Abstract
The effects of exogenous insulin or vanadate (an insulin mimetic) on the disposal of dietary [14C]lipid between oxidation to 14CO2, deposition in adipose tissue or uptake by mammary gland and transfer to suckling pups were studied in virgin and lactating rats. After an oral load of [1-14C]triolein, virgin rats treated with a supraphysiological dose of insulin over 24 h showed a decrease (58%) in 14CO2 production and increased accumulation of [14C]lipid in carcass and white adipose tissue. There was a 2.5-fold increase in lipoprotein lipase activity in the latter. Chronic vanadate administration (12 days) had no effect on these parameters. In lactating rats, the stimulation of the deposition of [14C]lipid in adipose tissue by exogenous insulin was about 10% of that in virgin rats. In prolactin-deficient lactating rats there was no stimulation of [14C]lipid deposition in adipose tissue by insulin. However, both insulin and vanadate treatment increased the accumulation of [14C]lipid in mammary gland to the values seen in the mammary glands plus pups of normal lactating rats. Lipoprotein lipase activity in the gland was also restored to normal values. It is concluded that in lactation there is resistance to insulin stimulation of dietary lipid deposition in adipose tissue, and that this is not due to circulating prolactin. In addition, exogenous insulin plays a role in the regulation of lipoprotein lipase and hence of dietary lipid uptake into lactating mammary gland.
Full text
PDF
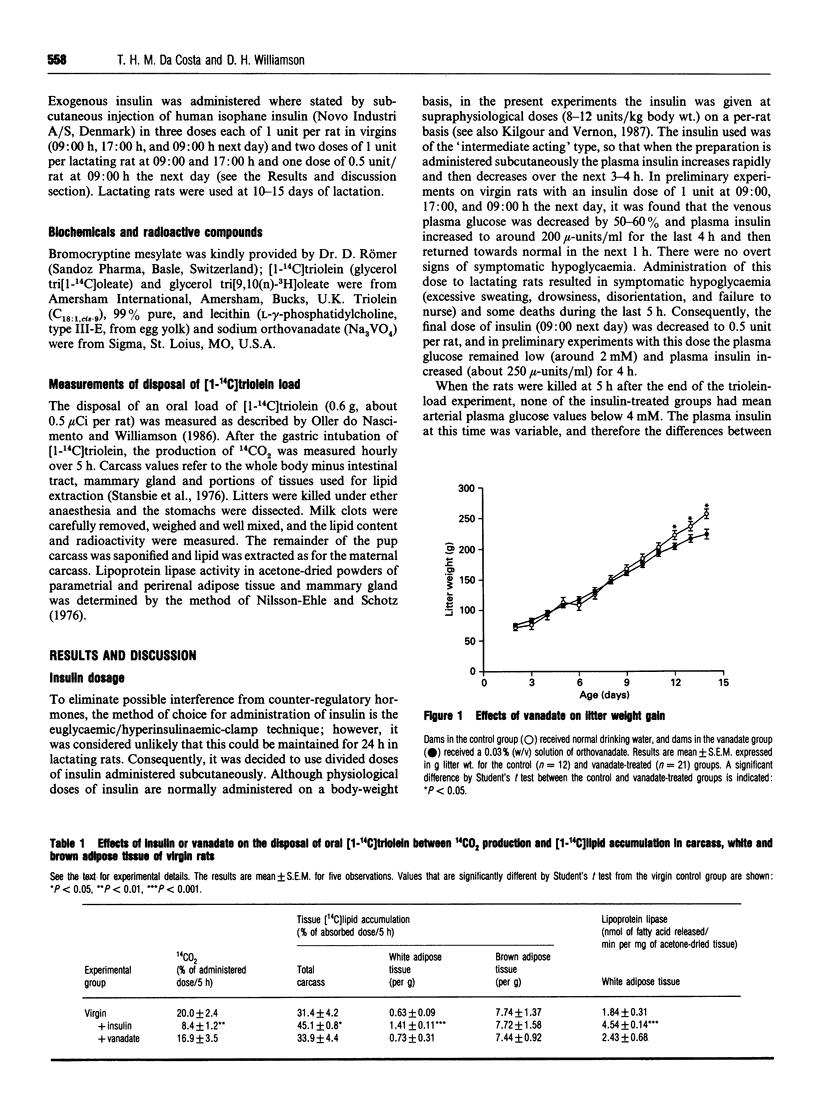
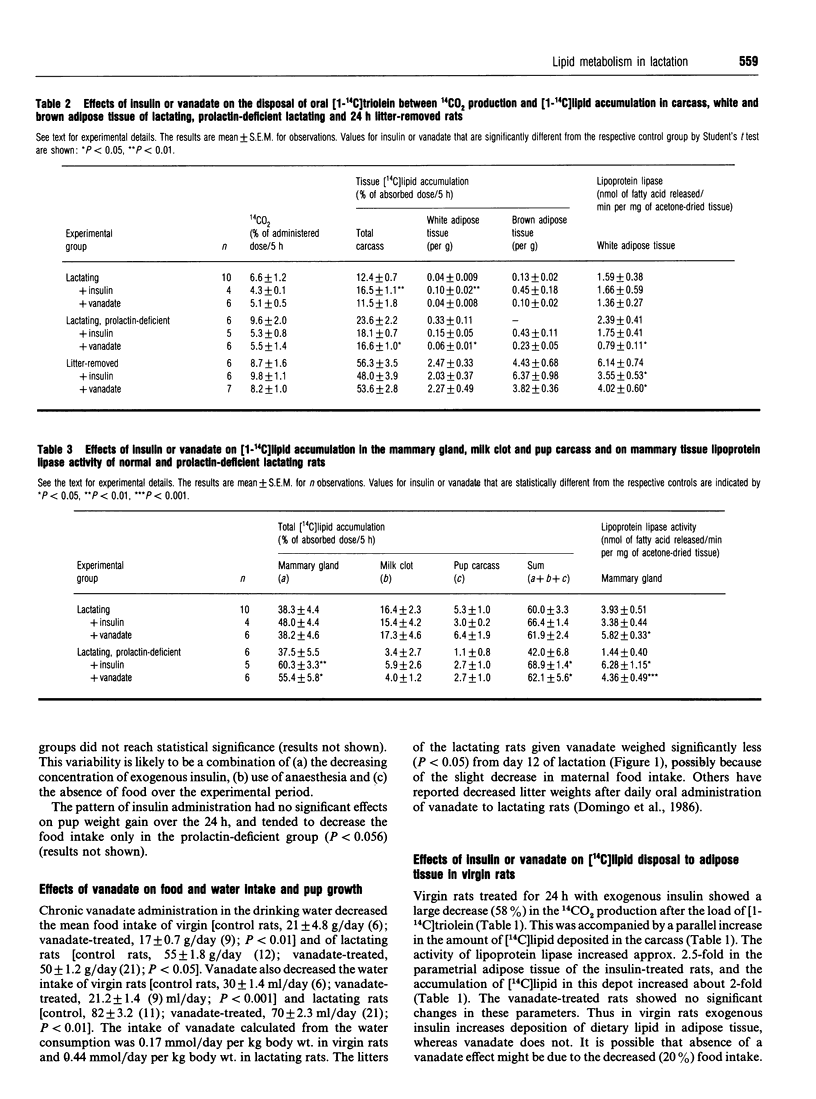


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Robinson A. M., Girard J. R., Williamson D. H. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: a possible regulatory role of prolactin. Biochem J. 1979 Jun 15;180(3):689–692. doi: 10.1042/bj1800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O., Bailbe D., Portha B. In vivo insulin resistance in streptozotocin-diabetic rats--evidence for reversal following oral vanadate treatment. Diabetologia. 1989 Mar;32(3):185–190. doi: 10.1007/BF00265092. [DOI] [PubMed] [Google Scholar]
