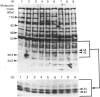
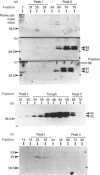
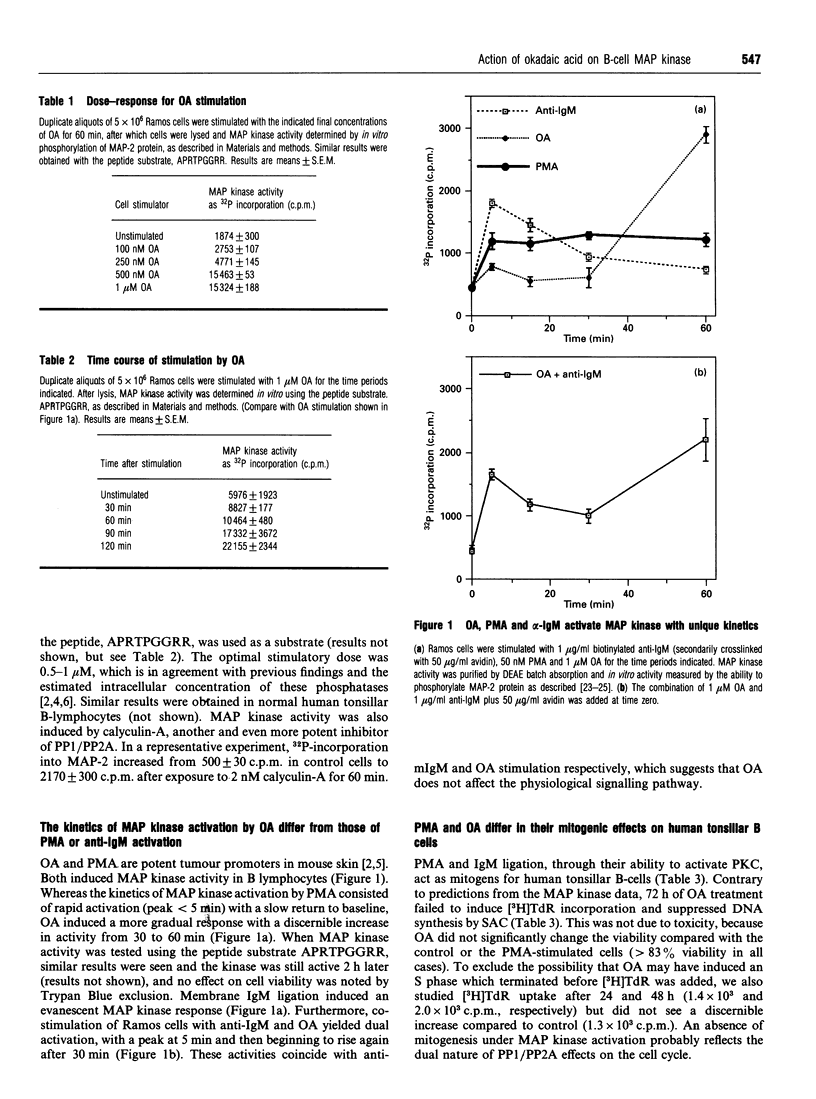
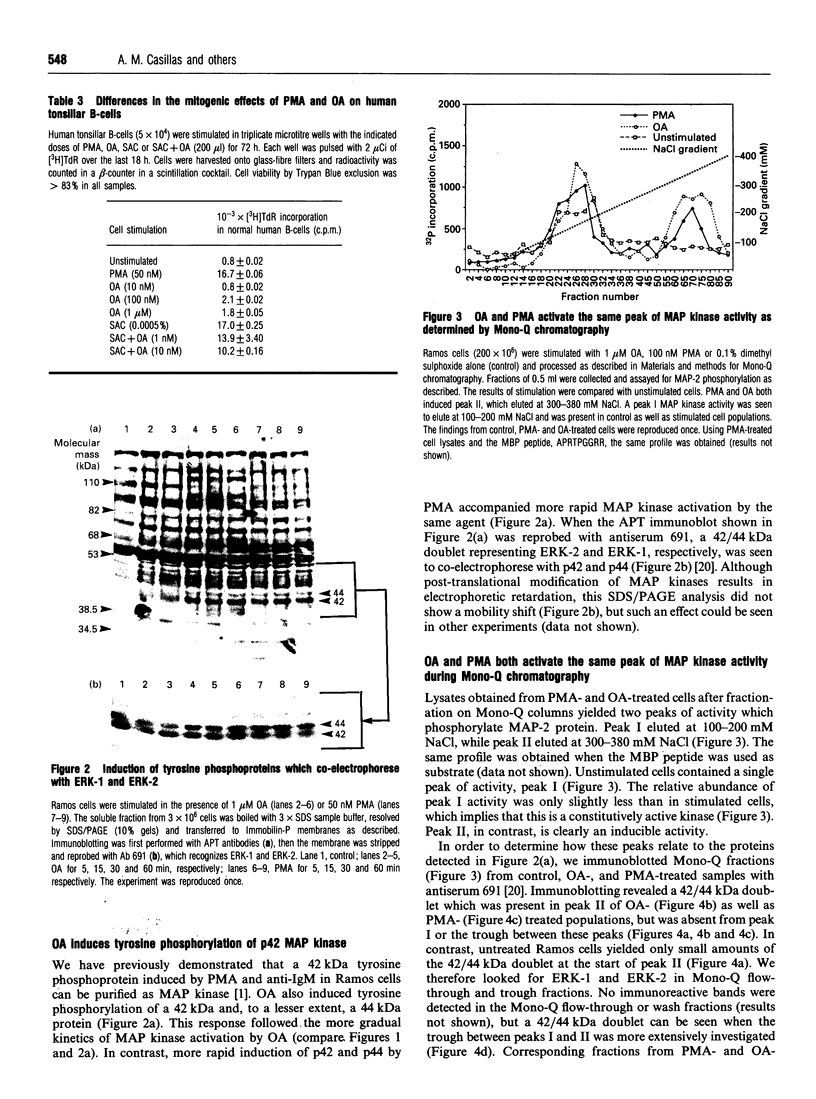
Abstract
Ligation of the membrane immunoglobulin M receptor as well as stimulation with the protein kinase C agonist phorbol 12-myristate 13-acetate leads to a B-lymphocyte proliferation and differentiation. Both stimuli activate p42 mitogen-activated protein (MAP) kinase in human B-lymphocytes [Casillas, Hanekom, Williams, Katz and Nel (1991) J. Biol. Chem. 266, 19088-19094]. MAP kinase activation is dependent on tyrosine as well as threonine phosphorylation of the kinase and its activity is inhibited by tyrosine as well as threonine/serine phosphatases. Okadaic acid, a specific inhibitor of type 1 and 2A serine/threonine phosphatases, induced MAP kinase activity in a potent and dose-dependent fashion, but failed to induce [3H]thymidine incorporation into normal human tonsil B-cells. Moreover, in combination with membrane immunoglobulin M ligation, okadaic acid decreased rather than increased [3H]thymidine incorporation. The kinetics of MAP kinase activation by okadaic acid differed from phorbol 12-myristate 13-acetate and anti-membrane immunoglobulin M stimulation. Okadaic acid induced tyrosine phosphorylation of 42 kDa and 44 kDa proteins which co-electrophoresed and co-chromatographed with ERK-2 and ERK-1 respectively. Ramos cells also contained a constitutively active 46 kDa MAP kinase which appeared as a separate peak in chromatography and could be immunoprecipitated by an antiserum against a rat ERK-1 fusion protein.
Full text
PDF

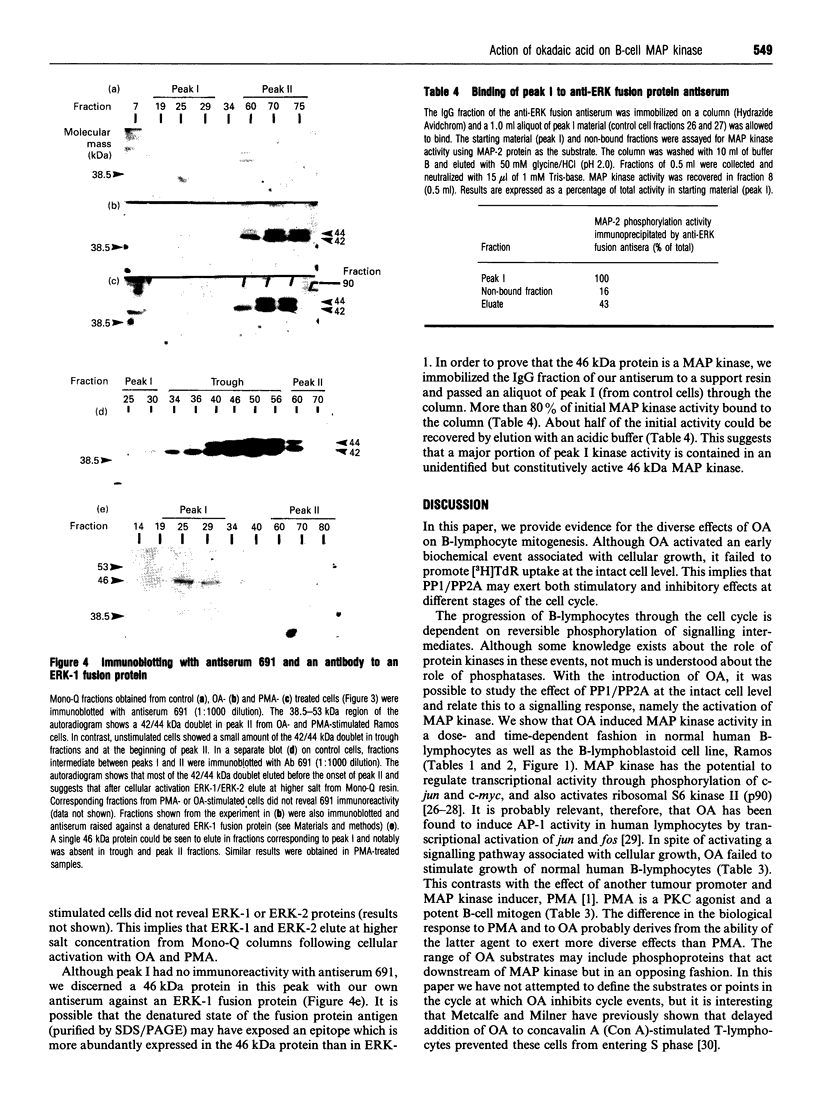

