Abstract
Acute stressors tend to shift preferences toward comfort foods, yet they do not ubiquitously increase the amount of food consumed. Moreover, although many individuals eat more under stress, others eat less or show no change. Although the precise mechanisms explaining this variability in stress-related eating are unknown, they may be driven by individual differences in the rewarding effects of comfort eating, which are enhanced by greater lifetime stressor exposure. To investigate this possibility, we examined whether differences in lifetime stressor exposure predicted reductions in negative affect following snacking (i.e., negative reinforcement) and if this effect was specific to stress-related snacking or snacking in general. Participants were 26 women (23% non-White) between 20 and 45 years old (M = 31), with a mean body mass index of 26, who completed three laboratory visits. Participants completed an assessment of lifetime stressor exposure (i.e., STRAIN) on the first visit and, on two subsequent laboratory visits in counterbalanced order, were given snacks after an acute social stress task (i.e., TSST) or rest period. Greater lifetime stressor exposure was related to greater post-ingestive decreases in negative affect following the acute social stressor but not following the rest period. If stress-related eating is more comforting for women with greater lifetime stressors and contributes to a stronger stress-eating association, then this may inform obesity-related clinical treatments that target behaviors and cognitions related to reward-based learning.
1. Introduction
Life stressors are common in the United States and strongly associated with changes in mood and eating behaviors that can lead to health problems if not addressed (Adam & Epel, 2007; Chao et al., 2017; Epel et al., 2012; Sinha, 2018). Following acute stressors, for example, personal preferences tend to shift toward comfort foods (i.e., foods self-reported to reduce negative affect that are high in fat, sugar, carbohydrates, or sodium; Boggiano, 2016; Boggiano et al., 2017; Chao et al., 2020; Tryon, DeCant, et al., 2013; Zellner et al., 2006). However, stressors do not ubiquitously increase the amount of food eaten. Whereas many individuals increase their food intake under stress (APA, 2015), for example, others decrease intake or show no change (Adam & Epel, 2007; Epel et al., 2012; Hill et al., 2021). Although the precise causes of this variability are unknown, individual differences in reward and affective processing may play a role.
1.1. Rewarding effects of stress-eating
Inconsistencies in the literature regarding stress-related eating may be driven by individual differences in the rewarding effects of comfort eating. Comfort foods high in sugar, fat, carbohydrates, or sodium are hedonically rewarding. Eating comfort foods increases opioid release in brain reward pathways and may protect against the detrimental effects of stress by enhancing feelings of pleasure, and reducing the behavioral and neuroendocrine responses to stress (Epel et al., 2012; Finch & Tomiyama, 2014; Foster et al., 2009; La Fleur et al., 2005; Pecoraro et al., 2004). Adam and Epel (2007) describe a reward-based stress eating model in which repeated excitation of brain reward systems coinciding with stress-eating leads to changes in neural circuitry that promote future stress-eating. Similarly, the affect regulation model states that a reduction in negative affect following binge eating negatively reinforces the eating behavior (Hawkins & Clement, 1984), and Skinner’s reinforcement learning theory (Skinner, 1963) suggests that dampening stress and negative affect are rewarding consequences of eating and can promote future food intake (Epel et al., 2012; Macht et al., 2005; Yeomans et al., 2004). These theories suggest that individuals who have post-ingestive negative reinforcement (i.e., decreases in negative affect) are more likely to eat in response to stress and negative emotions in the future. To our knowledge, only one study to date has data to directly support these theories in the laboratory (Klatzkin et al., 2022). In this study, participants ate snack food in response to a stressor, and negative affect was then measured. Indeed, it was found that there was greater post-ingestive negative reinforcement following greater snack food intake; however, because snack food intake occurred before the measure of negative reinforcement, directionality of the effect could not be directly supported.
1.2. Is comfort food comforting?
Individuals often self-report eating comfort foods to increase pleasure and dampen negative emotions (Boggiano, 2016; Boggiano et al., 2017), yet the scientific literature is inconsistent regarding whether eating for comfort actually works. Tomiyama, Finch, and Cummings (2015) argued that it is important to know whether, and for whom, eating comfort foods is truly comforting due to the need to personalize obesity-related treatment and intervention efforts.
Animal studies have consistently found that palatable foods decrease anxiety and depressive responses to stressors (Tomiyama et al., 2015). However, there is a dearth of studies that have assessed these processes in humans. Both naturalistic and laboratory studies have reported decreased negative affect following comfort food intake (Finch & Tomiyama, 2014; Macht & Mueller, 2007; Wouters et al., 2018), yet the results are mixed and indicate that eating comfort foods may not consistently reduce negative emotions (Cummings et al., 2022; Finch et al., 2019; Franja et al., 2021; Haedt-Matt & Keel, 2011b; McKay et al., 2021; Mikhail, 2021). Macht and Mueller (2007) found that eating a small amount of chocolate reduced negative emotional reactivity to a negative film clip in healthy controls; yet, Bongers and colleagues (2013) reported that feeling better after eating may not be specific to a negative mood state, as participants reported decreases in post-ingestive negative affect following positive, negative, and neutral mood induction (Bongers et al., 2013). Furthermore, one study found that neither eating palatable nor healthy comfort foods dampened physiological or negative mood responses to a laboratory-based stressor (Finch et al., 2019).
In participants with binge eating disorder (BED) and obesity, there is evidence for an overall (short-term) mood improvement following food intake (Leehr et al., 2015; Schulz & Laessle, 2012; Telch & Agras, 1996), although a meta-analytic review of naturalistic studies found that self-reported negative affect increased after binge eating episodes in individuals with BED and bulimia nervosa (Haedt-Matt & Keel, 2011a). This inconsistency in the literature suggests that eating in response to stress and negative emotions may be more rewarding for some people than others.
1.3. Stress-eating may be more rewarding for individuals with greater lifetime stressors
Whereas chronic stressor exposure enhances the rewarding effects of eating palatable foods following stress in rodent models, we are not aware of any studies that have investigated this effect in humans (Dallman et al., 2003; Finch & Tomiyama, 2014; Tomiyama et al., 2015). Chronic stressor exposure dysregulates cortisol levels, dopamine receptors in the nucleus accumbens, and dopaminergic responses to acute stress, leading to greater responsivity to reward (e.g., comfort foods; Epel et al., 2012; Sinha, 2018; Tryon et al., 2013; Wei et al., 2019). Moreover, a greater feeling of reward following stress-related eating for individuals with higher chronic stress may act as a form of self-medication that increases the potency of stress-induced negative emotions as a trigger for eating and may contribute to the greater susceptibility to obesity in this population (Adam & Epel, 2007; Dallman et al., 2003; van der Valk et al., 2018). We previously reported that greater perceived life stress is associated with greater reductions in negative affect (i.e., negative reinforcement) after stress-eating in the laboratory (Klatzkin et al., 2018). However, this study assessed perceived life stress and only over the past month. Moreover, all participants were students, which may limit the generalizability of the results.
