: University of California at Berkeley; 1998. [Google Scholar]
- 35.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HAB, Kuczynski KJ, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33(5 Pt 1): 1381–402. [PMC free article] [PubMed] [Google Scholar]
- 37.Berry C, Quinn K, Shalowitz M, Wolf R. Validation of the Crisis in Family Systems—Revised, a contemporary measure of life stressors. Psychol Rep. 2001;88(3):713–24. [DOI] [PubMed] [Google Scholar]
- 38.Jackson EM. Stress relief: the role of exercise in stress management. ACSMs Health Fit J. 2013;17(3):14–9.24124347 [Google Scholar]
- 39.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. [DOI] [PubMed] [Google Scholar]
- 40.Kayman S, Bruvold W, Stern JS. Maintenance and relapse after weight loss in women: behavioral aspects. Am J Clin Nutr. 1990;52(5):800–7. [DOI] [PubMed] [Google Scholar]
- 41.Gormally J, Rardin D. Weight loss and maintenance and changes in diet and exercise for behavioral counseling and nutrition education. J Couns Psychol. 1981;28(4):295–304. [Google Scholar]
- 42.Taylor SE. Tend and befriend: biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci. 2006;15(6):273–7. [Google Scholar]
- 43.Taylor S, Friedman H, Silver R. Foundations of health psychology. Social support. 2007:145–71. [Google Scholar]
- 44.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend- and-befriend, not fight-or-flight. Psychol Rev. 2000;107(3):411–29. [DOI] [PubMed] [Google Scholar]