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Yancopoulos G. D., Gregory J. S., Slaughter C., Moomaw C., Hsu J., Cobb M. H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990 Jul 6;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- Casillas A., Hanekom C., Williams K., Katz R., Nel A. E. Stimulation of B-cells via the membrane immunoglobulin receptor or with phorbol myristate 13-acetate induces tyrosine phosphorylation and activation of a 42-kDa microtubule-associated protein-2 kinase. J Biol Chem. 1991 Oct 5;266(28):19088–19094. [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Ettehadieh E., Sanghera J. S., Pelech S. L., Hess-Bienz D., Watts J., Shastri N., Aebersold R. Tyrosyl phosphorylation and activation of MAP kinases by p56lck. Science. 1992 Feb 14;255(5046):853–855. doi: 10.1126/science.1311128. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Weiel J. E., Litchfield D. W., Tsukitani Y., Fischer E. H., Krebs E. G. Okadaic acid mimics the action of insulin in stimulating protein kinase activity in isolated adipocytes. The role of protein phosphatase 2a in attenuation of the signal. J Biol Chem. 1990 Sep 25;265(27):16571–16580. [PubMed] [Google Scholar]
- Lane P. J., Ledbetter J. A., McConnell F. M., Draves K., Deans J., Schieven G. L., Clark E. A. The role of tyrosine phosphorylation in signal transduction through surface Ig in human B cells. Inhibition of tyrosine phosphorylation prevents intracellular calcium release. J Immunol. 1991 Jan 15;146(2):715–722. [PubMed] [Google Scholar]
- Lane P. J., McConnell F. M., Schieven G. L., Clark E. A., Ledbetter J. A. The role of class II molecules in human B cell activation. Association with phosphatidyl inositol turnover, protein tyrosine phosphorylation, and proliferation. J Immunol. 1990 May 15;144(10):3684–3692. [PubMed] [Google Scholar]
- Metcalfe S., Milner J. Phosphatases PP1 and PP2A act after the G0/G1 interface in lymphocyte activation. Immunol Lett. 1990 Nov;26(2):177–182. doi: 10.1016/0165-2478(90)90142-d. [DOI] [PubMed] [Google Scholar]