1.4. Present Study
To address these gaps in the literature, we used the Stress and Adversity Inventory (Slavich & Shields, 2018) to comprehensively assess participants’ exposure to acute and chronic stressors over the entire life course, and sampled a diverse group of participants from the Memphis community to investigate how lifetime stressor exposure is related to the rewarding effect of eating in women. As eating may reduce negative affect in general, irrespective of the presence of a stressor or negative mood (Bongers et al., 2013), we assessed post-ingestive changes in negative affect following a laboratory-based stressor or a rest period (i.e., within-subjects design). In doing so, we sought to determine whether individual differences in lifetime stressor exposure lead to reductions in negative affect following snacking and, if so, if this effect is specific to snacking during stress or snacking in general. Based on the research reviewed above, we hypothesized that greater lifetime stressor exposure would be related to greater post-ingestive decreases in negative affect following a laboratory-based stressor, but not following a rest period.
2. Method
2.1. Participants
The present report represents a secondary analysis of data used in a prior study (Klatzkin et al., 2023). Participants were 26 women (mean combined household income category = $50,000 to $75,000) between 20 and 45 years old (M = 31.4, SD = 5.8), with a mean body mass index of 26.2 (SD = 6.4), who responded to advertisements for a study investigating the effects of stress physiology on taste experiences. 77% of participants identified as non-Hispanic white and the remaining 23% identified as either Black, African, or African American (11%), Native American (4%), Asian (4%), or Hispanic/Latinx (4%). We recruited women in Memphis, Tennessee via a partnership with a local community center. Only women were recruited for this study, as women tend to eat more following stress and show a stronger association between stress and obesity than men (Konttinen et al., 2010; Udo et al., 2014).
Participants were excluded if they self-reported current or prior cardiovascular disease, diabetes, or blood pressure above 160/95mmHg; were currently taking blood pressure, stimulant, or psychoactive medications; were in current treatment for eating or weight problems; were regular smokers; or were pregnant, lactating, or menopausal. The research was approved by the Institutional Review Board at Rhodes College. Participants provided written informed consent and were paid for their time.
2.2. Procedure
Women responding to the advertisements completed preliminary screening questions aimed at assessing demographic information in addition to the exclusionary criteria described above. Participants also answered a battery of questions that included assessments of lifetime stressor exposure as well as eating-related behaviors and cognitions (see Klatzkin et al., 2023). A total of 62 women completed the preliminary screening. Next, participants were asked to complete two laboratory testing sessions in counterbalanced order: a stress day during which participants underwent a social stress test prior to eating snacks, and a rest day in which participants rested prior to eating snacks. From September 2019 to March 2020, only 26 participants completed both the rest day and the stress day testing sessions before data collection ended due to COVID-19. Data from these 26 participants comprise the present report. Our prior report using the same dataset included all 44 women who completed stress day testing between September 2019 to March 2020 as well as between January through May 2022 (Klatzkin et al., 2023). Only 26 of these 44 participants completed rest day testing.
Each laboratory testing session began between 3:00 pm and 5:30 pm (Figure 1). The order of rest and stress laboratory sessions was counterbalanced between participants and were separated by an average of 7 days. The women who completed the rest day first (n = 14) did not differ from women who completed the stress day first (n = 12) on lifetime stressor exposure or negative affect ratings at baseline, stress, or post-snack time points (ps > 0.34). The rest and stress days were the same with the exception that on the rest day, stress testing was replaced with a rest period of the same length during which participants listened to classical music and had the option to read popular science magazines. On the day of the study, participants did not wake from sleep less than two hours prior to the testing session, take any antihistamines, psychotropic medications, or neural stimulants, exercise strenuously (i.e., cardiovascular exercise for more than a few minutes), drink more than a single caffeinated beverage, eat or drink (except water) two hours prior to the study, or consume any alcohol 12 hours prior to the study. Participants were also asked to arrive “not too hungry, but not too full” and to “make sure to eat some food at least 2 hours before the study visit to avoid excess hunger.” Research assistants confirmed compliance with study requirements upon arrival to the laboratory; else, participants were rescheduled.
Figure 1.
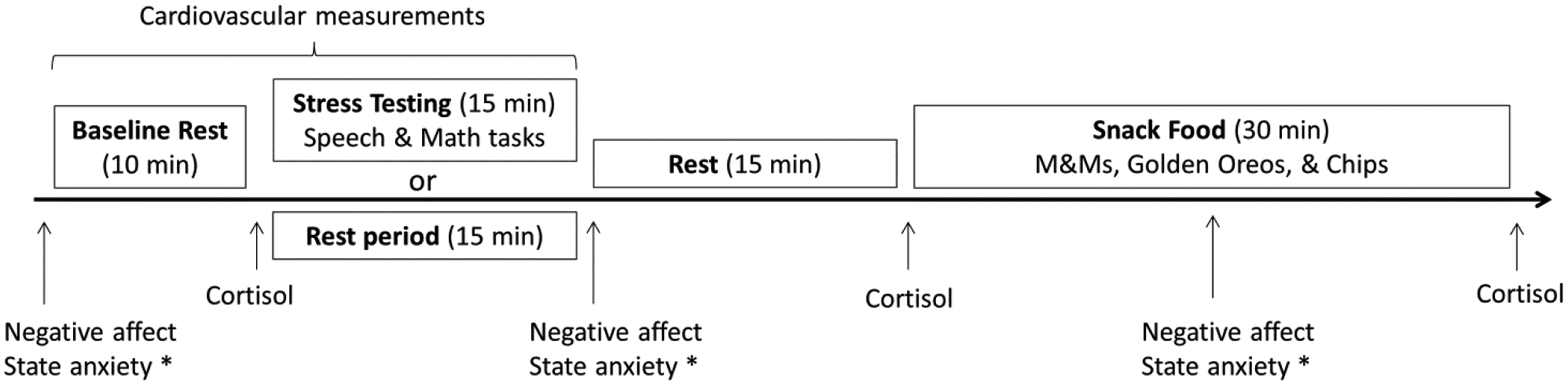
Laboratory protocol for stress and rest days
* Participants rated their hunger, desire to eat, and wanting/liking of snack foods, but results for these measures are not reported here.
2.3. Psychological Measures—Preliminary screening
2.3.1. Lifetime Stressor Exposure
The Stress and Adversity Inventory (Slavich & Shields, 2018) was used to assess participants’ exposure to acute and chronic stressors occurring over the entire life course (see http://www.strainsetup.com). The STRAIN is a National Institute of Mental Health-recommended instrument that assesses a person’s cumulative exposure to 55 different acute life events (e.g., deaths of relatives, job losses, negative health events, etc.) and chronic difficulties (e.g., ongoing health problems, work problems, relationship problems, financial problems, etc.). Included in this list are 26 pre-defined acute life events and 29 pre-defined chronic difficulties that are known to impact health (e.g., have you ever experienced exclusion or unfair treatment at a job - for example, because of your gender, sexual orientation, race, or ethnicity?). The STRAIN has excellent test-rest reliability, construct validity, discriminate validity, and has been shown to predict a variety of biological, clinical, and behavioral outcomes including impulsivity, coping, and risky behaviors (Cazassa et al., 2020; Lam et al., 2019; McMullin et al., 2021; Murphy et al., 2023; Olvera Alvarez et al., 2019; Slavich & Shields, 2018). In the present study, we used the STRAIN’s total count of lifetime stressors (including both acute and chronic lifetime stressors) to test our hypothesis. Higher scores indicate greater number of stressors experienced.
2.4. Laboratory Protocol
2.4.1. Baseline Rest
Researchers placed an automated blood pressure cuff on the non-dominant arm of the participant. Participants then completed questionnaires that assessed state anxiety, and positive and negative affect. We then assessed cardiovascular measures of systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR).
