Abstract
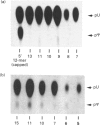
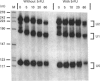
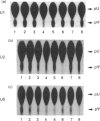
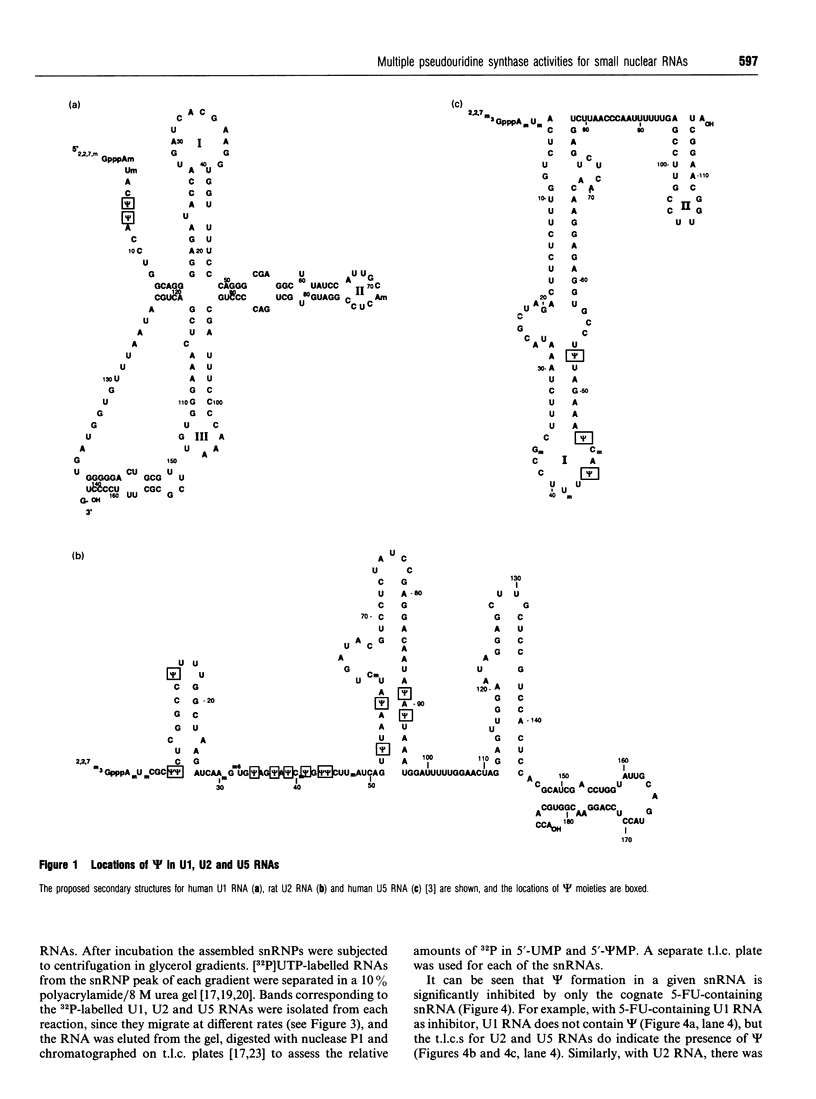
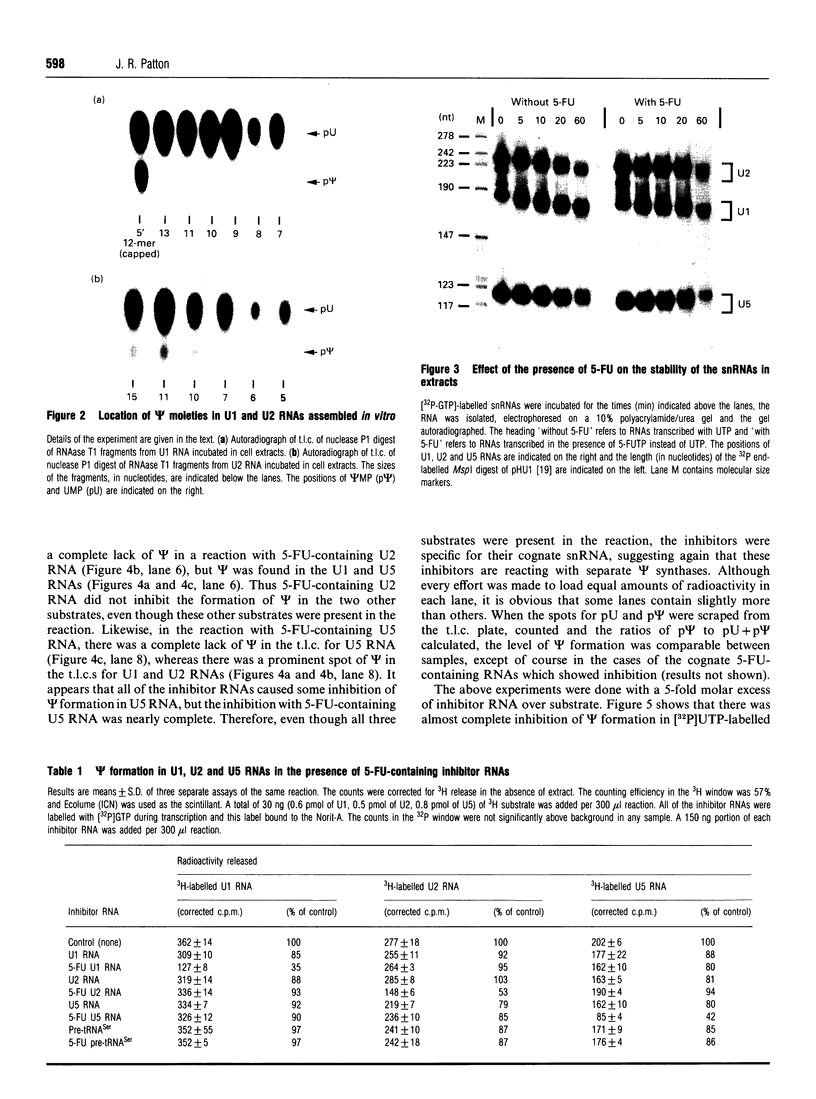
The formation of pseudouridine (psi) in human U1, U2 and U5 small nuclear RNAs (snRNAs) was investigated using HeLa cell extracts. Unmodified snRNAs were synthesized in vitro and the extent of psi formation was determined after incubation in cell extracts. The formation of psi on labelled substrates was monitored in the presence of 5-fluorouracil (5-FU)-containing snRNAs as inhibitors of psi formation. The conversion of uridine to psi was inhibited only when the cognate 5-FU-containing inhibitor snRNA was included in the reaction. For example, 5-FU-containing U1 RNA inhibited psi formation in unmodified U1 RNA, but not in (unmodified) U2 or U5 RNAs. The results suggest that there are at least three activities that form psi in these snRNAs. The 5-FU-containing RNAs were stable during incubation in the cell extracts. A 12-fold molar excess of unlabelled U1 RNA did not inhibit psi formation on a labelled U1 RNA substrate, whereas a 3-fold molar excess of 5-FU-containing U1 RNA nearly abolished psi formation on the U1 substrate. The fact that 5-FU-containing snRNAs are potent inhibitors of psi formation in these pre-mRNA splicing cofactors raises the possibility that this is related to the cytotoxicity of fluoropyrimidines in cancer chemotherapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. D., Takimoto C. H., Cadman E. C. Fluoropyrimidine-mediated changes in small nuclear RNA. J Biol Chem. 1986 Jan 5;261(1):21–24. [PubMed] [Google Scholar]
- Bach M., Winkelmann G., Lührmann R. 20S small nuclear ribonucleoprotein U5 shows a surprisingly complex protein composition. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6038–6042. doi: 10.1073/pnas.86.16.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C. B., Colantuoni V., Sbordone L., Cortese R., Blasi F. Biochemical and regulatory properties of Escherichia coli K-12 hisT mutants. J Bacteriol. 1977 Apr;130(1):4–10. doi: 10.1128/jb.130.1.4-10.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Roth J. R., Ames B. N. Histidine regulation in Salmonella typhimurium. 