- Miyasaka T., Chao M. V., Sherline P., Saltiel A. R. Nerve growth factor stimulates a protein kinase in PC-12 cells that phosphorylates microtubule-associated protein-2. J Biol Chem. 1990 Mar 15;265(8):4730–4735. [PubMed] [Google Scholar]
- Miyasaka T., Miyasaka J., Saltiel A. R. Okadaic acid stimulates the activity of microtubule associated protein kinase in PC-12 pheochromocytoma cells. Biochem Biophys Res Commun. 1990 May 16;168(3):1237–1243. doi: 10.1016/0006-291x(90)91161-k. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Hultin L. Protein kinase C plays a role in the induction of tyrosine phosphorylation of lymphoid microtubule-associated protein-2 kinase. Evidence for a CD3-associated cascade that includes pp56lck and that is defective in HPB-ALL. J Immunol. 1991 Sep 15;147(6):1933–1939. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Rheeder A., Williams K., Pollack S., Katz R., Landreth G. E. Stimulation of MAP-2 kinase activity in T lymphocytes by anti-CD3 or anti-Ti monoclonal antibody is partially dependent on protein kinase C. J Immunol. 1990 Apr 1;144(7):2683–2689. [PubMed] [Google Scholar]
- Nel A. E., Navailles M., Rosberger D. F., Landreth G. E., Goldschmidt-Clermont P. J., Baldwin G. J., Galbraith R. M. Phorbol ester induces tyrosine phosphorylation in normal and abnormal human B lymphocytes. J Immunol. 1985 Nov;135(5):3448–3453. [PubMed] [Google Scholar]
- Nel A. E., Pollack S., Landreth G., Ledbetter J. A., Hultin L., Williams K., Katz R., Akerley B. CD-3-mediated activation of MAP-2 kinase can be modified by ligation of the CD4 receptor. Evidence for tyrosine phosphorylation during activation of this kinase. J Immunol. 1990 Aug 1;145(3):971–979. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Thévenin C., Kim S. J., Kehrl J. H. Inhibition of protein phosphatases by okadaic acid induces AP1 in human T cells. J Biol Chem. 1991 May 25;266(15):9363–9366. [PubMed] [Google Scholar]