2.4.2. Trier Social Stress Test (TSST)
The researcher informed the participants that they would be undergoing a mental stress test (i.e., the TSST) that includes giving a speech and performing serial subtraction while being audio and visually recorded, which has been shown to reliably induce a robust stress response (Kirschbaum et al., 1993). The researcher then asked participants to take 5 min to prepare their speech that should describe why they would be the best candidate for their ideal job. Immediately following the preparation period, the selection committee returned to the testing room and asked the participants to deliver their speech for 5 min. Finally, the researcher asked the participants to perform mental math for 5 min by serially subtracting 7 from 2000 aloud as quickly and accurately as possible. Cardiovascular and cortisol reactivity were assessed throughout the TSST (see section 2.5 below).
Following the TSST, participants were told that the recordings of their performance would be analyzed while they completed questionnaires assessing state anxiety and positive and negative affect. Following questionnaire completion, the researcher returned to inform the participant that “there has been a problem with the recording, and it may be necessary to redo the task”. This information was given to prolong the stressor until 15 min after the end of the TSST when cortisol levels peak post-stress. Following saliva collection, the researcher informed the participant that the problem with the recording had been fixed and that they would not be required to redo the stress tasks.
2.4.3. Snack Food
Following saliva collection, participants began the bogus taste test, a validated measure of food intake (Robinson et al., 2017). Participants were given three clear bowls filled with either M&Ms (250g, 9 servings, 1250 calories), mini golden Oreos (150g, 5.2 servings, 724 calories), or potato chips (100g, 3.6 servings, 570 calories). The researcher told the participant the following, “We are interested in how stress affects the perceived taste and texture of snack foods. When we return, we will ask you to rate each of these foods across various tastes and textures. Please sample each snack so that you will be able to provide these ratings. Feel free to eat as much as you would like, and to ask for more if you want it. We’ll be back in 15 min with more questionnaires and to collect your ratings.” Participants were then left alone for 15 min to consume the snacks while free to move about the private testing room.
After 15 minutes of the 30-minute snack period, participants again completed assessments measuring state anxiety and positive and negative affect. Participants also rated the degree to which they found each snack food to be salty, sweet, crunchy, and enjoyable. Researchers weighed each bowl before and after food consumption to determine food intake.
2.4.4. Post-snack
Following the 30-minute snack period, participants rinsed their mouths out with water and provided a final saliva sample. Finally, during the second laboratory session only, a student researcher assessed height (cm) and weight (kg) to calculate BMI (kg/m2) using a Seca 769 digital column scale and stadiometer and waist circumference with an anthropometric tape measure. We chose to measure weight at the conclusion of all study visits to ensure that the priming knowledge of one’s weight would not influence eating behaviors.
2.5. Physiological Measures
The Oscar 2 oscillometric ambulatory blood pressure monitor (SunTech Medical Instruments, Inc., Raleigh, NC) provided automated measurement of systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) while participants were in a comfortable seated position. Blood pressure and HR measures were taken at minutes 0, 5, and 10 of baseline and minutes 0, 2, and 4 of both the speech and serial subtraction periods. The cardiovascular data recorded at minute 10 of baseline constituted the baseline values of SBP, DBP, and HR. The peak value of SBP, DBP, and HR for each participant during each stress task constituted the speech and math stress values.
Saliva was collected in 1.5 mL Eppendorf tubes at the end of the baseline rest period, and 15 and 45 min following the end of the TSST or rest period (Figure 1). Participants passively drooled into the tube for a maximum of 2 min per sample. Saliva samples were frozen within 30 min of collection at −20 °C until assayed. The mean intra-assay coefficient of variation was 9.14% and the inter-assay coefficient was 4.83%.
2.6. Subjective Psychological Measures—Baseline, Post-Stress/Rest, and Post-Snack
2.6.1. Positive and negative affect:
Affect was quantified with the Positive and Negative Affect Schedule (PANAS), a 20-item multiple-choice survey validated in a university population (Watson et al., 1988). Participants choose from 1 (Very Slightly or Not At All) to 5 (Extremely) for each word describing a different feeling or emotion felt at the present moment (e.g. distressed, hostile, nervous). The positive subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more positive affect. The negative subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more negative affect. Cronbach’s alpha for the 10 items on the positive affect subscale (α = 0.92) and the 10 items on the negative affect subscale (α = 0.65) of the PANAS were very high and adequate, respectively.
2.6.2. State anxiety:
The State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) is a 20-item self-report questionnaire assessing current anxiety (e.g., I feel nervous and restless). The STAI-State ranges from 20–80, with higher scores indicating greater anxiety. Cronbach’s alpha for STAI was very good, α = 0.89.
2.7. Data analysis
To determine the effectiveness of the laboratory-based stress manipulation, we assessed changes in a variety of physiological (heart rate, blood pressure, and cortisol) and psychological (self-reported anxiety and negative affect) outcomes using a repeated measures ANOVA with baseline and post-stress as the within-subject factor.
To investigate whether post-ingestive reduction in negative affect (i.e., change in negative affect from post-stress to post-snack) is predicted by lifetime stressor exposure, baseline negative affect, age, and snack intake, we used two linear regression analyses: one to analyze data collected on the stress day and one to analyze data collected on the rest day. We included snack food intake in the model because we were interested in determining how lifetime stressor exposure impacts the reduction of negative affect following snacking regardless of the amount of food consumed, and because greater snack food intake may cause greater post-ingestive decreases in negative affect. Given that the STRAIN assesses stressors over the entire life course, we also included age in the analyses. Furthermore, we included baseline negative affect in the analysis because this variable could be influenced by various circumstances in a person’s day prior to the laboratory protocol and potentially influence subsequent negative ratings. The data were analyzed using IBM SPSS Statistics (Version 25).
For the purposes of visualizing the linear regression results, we split our continuous variable of lifetime stressor exposure into Low and High using 1 standard deviation above and below the mean and then used the regression coefficients to create Figure 4 using the formula: y = a +b1*x + b2*x +b3* x + b4*x.
Figure 4.