- Brichard S. M., Pottier A. M., Henquin J. C. Long term improvement of glucose homeostasis by vanadate in obese hyperinsulinemic fa/fa rats. Endocrinology. 1989 Nov;125(5):2510–2516. doi: 10.1210/endo-125-5-2510. [DOI] [PubMed] [Google Scholar]
- Burnol A. F., Guerre-Millo M., Lavau M., Girard J. Effect of lactation on insulin sensitivity of glucose metabolism in rat adipocytes. FEBS Lett. 1986 Jan 6;194(2):292–296. doi: 10.1016/0014-5793(86)80103-x. [DOI] [PubMed] [Google Scholar]
- Burnol A. F., Leturque A., Ferré P., Girard J. Glucose metabolism during lactation in the rat: quantitative and regulatory aspects. Am J Physiol. 1983 Oct;245(4):E351–E358. doi: 10.1152/ajpendo.1983.245.4.E351. [DOI] [PubMed] [Google Scholar]
- Domingo J. L., Paternain J. L., Llobet J. M., Corbella J. Effects of vanadium on reproduction, gestation, parturition and lactation in rats upon oral administration. Life Sci. 1986 Sep 1;39(9):819–824. doi: 10.1016/0024-3205(86)90460-1. [DOI] [PubMed] [Google Scholar]
- Flint D. J., Clegg R. A., Vernon R. G. Prolactin and the regulation of adipose-tissue metabolism during lactation in rats. Mol Cell Endocrinol. 1981 May;22(2):265–275. doi: 10.1016/0303-7207(81)90096-4. [DOI] [PubMed] [Google Scholar]
- Flint D. J. Regulation of insulin receptors by prolactin in lactating rat mammary gland. J Endocrinol. 1982 May;93(2):279–285. doi: 10.1677/joe.0.0930279. [DOI] [PubMed] [Google Scholar]
- Flint D. J., Sinnett-Smith P. A., Clegg R. A., Vernon R. G. Role of insulin receptors in the changing metabolism of adipose tissue during pregnancy and lactation in the rat. Biochem J. 1979 Aug 15;182(2):421–427. doi: 10.1042/bj1820421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh M., Clary T. R., Chernick S. S., Scow R. O. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970 Sep 8;210(3):473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- Heyliger C. E., Tahiliani A. G., McNeill J. H. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985 Mar 22;227(4693):1474–1477. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- Jones R. G., Ilic V., Williamson D. H. