Abstract
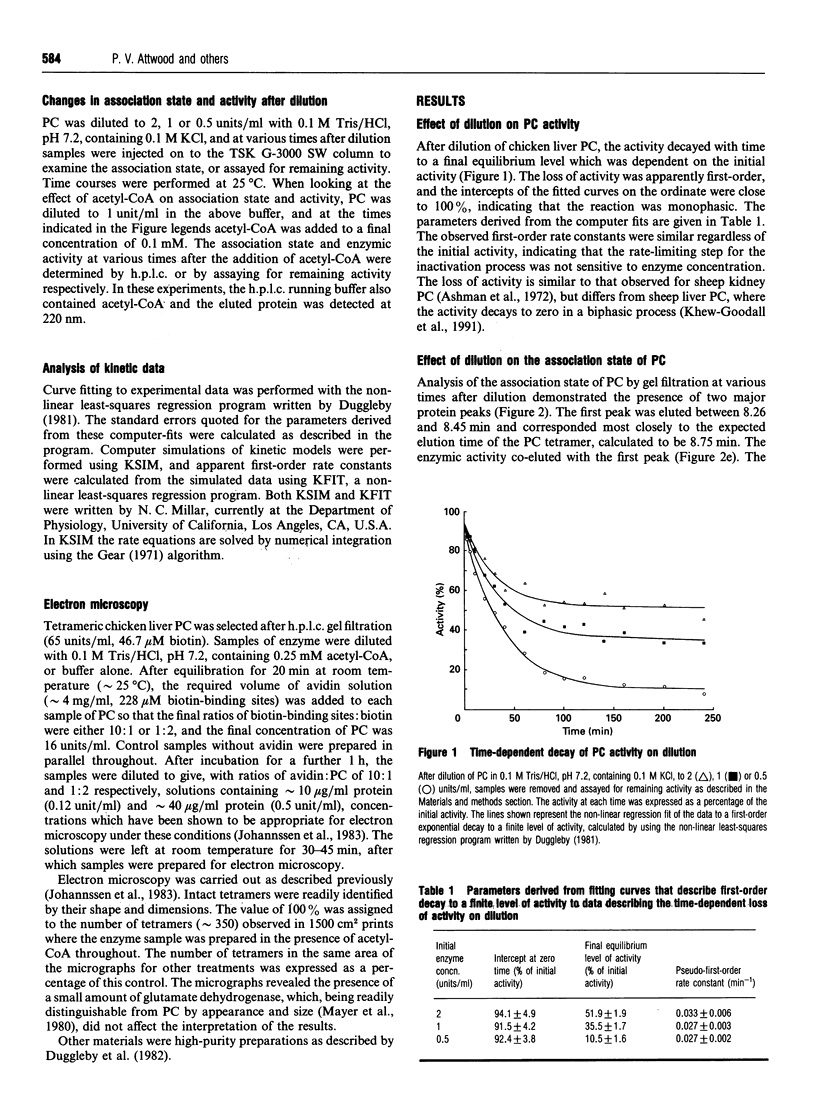
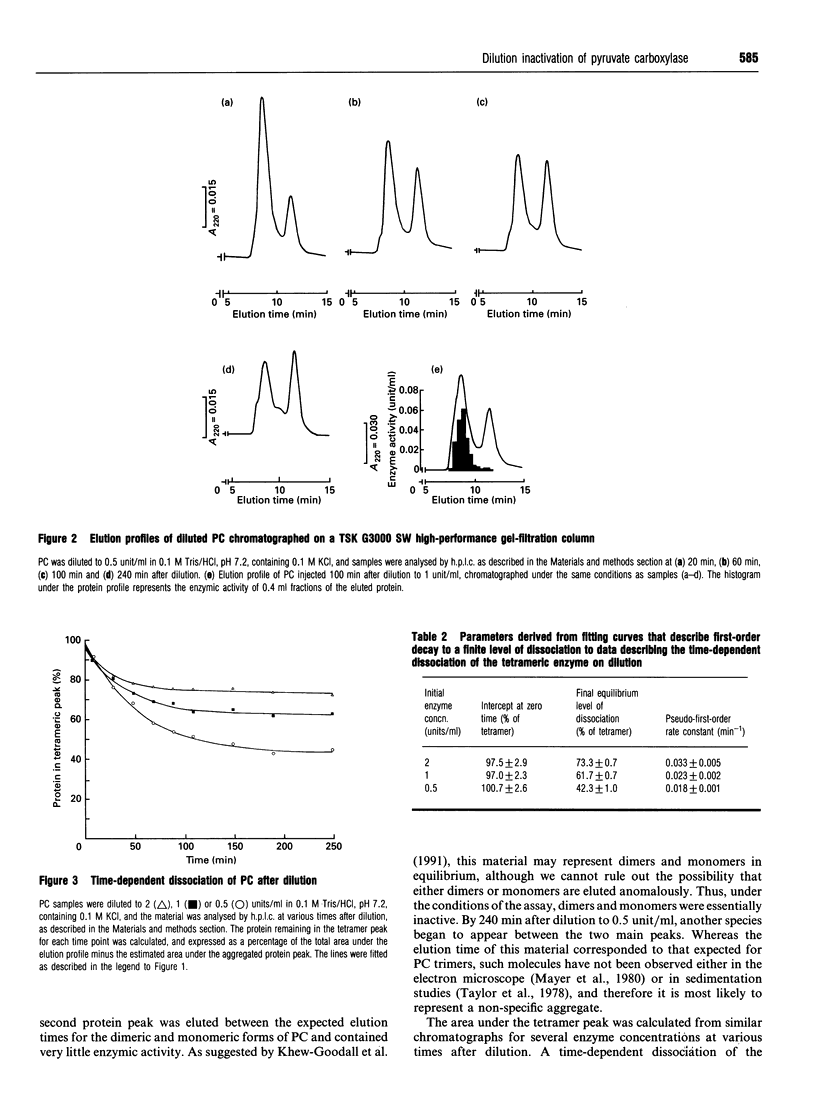
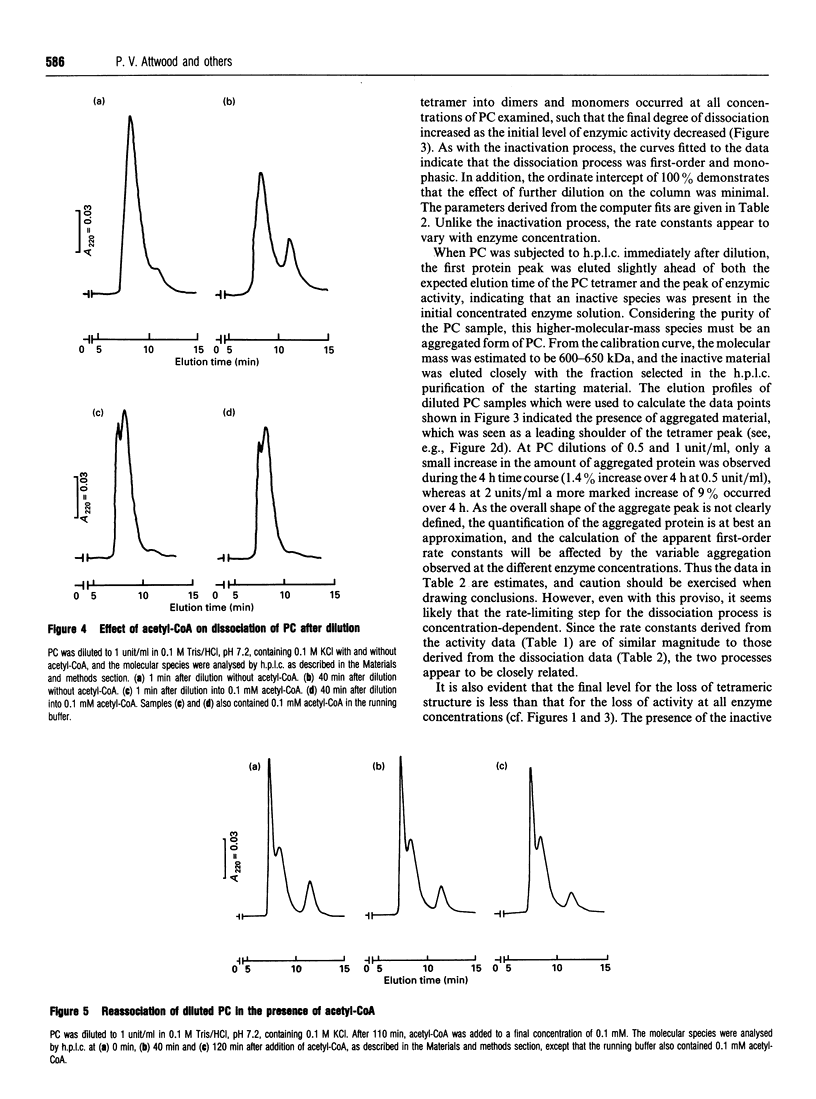
The time-dependent loss of enzymic activity and tetrameric structure of chicken liver pyruvate carboxylase (EC 6.4.1.1) after dilution below 2 units/ml was apparently monophasic and first-order. When examined over a range of initial enzyme concentrations, both activity and tetrameric structure decayed to equilibrium levels which were dependent on the initial concentration. The observed rate constants for the loss of enzymic activity (i) showed no apparent dependence on the initial enzyme concentration, and (ii) were of similar magnitude to the corresponding rate constants of dissociation. Computer simulations of the most likely kinetic model suggest that the predominant form of the dissociated enzyme is the monomer. Dilution of pyruvate carboxylase in the presence of the allosteric activator acetyl-CoA largely prevented