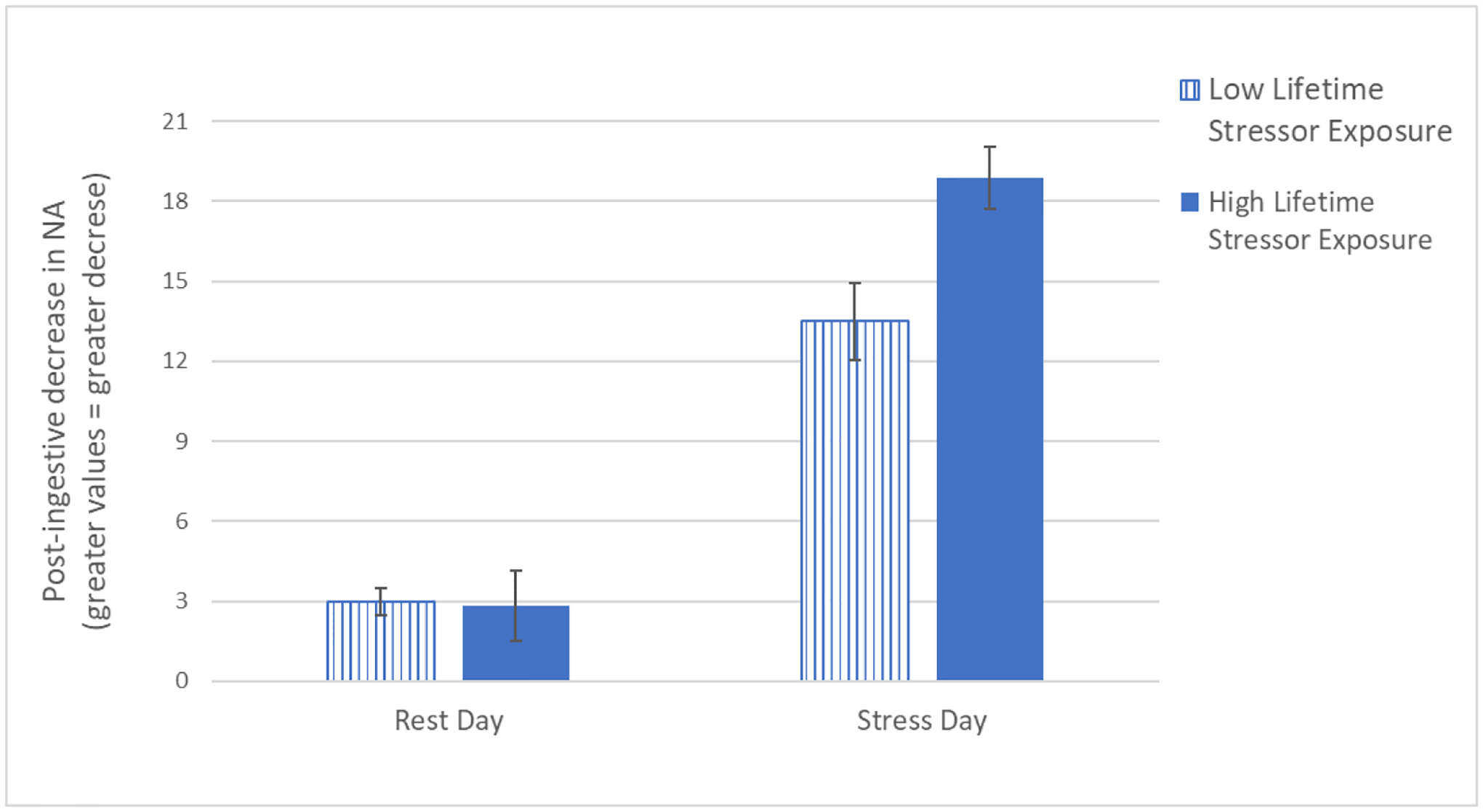
Visual depiction of the linear regression analyses predicting post-ingestive reductions in negative affect (NA) following rest and the laboratory-based acute social stressor. We split the continuous lifetime stressor exposure variable into Low and High using 1 standard deviation above and below the mean, and used the formula: y = a +b1*x + b2*x +b3* x + b4*x. Experience more lifetime stressors was related to greater post-ingestive reductions in negative affect, but only on the stress day, F(4, 21) = 4.03, p = 0.01, R2 = 0.43.
We examined potential outliers in all variables used in the analyses and determined that one participant showed extreme changes in post-ingestive negative affect following the stressor (i.e., a decrease that was greater than the third quartile plus 1.5 times the interquartile range). Additionally, one participant showed extreme post-ingestive changes in negative affect following the rest period (i.e., an increase that was greater than the third quartile plus 1.5 times the interquartile range). We replaced these data points with the group mean plus two times the standard deviation (Field, 2019). The two outliers were adjusted from 22 to 16.59 (stress day) and from −13 to −11 (rest day).
Tests to determine if the data met the assumption of collinearity indicated that multicollinearity was not a concern (Lifetime stressor exposure, Tolerance = 0.88, VIF = 1.14; Age, Tolerance = 0.94, VIF = 1.07; Baseline negative affect, Tolerance = 0.96, VIF = 1.04; Snack intake, Tolerance = 0.90, VIF = 1.11). The data also met the assumption of independent errors (Durbin-Watson value = 1.98). The histogram of standardized residuals indicated that the data contained approximately normally distributed errors, as did the normal P-P plot of standardized residuals. The scatterplot of standardized residuals showed that the data met the assumptions of homogeneity of variance and linearity.
3. Results
Participants (23% non-white) were on average 31.5 (SD = 5.8) years of age with a body mass index of 26.3 (SD = 6.5) and reported an average of 18.7 (SD = 10.1) total lifetime stressors. The laboratory-based social stress manipulation was effective, as the TSST induced significant increases in state anxiety, F(1,24) = 37.3, p < .001, negative affect, F(1,24) = 27.8, p < .001, cortisol, F(1,23) = 10.1, p = 0.004, SBP, F(1,24) = 131.4, p < .001, DBP, F(1,24) = 193.3, p < .001, and HR, F(1,24) = 80.89, p < .001 (Table 1). Mean snack food intake on the rest day did not differ from the mean snack intake on the stress day, F(1, 24) = 0.48, p = 0.49 (Table 2).
Table 1.
Mean (± SD) variables of interest from our sample of women compared between stress and rest days (n = 26)
| Rest day | Stress day | ||||
|---|---|---|---|---|---|
| Baseline | Rest minus baseline | Baseline | Stress minus baseline | Change scores on stress vs. rest day | |
| Negative affect | 14.1 (±6.3) | −2.7 (±2.9) | 12.7 (±2.7) | 6.7 (±6.9) | F(1, 24) = 35.7, p < 0.0001 |
| State anxiety | 36.6 (±11.2) | −7.4 (±8.5) | 35.2 (±6.9) | 15.2 (±12.8) | F(1, 24) = 48.3, p < 0.0001 |
| Systolic blood pressure | 116.6 (±9.7) | 4.7 (±7.9) | 118.4 (±9.4) | 31.2 (±13.4) | F(1, 24) = 71.4, p < 0.0001 |
| Diastolic blood pressure | 70.4 (±6.3) | 3.0 (±4.2) | 69.6 (±7.2) | 24.3 (±8.7) | F(1, 24) = 122.4, p < 0.0001 |
| Heart rate | 66.0 (±11.7) | 2.6 (±5.2) | 64.4 (±9.9) | 31.8 (±17.4) | F(1, 24) = 68.7, p < 0.0001 |
| Cortisol | 0.24 (±0.1) | % increase = −17.5 (±23.9) | 0.22 (±0.17) | % increase = 85.0 (±159.4) | F(1, 23) = 9.15, p = 0.006 |
Table 2.