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem J. 1984 Jun 1;220(2):455–460. doi: 10.1042/bj2200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgour E., Vernon R. G. Tissue-specific changes in the ability of insulin and noradrenaline to activate pyruvate dehydrogenase in vivo during lactation in the rat. Biochem J. 1987 Apr 1;243(1):69–74. doi: 10.1042/bj2430069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madon R. J., Ensor D. M., Flint D. J. Hypoinsulinaemia in the lactating rat is caused by a decreased glycaemic stimulus to the pancreas. J Endocrinol. 1990 Apr;125(1):81–88. doi: 10.1677/joe.0.1250081. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Farfel Z., Sack J., Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J Biol Chem. 1987 May 15;262(14):6658–6662. [PubMed] [Google Scholar]
- Neville M. C., Waxman L. J., Jensen D., Eckel R. H. Lipoprotein lipase in human milk: compartmentalization and effect of fasting, insulin, and glucose. J Lipid Res. 1991 Feb;32(2):251–257. [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Oller do Nascimento C. M., Ilic V., Williamson D. H. Re-examination of the putative roles of insulin and prolactin in the regulation of lipid deposition and lipogenesis in vivo in mammary gland and white and brown adipose tissue of lactating rats and litter-removed rats. Biochem J. 1989 Feb 15;258(1):273–278. doi: 10.1042/bj2580273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller do Nascimento C. M., Williamson D. H. Tissue-specific effects of starvation and refeeding on the disposal of oral [1-14C]triolein in the rat during lactation and on removal of litter. Biochem J. 1988 Sep 1;254(2):539–546. doi: 10.1042/bj2540539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugazhenthi S., Khandelwal R. L. Insulinlike effects of vanadate on hepatic glycogen metabolism in nondiabetic and streptozocin-induced diabetic rats. Diabetes. 1990 Jul;39(7):821–827. doi: 10.2337/diab.39.7.821. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera M., Tanaka K., Morita T., Ueki H. Increasing effect of vanadate on lipoprotein lipase activity in isolated rat fat pads. Arch Biochem Biophys. 1990 Jun;279(2):291–297. doi: 10.1016/0003-9861(90)90494-j. [DOI] [PubMed] [Google Scholar]
- Shechter Y. Insulin-mimetic effects of vanadate. Possible implications for future treatment of diabetes. Diabetes. 1990 Jan;39(1):1–5. doi: 10.2337/diacare.39.1.1. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]


