Abstract
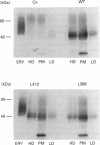
The tryptophan residues 388 and 412 in the glucose transporter GLUT1 were altered to leucine (L) by site-directed mutagenesis and were transiently expressed in COS-7 cells. As assessed by immunoblotting, comparable numbers of glucose transporters were present in plasma membranes from cells transfected with wild-type GLUT1, GLUT1-L388 or GLUT1-L412. Transfection of the wild-type GLUT1 gave rise to a 3-fold increase in the reconstituted glucose transport activity recovered from plasma membranes. In contrast, transfection of GLUT1-L412 failed to increase the reconstituted transport activity, whereas transfection of GLUT1-L388 produced only a 70% increase. Photolabelling of GLUT1-L412 with 3-[125I]iodo-4-azidophenethylamido-7-O-succinyldeacetyl (125IAPS)-forskolin was not different from that of the wild-type GLUT1, whereas the GLUT1-L388 incorporated 70% less photolabel than did the wild-type GLUT1. These data suggest a dissociation of the binding sites of forskolin and glucose in GLUT1. Whereas both tryptophan-388 and tryptophan-412 appear indispensable for the function of the transporter, only tryptophan-388 is involved in the binding of the inhibitory ligand forskolin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairns M. T., Alvarez J., Panico M., Gibbs A. F., Morris H. R., Chapman D., Baldwin S. A. Investigation of the structure and function of the human erythrocyte glucose transporter by proteolytic dissection. Biochim Biophys Acta. 1987 Dec 11;905(2):295–310. doi: 10.1016/0005-2736(87)90458-5. [DOI] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. C., Strube M., Leingang K., Keller K., Mueckler M. M. Amino acid substitutions at tryptophan 388 and tryptophan 412 of the HepG2 (Glut1) glucose transporter inhibit transport activity and targeting to the plasma membrane in Xenopus oocytes. J Biol Chem. 1992 Apr 15;267(11):7770–7776. [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hellwig B., Brown F. M., Schürmann A., Shanahan M. F., Joost H. G. Localization of the binding domain of the inhibitory ligand forskolin in the glucose transporter GLUT-4 by photolabeling, proteolytic cleavage and a site-specific antiserum. Biochim Biophys Acta. 1992 Nov 9;1111(2):178–184. doi: 10.1016/0005-2736(92)90309-a. [DOI] [PubMed] [Google Scholar]
- Hellwig B., Joost H. G. Differentiation of erythrocyte-(GLUT1), liver-(GLUT2), and adipocyte-type (GLUT4) glucose transporters by binding of the inhibitory ligands cytochalasin B, forskolin, dipyridamole, and isobutylmethylxanthine. Mol Pharmacol. 1991 Sep;40(3):383–389. [PubMed] [Google Scholar]
- Holman G. D., Parkar B. A., Midgley P. J. Exofacial photoaffinity labelling of the human erythrocyte sugar transporter. Biochim Biophys Acta. 1986 Feb 13;855(1):115–126. doi: 10.1016/0005-2736(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Photolabelling of the hexose transporter at external and internal sites: fragmentation patterns and evidence for a conformational change. Biochim Biophys Acta. 1987 Mar 12;897(3):395–405. doi: 10.1016/0005-2736(87)90437-8. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Habberfield A. D., Simpson I. A., Laurenza A., Seamon K. B. Activation of adenylate cyclase and inhibition of glucose transport in rat adipocytes by forskolin analogues: structural determinants for distinct sites of action. Mol Pharmacol. 1988 Apr;33(4):449–453. [PubMed] [Google Scholar]
- Joost H. G., Steinfelder H. J. Forskolin inhibits insulin-stimulated glucose transport in rat adipose cells by a direct interaction with the glucose transporter. Mol Pharmacol. 1987 Mar;31(3):279–283. [PubMed] [Google Scholar]
- Karim A. R., Rees W. D., Holman G. D. Binding of cytochalasin B to trypsin and thermolysin fragments of the human erythrocyte hexose transporter. Biochim Biophys Acta. 1987 Sep 3;902(3):402–405. doi: 10.1016/0005-2736(87)90208-2. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Huecksteadt T. P., Foley J. E. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J Biol Chem. 1983 Nov 25;258(22):13685–13692. [PubMed] [Google Scholar]
- Katagiri H., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Ishihara H., Akanuma Y., Takaku F., Oka Y. Substitution of leucine for tryptophan 412 does not abolish cytochalasin B labeling but markedly decreases the intrinsic activity of GLUT1 glucose transporter. J Biol Chem. 1991 Apr 25;266(12):7769–7773. [PubMed] [Google Scholar]
- Kim H. D., Sergeant S., Shukla S. D. Glucose transport in human platelets and its inhibition by forskolin. J Pharmacol Exp Ther. 1986 Mar;236(3):585–589. [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Robinson F. W., Blevins T. L., Suzuki K., Kono T. An improved method of reconstitution of adipocyte glucose transport activity. Anal Biochem. 1982 May 1;122(1):10–19. doi: 10.1016/0003-2697(82)90244-5. [DOI] [PubMed] [Google Scholar]
- Schürmann A., Monden I., Joost H. G., Keller K. Subcellular distribution and activity of glucose transporter isoforms GLUT1 and GLUT4 transiently expressed in COS-7 cells. Biochim Biophys Acta. 1992 Jul 15;1131(3):245–252. doi: 10.1016/0167-4781(92)90022-r. [DOI] [PubMed] [Google Scholar]
- Schürmann A., Rosenthal W., Hinsch K. D., Joost H. G. Differential sensitivity to guanine nucleotides of basal and insulin-stimulated glucose transporter activity reconstituted from adipocyte membrane fractions. FEBS Lett. 1989 Sep 25;255(2):259–264. doi: 10.1016/0014-5793(89)81102-0. [DOI] [PubMed] [Google Scholar]
- Sergeant S., Kim H. D. Inhibition of 3-O-methylglucose transport in human erythrocytes by forskolin. J Biol Chem. 1985 Nov 25;260(27):14677–14682. [PubMed] [Google Scholar]
- Shanahan M. F., Morris D. P., Edwards B. M. [3H]forskolin. Direct photoaffinity labeling of the erythrocyte D-glucose transporter. J Biol Chem. 1987 May 5;262(13):5978–5984. [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Clark R. B., Ruoho A. E. Identification of the glucose transporter in mammalian cell membranes with a 125I-forskolin photoaffinity label. Biochem J. 1988 Nov 1;255(3):983–990. doi: 10.1042/bj2550983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Ruoho A. E. Derivatization of the human erythrocyte glucose transporter using a novel forskolin photoaffinity label. J Biol Chem. 1987 Dec 25;262(36):17683–17689. [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Seamon K. B., Ruoho A. E. Localization of the forskolin photolabelling site within the monosaccharide transporter of human erythrocytes. Biochem J. 1990 Nov 15;272(1):151–158. doi: 10.1042/bj2720151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland M., Schürmann A., Schmidt W. E., Joost H. G. Development of the hormone-sensitive glucose transport activity in differentiating 3T3-L1 murine fibroblasts. Role of the two transporter species and their subcellular localization. Biochem J. 1990 Sep 1;270(2):331–336. doi: 10.1042/bj2700331. [DOI] [PMC free article] [PubMed] [Google Scholar]