the subsequent dissociation of the tetrameric molecule. In addition, acetyl-CoA was able to cause a degree of activation and reassociation when added after dilution inactivation had been allowed to occur. Electron-microscopic observation showed the treatment with avidin before dilution markedly decreased the degree of dissociation of the enzyme tetramer. This structure-stabilizing effect of avidin was dependent on preincubation of the concentrated enzyme solution with acetyl-CoA. We propose that, over a range of protein concentrations, the tetrameric enzyme exists in two forms that are in equilibrium, and that acetyl-CoA alters the equilibrium to favour the more compact form.
Full text
PDF
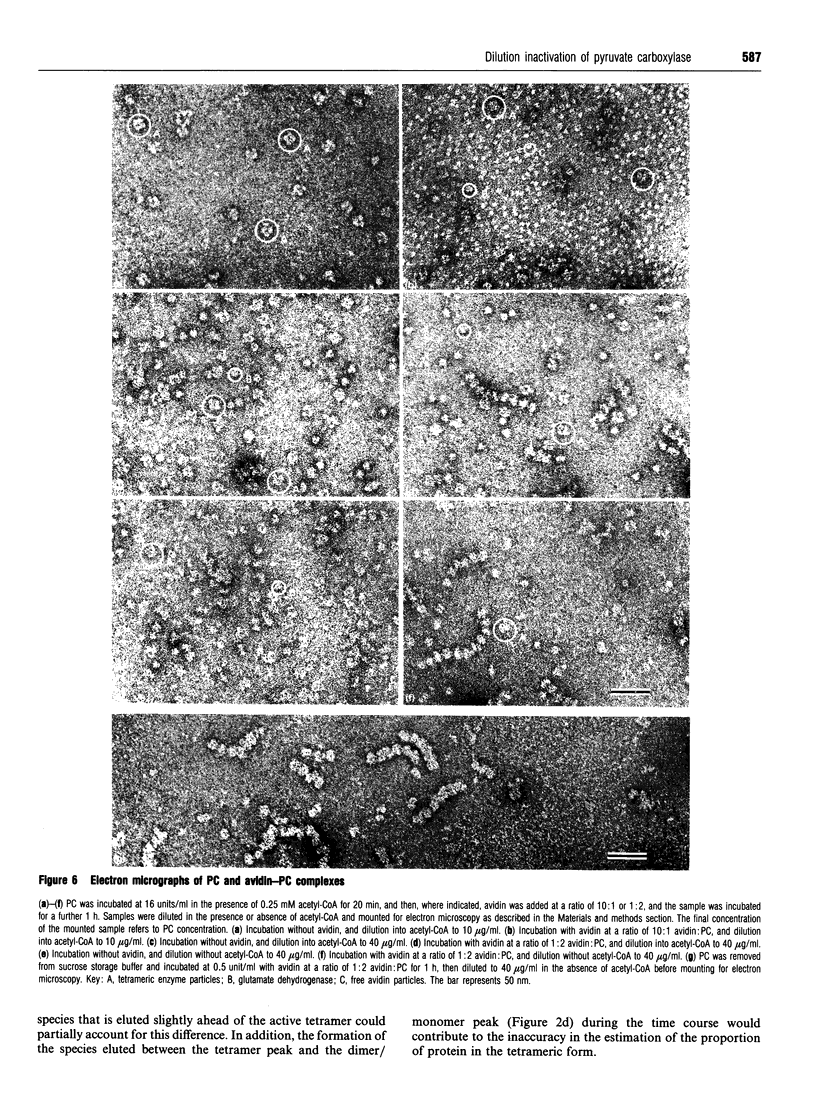
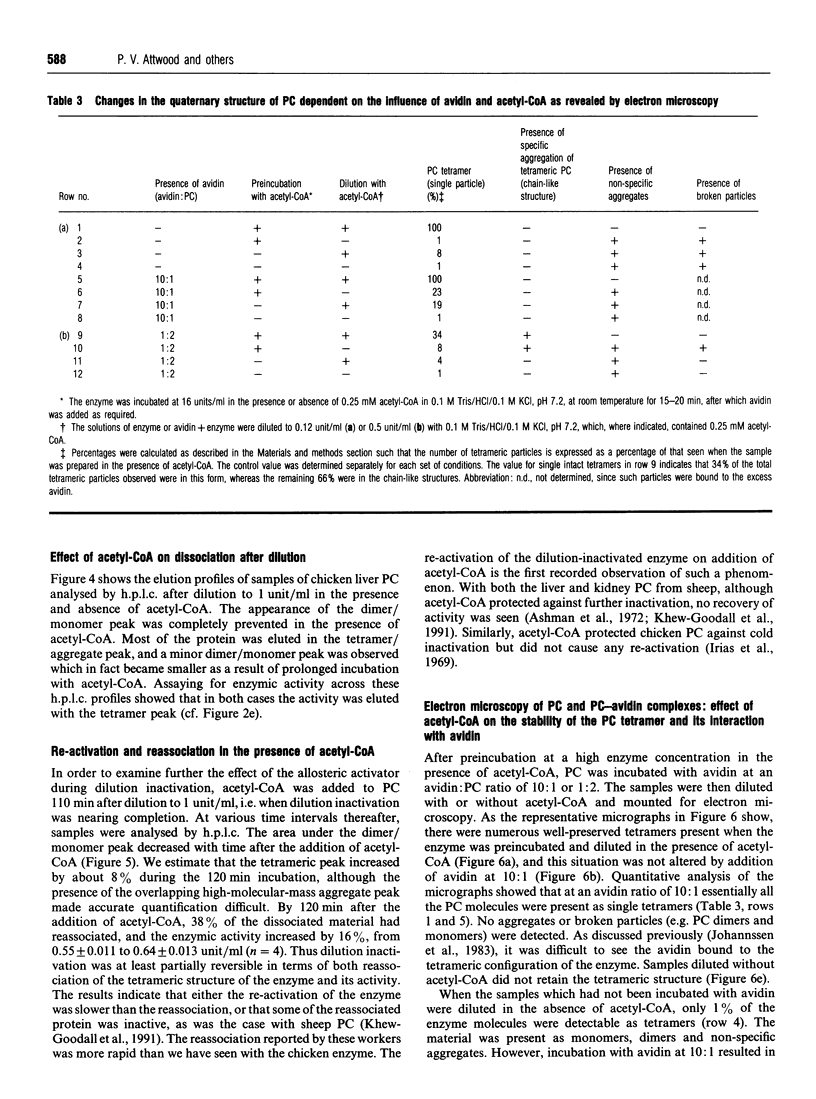

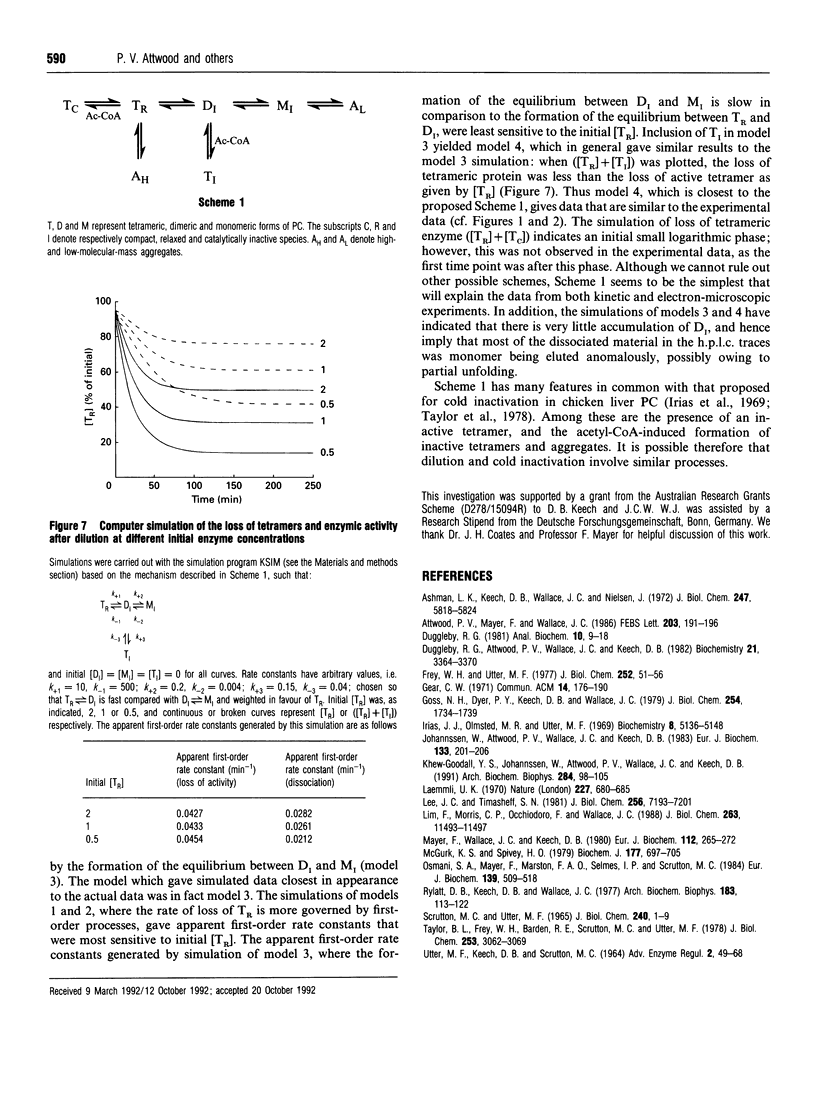