Mean (± SD) negative affect (min and max possible scores = 10–50) and snack intake from our sample of women compared between stress and rest days (n = 26).
| Rest day | Stress day | Rest vs. Stress | |
|---|---|---|---|
| Baseline negative affect | 14.1 (±6.3) | 12.7 (±2.7) | F(1, 24) = 1.9, p = 0.19 |
| Post-rest or post-stress negative affect | 11.1 (±3.2) | 19.4 (±7.2) | F(1, 24) = 59.2, p < 0.0001 |
| Post-eating negative affect | 12.1 (±3.7) | 14.2 (±5.0) | F(1, 24) = 6.2, p = 0.02 |
| Snack intake | 494.9 (±270.6) | 464.3 (±276.4) | F(1, 24) = 0.5, p = 0.49 |
As hypothesized, linear regression analyses revealed that greater lifetime stressor exposure was associated with greater post-ingestive decreases in negative affect following the laboratory-based stressor, but not following a rest period (Tables 3, 4; Figures 2–4). On the stress day, lifetime stressor exposure, Beta = 0.453, p = 0.017 (Table 3; Figures 2 and 4), significantly predicted post-ingestive reductions in negative mood, as did snack intake, Beta = −0.370, p = 0.045; F(4, 21) = 4.03, p = 0.01, R2 = 0.43. In contrast, on the rest day, only snack intake predicted post-ingestive reductions in negative mood, Beta = −0.42, p = 0.05 (Table 4; Figures 3 and 4), F(4, 21) = 1.6, p = 0.21, R2 = 0.24. Mean (± SD) negative affect ratings at baseline, stress, and post-snack on rest and stress days are depicted in Table 2.
Table 3.
Multivariate linear regression analysis predicting post-ingestive reductions in negative mood following the laboratory-based acute social stressor, F(4, 21) = 4.03, p = 0.01.
| B | SE B | β | |
|---|---|---|---|
| Baseline negative affect on stress day (min = 10, max = 19) | 0.050 | 0.332 | 0.025 |
| Age (years) | −0.257 | 0.159 | −0.275 |
| Snack intake following a stressor (kcal) | −0.007 | 0.003 | −0.370* |
| Lifetime stressor exposure (min = 4, max = 57) | 0.197 | 0.076 | 0.453+ |
| R2 | 0.434 | ||
| Adjusted R2 | 0.326 | ||
p = 0.045
p = 0.017
Table 4.
Multivariate linear regression analysis predicting post-ingestive reductions in negative mood following a rest period, F(4, 21) = 1.6, p = 0.21.
| B | SE B | β | |
|---|---|---|---|
| Baseline negative affect on rest day (min = 10, max = 41) | 0.100 | 0.098 | 0.198 |
| Age (years) | −0.076 | 0.110 | −0.137 |
| Snack intake following rest (kcal) | −0.005 | 0.002 | −0.421* |
| Lifetime stressor exposure (min = 4, max = 57) | −0.025 | 0.051 | −0.097 |
| R2 | 0.236 | ||
| Adjusted R2 | 0.091 | ||
p = .05
Figure 2.
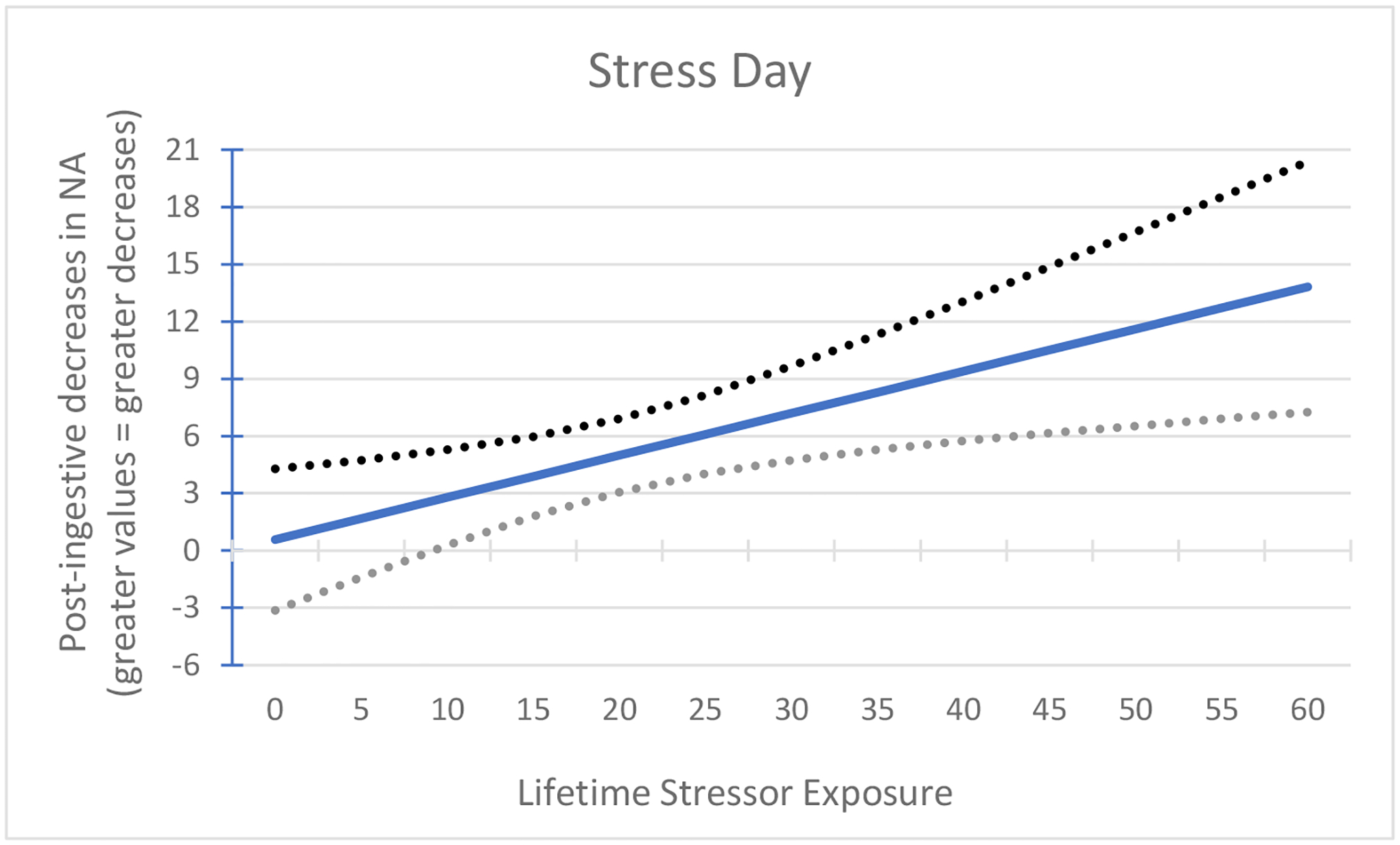
Association between lifetime stressor exposure and post-ingestive decreases in negative affect (NA) following the laboratory-based acute social stressor. On the stress day, greater lifetime stressor exposure significantly predicted greater post-ingestive reductions in negative mood, F(4, 21) = 4.03, p = 0.01.
Figure 3.