8. Mutations of the hisT gene. J Bacteriol. 1971 Oct;108(1):410–414. doi: 10.1128/jb.108.1.410-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Sprinzl M., Watanabe K., Albani M., Kersten H. Role of ribothymidine in the thermal stability of transfer RNA as monitored by proton magnetic resonance. Nucleic Acids Res. 1979 Apr;6(4):1571–1581. doi: 10.1093/nar/6.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong S. L., Dolnick B. J. 5-Fluorouracil substitution alters pre-mRNA splicing in vitro. J Biol Chem. 1988 Mar 25;263(9):4467–4473. [PubMed] [Google Scholar]
- Frendewey D. A., Kladianos D. M., Moore V. G., Kaiser I. I. Loss of tRNA 5-methyluridine methyltransferase and pseudouridine synthetase activities in 5-fluorouracil and 1-(tetrahydro-2-furanyl)-5-fluorouracil (ftorafur)-treated Escherichia coli. Biochim Biophys Acta. 1982 Apr 26;697(1):31–40. doi: 10.1016/0167-4781(82)90042-2. [DOI] [PubMed] [Google Scholar]
- Green C. J., Kammen H. O., Penhoet E. E. Purification and properties of a mammalian tRNA pseudouridine synthase. J Biol Chem. 1982 Mar 25;257(6):3045–3052. [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hamm J., Kazmaier M., Mattaj I. W. In vitro assembly of U1 snRNPs. EMBO J. 1987 Nov;6(11):3479–3485. doi: 10.1002/j.1460-2075.1987.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C., Danenberg P. V., Moran R. G. Fluorinated pyrimidines and their nucleosides. Adv Enzymol Relat Areas Mol Biol. 1983;54:58–119. [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammen H. O., Marvel C. C., Hardy L., Penhoet E. E. Purification, structure, and properties of Escherichia coli tRNA pseudouridine synthase I. J Biol Chem. 1988 Feb 15;263(5):2255–2263. [PubMed] [Google Scholar]
- Lutz-Freyermuth C., Keene J. D., Lutz-Reyermuth C. The U1 RNA-binding site of the U1 small nuclear ribonucleoprotein (snRNP)-associated A protein suggests a similarity with U2 snRNPs. Mol Cell Biol. 1989 Jul;9(7):2975–2982. doi: 10.1128/mcb.9.7.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., De Robertis E. M. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell. 1985 Jan;40(1):111–118. doi: 10.1016/0092-8674(85)90314-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullenbach G. T., Kammen H. O., Penhoet E. E. A heterologous system for detecting eukaryotic enzymes which synthesize pseudouridine in transfer ribonucleic acids. J Biol Chem. 1976 Aug 10;251(15):4570–4578. [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Ofengand J., Henes C. The function of pseudouridylic acid in transfer ribonucleic acid. II. Inhibition of amino acyl transfer ribonucleic acid-ribosome complex formation by ribothymidylyl-pseudouridylyl-cytidylyl-guanosine 3'-phosphate. J Biol Chem. 1969 Nov 25;244(22):6241–6253. [PubMed] [Google Scholar]
- Patton J. R., Habets W., van Venrooij W. J., Pederson T. U1 small nuclear ribonucleoprotein particle-specific proteins interact with the first and second stem-loops of U1 RNA, with the A protein binding directly to the RNA independently of the 70K and Sm proteins. Mol Cell Biol. 1989 Aug;9(8):3360–3368. doi: 10.1128/mcb.9.8.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R., Patterson R. J., Pederson T. Reconstitution of the U1 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1987 Nov;7(11):4030–4037. doi: 10.1128/mcb.7.11.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R., Pederson T. The Mr 70,000 protein of the U1 small nuclear ribonucleoprotein particle binds to the 5' stem-loop of U1 RNA and interacts with Sm domain proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(3):747–751. doi: 10.1073/pnas.85.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R. Pseudouridine modification of U5 RNA in ribonucleoprotein particles assembled in vitro. Mol Cell Biol. 1991 Dec;11(12):5998–6006. doi: 10.1128/mcb.11.12.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T. Interactions of transfer RNA pseudouridine synthases with RNAs substituted with fluorouracil. Nucleic Acids Res. 1991 Nov 25;19(22):6139–6144. doi: 10.1093/nar/19.22.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Olsson M. Transfer RNA pseudouridine synthases in Saccharomyces cerevisiae. J Biol Chem. 1990 May 25;265(15):8782–8787. [PubMed] [Google Scholar]
- Tsui H. C., Arps P. J., Connolly D. M., Winkler M. E. Absence of hisT-mediated tRNA pseudouridylation results in a uracil requirement that interferes with Escherichia coli K-12 cell division. J Bacteriol. 1991 Nov;173(22):7395–7400. doi: 10.1128/jb.173.22.7395-7400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]