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman L. K., Keech D. B., Wallace J. C., Nielsen J. Sheep kidney pyruvate carboxylase. Studies on its activation by acetyl coenzyme A and characteristics of its acetyl coenzyme A independent reaction. J Biol Chem. 1972 Sep 25;247(18):5818–5824. [PubMed] [Google Scholar]
- Attwood P. V., Mayer F., Wallace J. C. Avidin as a probe of the conformational changes induced in pyruvate carboxylase by acetyl-CoA and pyruvate. FEBS Lett. 1986 Jul 28;203(2):191–196. doi: 10.1016/0014-5793(86)80740-2. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Attwood P. V., Wallace J. C., Keech D. B. Avidin is a slow-binding inhibitor of pyruvate carboxylase. Biochemistry. 1982 Jul 6;21(14):3364–3370. doi: 10.1021/bi00257a018. [DOI] [PubMed] [Google Scholar]
- Frey W. H., 2nd, Utter M. F. Binding of acetyl-CoA to chicken liver pyruvate carboxylase. J Biol Chem. 1977 Jan 10;252(1):51–56. [PubMed] [Google Scholar]
- Goss N. H., Dyer P. Y., Keech D. B., Wallace J. C. An electron microscopic study of pyruvate carboxylase. J Biol Chem. 1979 Mar 10;254(5):1734–1739. [PubMed] [Google Scholar]