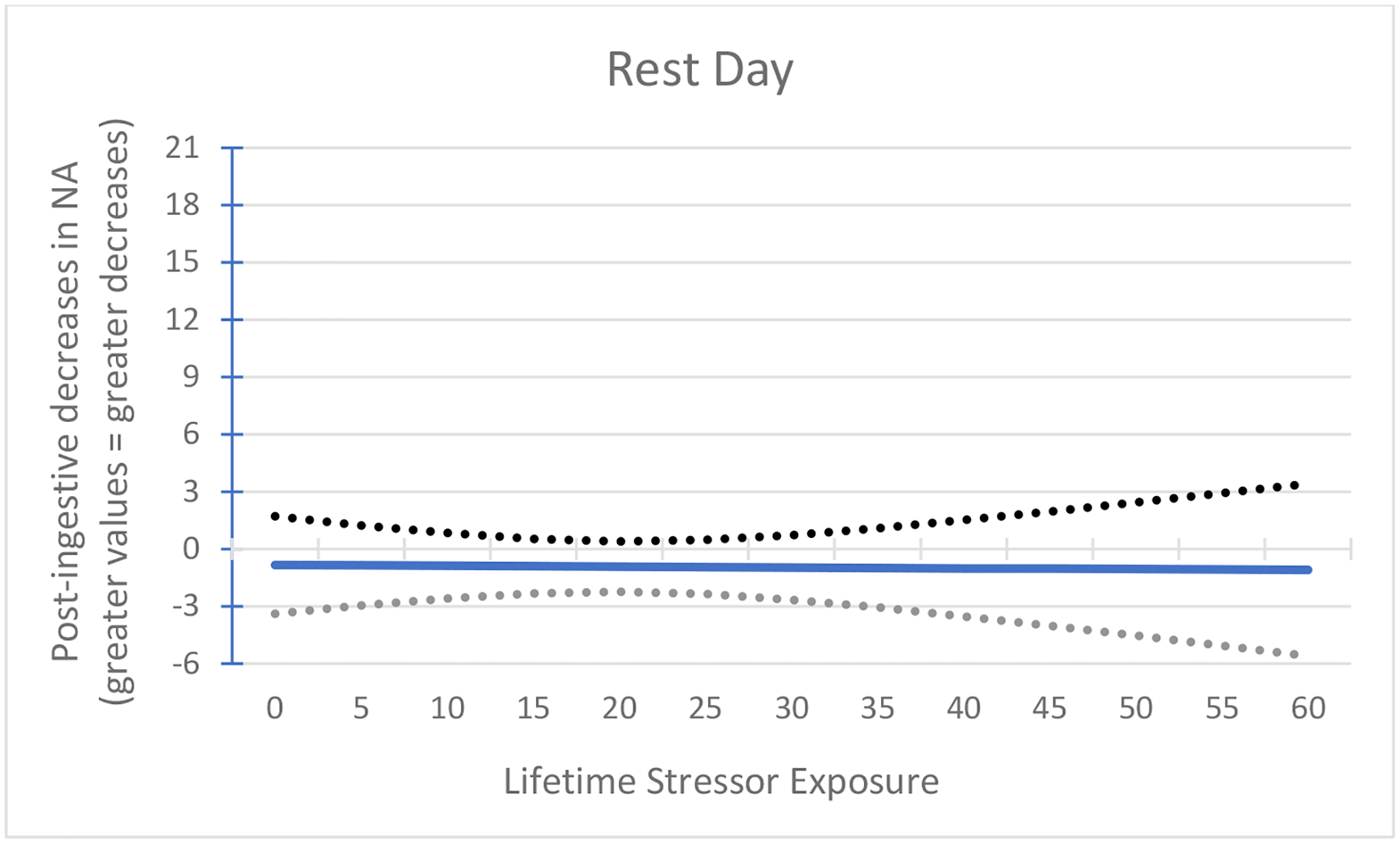
Association between lifetime stressor exposure and post-ingestive decreases in negative affect (NA) following rest. On the rest day, lifetime stressor exposure did not predict post-ingestive reductions in negative mood, F(4, 21) = 1.6, p = 0.21.
The lack of an association between lifetime stressor exposure and negative reinforcement (i.e., decrease in negative affect) in response to snacking on the rest day may have been due to a floor effect of negative affect ratings during the rest period. To partially address this issue, we performed a post-hoc analysis to test whether lifetime stressor exposure was associated with the difference in negative reinforcement between stress and rest days, controlling for age. Greater lifetime stressor exposure, Beta = 0.606, p = 0.002, was associated with greater differences in post-ingestive decreases in negative affect between stress and rest days, F(2,25) = 6.192, p = 0.007, R2 = 0.35, adjusted R2 = 0.293. Although a floor effect may have contributed to the lack of significant effects on the rest day, these findings provide further support that the association between lifetime stressor exposure and post-ingestive reductions in negative affect specific to snacking following a stressor rather than snacking in general.
4. Discussion
Although sizable variability exists with regard to eating behaviors under stress, the mechanisms underlying this variability have yet to be elucidated (Adam & Epel, 2007; Epel et al., 2012; Hill et al., 2021). Existing theories of stress and reward posit that greater negative reinforcement from eating under stress strengthens the association between stress and eating and increases the likelihood of stress-eating in the future (Adam & Epel, 2007; Epel et al., 2012; Hawkins & Clement, 1984; Macht, 2008; Skinner, 1963). Because chronic stress is associated with greater responsivity to cues associated with food reward as well as obesity (Adam & Epel, 2007; Dallman et al., 2003; Epel et al., 2012; Sinha, 2018; Tryon, Carter, et al., 2013), we hypothesized that greater lifetime stressor exposure would predict greater negative reinforcement following a laboratory-based stressor, but not following a rest period. The present data support this hypothesis. Specifically, we found that greater lifetime stressor exposure was associated with greater reductions in negative affect following snacking under stress, but not following snacking at rest. The inconsistency in the literature regarding the amount of food consumed following stress as well as the ability of comfort food to dampen stress and negative emotions may be driven by failure to assess individual differences in lifetime stressors.
Somewhat in contrast to the findings of Bongers and colleagues (2013) that eating reduces negative affect irrespective of the presence of a stressor or negative mood, the present data suggest that the association between lifetime stressor exposure and post-ingestive reductions in negative affect is specific to snacking following a stressor rather than snacking in general. Therefore, one explanation for the association between chronic stress and obesity may be greater stress-induced rewarding effects of eating, as opposed to greater rewarding effects of eating in general.
Whereas cautious interpretation is warranted given the small sample size, these results indicate that the theory-driven emotion regulation cycle (Figure 5) may be enhanced for women with greater lifetime stressor exposure. This model states that greater negative reinforcement following stress-eating (Box C) strengthens the learned association between stress-induced negative affect (Box A) and eating (Box B), enhancing the positive feedback loop to increase the likelihood of future stress-related eating via reinforcement learning. Specifically, greater lifetime stressor exposure was associated with greater negative reinforcement following stress-related eating (Box C). Evidence supporting a “turned up” reward-based eating cycle in women with greater life stress has also been reported in a prior study finding that women with higher perceived stress over the past three months reported greater negative affect (Box A) and greater post-ingestive decreases in negative affect (Box C) following a laboratory-based stressor (Klatzkin et al., 2018). In contradiction to the model (Figure 5), increased snack food intake following stress (Box B) did not increase negative reinforcement (Box C) for the participants in the present study (Table 3). However, it may not be the amount of food intake post-stress, but instead the heightened motivation to eat, reward sensitization, or reward learning, particularly for women with greater lifetime stressors, that leads to greater negative reinforcement, and ultimately, greater rates of obesity (Chao et al., 2015; Dallman et al., 2003; Epel et al., 2012). Further studies are needed to investigate the relative impact of each factor of the emotion regulation model on negative reinforcement (Box C).
Figure 5.
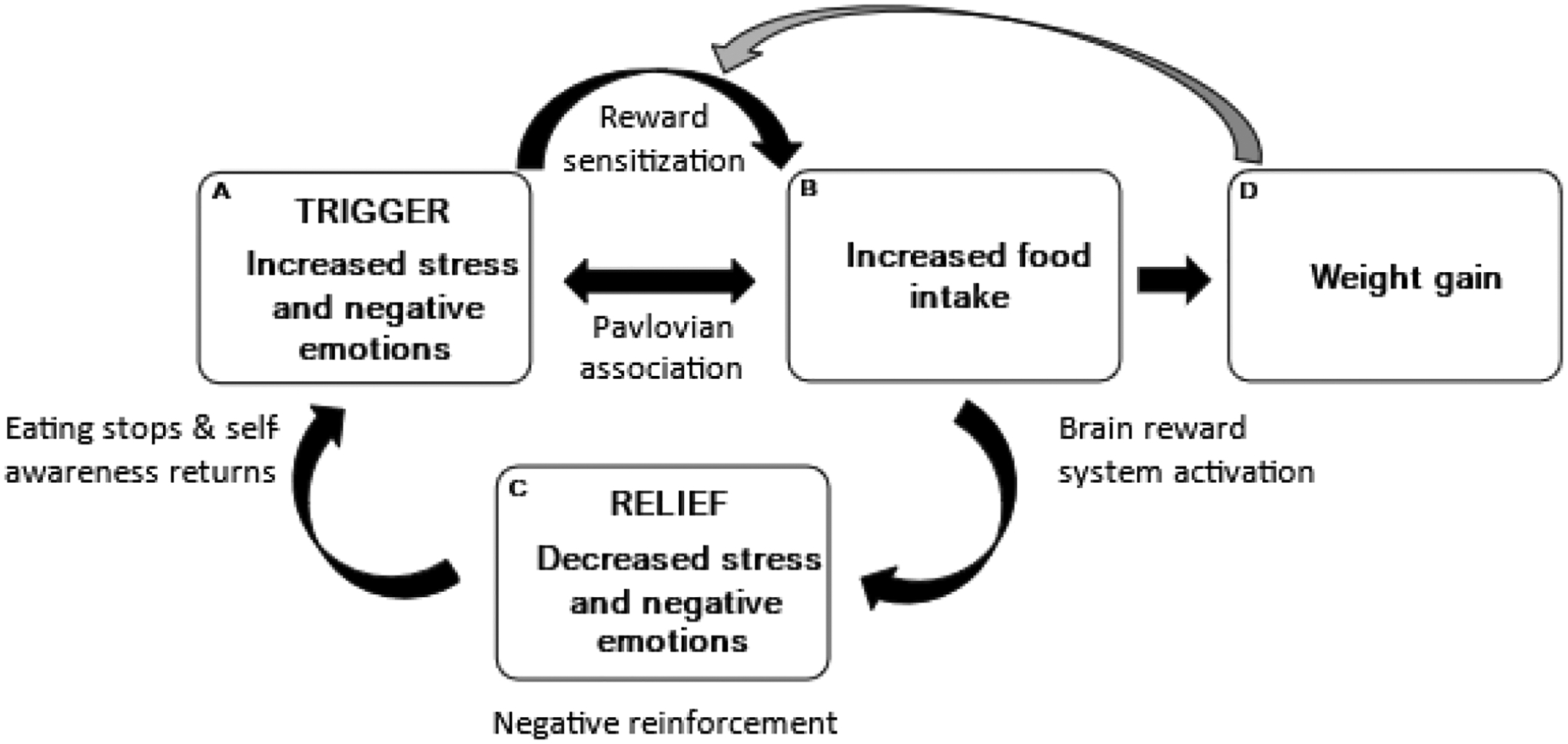
Results of the present study indicate that greater lifetime stressor exposure may enhance Box C of the emotion regulation model. The model depicts a feed-forward cycle in which greater stress and negative emotions (i.e., trigger; box A) sensitize the brain reward system and lead to more food intake (box B) and weight gain (box D). Comfort food intake (box B) causes further activation of the brain reward system and leads to reduced stress and negative emotions (i.e., relief; box C). However, this short-term negative reinforcement is not sustained, as stress and negative emotions (box A) return upon the cessation of eating. Over time, stressors and negative emotions (box A) are more likely to trigger food intake because of positive feedback from factors such as conditioning, brain reward processes, enhanced emotion regulation motives, and weight gain. Reproduced from Klatzkin et al. (2021).
4.1. Strengths and limitations
This study has several strengths. First, we assessed and controlled for snack food intake following both rest and stress. Obtaining significant results with the inclusion of this covariate in our linear regression analyses revealed that greater lifetime stressors predicted greater negative reinforcement in our sample regardless of how much food was eaten. Therefore, an enhanced stress-eating cycle for women with greater lifetime stressors may not be due to eating more comfort food under stress, but to greater rewarding effects of stress-related eating. In other words, the quantity of comfort food intake may not be as meaningful in strengthening the stress-eating cycle for women with greater lifetime stressors as much as the amount of negative reinforcement (i.e., emotional relief) achieved. Greater negative reinforcement from stress-related eating may drive the association between chronic stress and obesity by promoting a stronger cycle of stress-eating and reward (Figure 5) regardless of the quantity of comfort food consumed. A second strength of our study is that we examined lifetime stressor exposure via a well-validated instrument for measuring all the acute and chronic stressors that individuals have experienced over the life course (i.e., the STRAIN). Third, we used a validated, laboratory-based acute social stress task (i.e., the TSST) and confirmed stress induction via multiple physiological and self-reported manipulation checks. Finally, we used a valid measure of food intake (i.e., the bogus taste test; Robinson et al., 2017) using a within-subjects design in a sample of women from the Memphis community.
In terms of limitations, the present results should be interpreted with caution given the small sample size. Additional studies are needed to examine the generalizability of these results across the weight and gender spectrum, as well as to determine if a floor effect in stress-induced negative affect explains the association between greater lifetime stressor exposure and reductions in post-ingestive negative affect on the stress day, as well as the lack of such an association on the non-stress day. Moreover, we did not assess naturalistic eating in response to a stressor where the dynamics would be quite different from a laboratory setting (Cummings et al., 2022), and although the STRAIN is not sensitive to self-report biases associated with social desirability and personality (e.g., neuroticism) (Slavich & Shields, 2018), the influence of such effects on participants’ reporting of lifetime stressors cannot be ruled out. Finally, this study was not able to test whether a stronger reduction in negative affect after snack food intake in women with greater lifetime stressors leads to future increases in stress-induced comfort food intake. Ultimately, we need longitudinal studies that assess the emotion regulation model (Figure 5) more directly and holistically to further support the preliminary evidence that the stress-eating cycle is strengthened in women with greater lifetime stressor exposure.
4.2. Conclusions
In conclusion, the present data showing that greater lifetime stressor exposure predicts greater rewarding effects of stress-related eating may help to explain existing literature linking chronic stress, stress-eating, and obesity (Goens et al., 2023). These findings can thus help inform theory and research on stress, eating, and health. Should additional studies with larger samples replicate this association, these findings may inform obesity-related clinical treatments that target behaviors and cognitions related to reward learning.
Highlights.
Acute stressors shift preferences toward comfort foods, yet stress-eating varies.
Variation in stress-eating may be explained by differences in lifetime stressor exposure.
Lifetime stressors predicted greater reductions in negative affect after stress-eating.
Lifetime stressors did not predict changes in negative affect after snacking at rest.
Greater reward from stress-eating may explain links between chronic stress and obesity.