- Irias J. J., Olmsted M. R., Utter M. F. Pyruvate carboxylase. Reversible inactivation by cold. Biochemistry. 1969 Dec;8(12):5136–5148. doi: 10.1021/bi00840a068. [DOI] [PubMed] [Google Scholar]
- Johannssen W., Attwood P. V., Wallace J. C., Keech D. B. Localisation of the active site of pyruvate carboxylase by electron microscopic examination of avidin-enzyme complexes. Eur J Biochem. 1983 Jun 1;133(1):201–206. doi: 10.1111/j.1432-1033.1983.tb07448.x. [DOI] [PubMed] [Google Scholar]
- Khew-Goodall Y. S., Johannssen W., Attwood P. V., Wallace J. C., Keech D. B. Studies on dilution inactivation of sheep liver pyruvate carboxylase. Arch Biochem Biophys. 1991 Jan;284(1):98–105. doi: 10.1016/0003-9861(91)90269-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The stabilization of proteins by sucrose. J Biol Chem. 1981 Jul 25;256(14):7193–7201. [PubMed] [Google Scholar]
- Lim F., Morris C. P., Occhiodoro F., Wallace J. C. Sequence and domain structure of yeast pyruvate carboxylase. J Biol Chem. 1988 Aug 15;263(23):11493–11497. [PubMed] [Google Scholar]
- Mayer F., Wallace J. C., Keech D. B. Further electron microscope studies on pyruvate carboxylase. Eur J Biochem. 1980 Nov;112(2):265–272. doi: 10.1111/j.1432-1033.1980.tb07202.x. [DOI] [PubMed] [Google Scholar]
- McGurk K. S., Spivey H. O. Ligand-induced conformational transitions and secondary structure composition of chicken liver pyruvate carboxylase. Biochem J. 1979 Feb 1;177(2):697–705. doi: 10.1042/bj1770697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Mayer F., Marston F. A., Selmes I. P., Scrutton M. C. Pyruvate carboxylase from Aspergillus nidulans. Effects of regulatory modifiers on the structure of the enzyme. Eur J Biochem. 1984 Mar 15;139(3):509–518. doi: 10.1111/j.1432-1033.1984.tb08035.x. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Keech D. B., Wallace J. C. Pyruvate carboxylase: isolation of the biotin-containing tryptic peptide and the determination of its primary sequency. Arch Biochem Biophys. 1977 Sep;183(1):113–122. doi: 10.1016/0003-9861(77)90425-8. [DOI] [PubMed] [Google Scholar]
- SCRUTTON M. C., UTTER M. F. PYRUVATE CARBOXYLASE. 3. SOME PHYSICAL AND CHEMICAL PROPERTIES OF THE HIGHLY PURIFIED ENZYME. J Biol Chem. 1965 Jan;240:1–9. [PubMed] [Google Scholar]
- Taylor B. L., Frey W. H., 2nd, Barden R. E., Scrutton M. C., Utter M. F. The use of the ultracentrifuge to determine the catalytically competent forms of enzymes with more than one oligomeric structure. Multiple reacting forms of pyruvate carboxylase from chicken and rat liver. J Biol Chem. 1978 May 10;253(9):3062–3069. [PubMed] [Google Scholar]
- Utter M. F., Keech D. B., Scrutton M. C. A possible role for acetyl CoA in the control of gluconeogenesis. Adv Enzyme Regul. 1964;2:49–68. doi: 10.1016/s0065-2571(64)80005-4. [DOI] [PubMed] [Google Scholar]