Funding:
G.M.S. was supported by grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. These organizations had no role in designing or planning this study; in collecting, analyzing, or interpreting the data; in writing the article; or in deciding to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest with respect to this work.
References
- Adam TC, & Epel ES (2007). Stress, eating and the reward system. Physiology & Behavior, 91(4), 449–458. 10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Bekhbat M, & Neigh GN (2017). Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain, Behavior, and Immunity. 10.1016/j.bbi.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano MM (2016). Palatable Eating Motives Scale in a college population: Distribution of scores and scores associated with greater BMI and binge-eating. Eating Behaviors, 21, 95–98. 10.1016/j.eatbeh.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Wenger LE, Burgess EE, Tatum MM, Sylvester MD, Morgan PR, & Morse KE (2017). Eating tasty foods to cope, enhance reward, socialize or conform: What other psychological characteristics describe each of these motives? Journal of Health Psychology, 22(3), 280–289. 10.1177/1359105315600240 [DOI] [PubMed] [Google Scholar]
- Bongers P, Jansen A, Havermans R, Roefs A, & Nederkoorn C (2013). Happy eating. The underestimated role of overeating in a positive mood. Appetite, 67, 74–80. 10.1016/j.appet.2013.03.017 [DOI] [PubMed] [Google Scholar]
- Cazassa MJ, da S. Oliveira M, Spahr CM, Shields GS, & Slavich GM (2020). The Stress and Adversity Inventory for Adults (Adult STRAIN) in Brazilian Portuguese: Initial Validation and Links With Executive Function, Sleep, and Mental and Physical Health. Frontiers in Psychology, 10. https://www.frontiersin.org/articles/10.3389/fpsyg.2019.03083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Grilo CM, White MA, & Sinha R (2015). Food cravings mediate the relationship between chronic stress and body mass index. Journal of Health Psychology, 20(6), 721–729. 10.1177/1359105315573448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AM, Fogelman N, Hart R, Grilo CM, & Sinha R (2020). A Laboratory-Based Study of the Priming Effects of Food Cues and Stress on Hunger and Food Intake in Individuals with Obesity. Obesity (Silver Spring, Md.), 28(11), 2090–2097. 10.1002/oby.22952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JR, Schiestl ET, Tomiyama AJ, Mamtora T, & Gearhardt AN (2022). Highly processed food intake and immediate and future emotions in everyday life. Appetite, 169, 105868. 10.1016/j.appet.2021.105868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, & Manalo S (2003). Chronic stress and obesity: A new view of “comfort food.” Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11696–11701. 10.1073/pnas.1934666100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, & Penninx BWJH (2010). Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depression and Anxiety, 27(11), 1057–1065. 10.1002/da.20738 [DOI] [PubMed] [Google Scholar]
- Epel ES, Tomiyama AJ, & Dallman MF (2012). Stress and reward: Neural networks, eating, and obesity. In Food and addiction: A comprehensive handbook (pp. 266–272). Oxford University Press. 10.1093/med:psych/9780199738168.003.0040 [DOI] [Google Scholar]
- Finch LE, Cummings JR, & Tomiyama AJ (2019). Cookie or clementine? Psychophysiological stress reactivity and recovery after eating healthy and unhealthy comfort foods. Psychoneuroendocrinology, 107, 26–36. 10.1016/j.psyneuen.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch LE, & Tomiyama AJ (2014). Stress-Induced Eating Dampens Physiological and Behavioral Stress Responses. 10.1016/B978-0-12-407869-7.00018-0 [DOI] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, & Dallman MF (2009). Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology, 150(5), 2325–2333. 10.1210/en.2008-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franja S, Wahl DR, Elliston KG, & Ferguson SG (2021). Comfort eating: An observational study of affect in the hours immediately before, and after, snacking. British Journal of Health Psychology, 26(3), 825–838. 10.1111/bjhp.12505 [DOI] [PubMed] [Google Scholar]
- Goens D, Virzi NE, Jung SE, Rutledge TR, & Zarrinpar A (2023). Obesity, Chronic Stress, and Stress Reduction. Gastroenterology Clinics of North America, 52(2), 347–362. 10.1016/j.gtc.2023.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK (2011a). Hunger and binge eating: A meta-analysis of studies using ecological momentary assessment. International Journal of Eating Disorders, 44(7), 573–578. 10.1002/eat.20868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK (2011b). Revisiting the affect regulation model of binge eating: A meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 137(4), 660–681. 10.1037/a0023660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RC, & Clement PF (1984). Binge eating: Measurement problems and a conceptual model. In The binge purge syndrome: Diagnosis, treatment, and research. (pp. 229–251). Springer. [Google Scholar]
- Hill D, Conner M, Clancy F, Moss R, Wilding S, Bristow M, & O’Connor DB (2021). Stress and eating behaviours in healthy adults: A systematic review and meta-analysis. Health Psychology Review, 0(0), 1–25. 10.1080/17437199.2021.1923406 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/119004 [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Baldassaro A, & Rashid S (2018). Physiological responses to acute stress and the drive to eat: The impact of perceived life stress. Appetite, 133, 393–399. 10.1016/j.appet.2018.11.019 [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Nadel T, Wilkinson LL, Gaffney K, Files H, Gray ZJ, & Slavich GM (2023). Lifetime stressor exposure, eating expectancy, and acute social stress-related eating behavior: A pre-registered study of the emotional eating cycle. Appetite, 185, 106494. 10.1016/j.appet.2023.106494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin RR, Nolan LJ, & Kissileff HR (2022). Self-reported emotional eaters consume more food under stress if they experience heightened stress reactivity and emotional relief from stress upon eating. Physiology & Behavior, 243, 113638. 10.1016/j.physbeh.2021.113638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen H, Männistö S, Sarlio-Lähteenkorva S, Silventoinen K, & Haukkala A (2010). Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite, 54(3), 473–479. 10.1016/j.appet.2010.01.014 [DOI] [PubMed] [Google Scholar]
- La Fleur SE, Houshyar H, Roy M, & Dallman MF (2005). Choice of Lard, But Not Total Lard Calories, Damps Adrenocorticotropin Responses to Restraint. Endocrinology, 146(5), 2193–2199. 10.1210/en.2004-1603 [DOI] [PubMed] [Google Scholar]
- Lam JCW, Shields GS, Trainor BC, Slavich GM, & Yonelinas AP (2019). Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress and Health, 35(1), 15–26. 10.1002/smi.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, & Giel KE (2015). Emotion regulation model in binge eating disorder and obesity—A systematic review. Neuroscience and Biobehavioral Reviews, 49, 125–134. 10.1016/j.neubiorev.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Macht M (2008). How emotions affect eating: A five-way model. Appetite, 50(1), 1–11. 10.1016/j.appet.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Macht M, Haupt C, & Ellgring H (2005). The perceived function of eating is changed during examination stress: A field study. Eating Behaviors, 6(2), 109–112. 10.1016/j.eatbeh.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Macht M, & Mueller J (2007). Immediate effects of chocolate on experimentally induced mood states. Appetite, 49(3), 667–674. 10.1016/j.appet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- McKay N, Przybysz J, Cavanaugh A, Horvatits E, Giorgianni N, & Czajka K (2021). The effect of unhealthy food and liking on stress reactivity. Physiology & Behavior, 229, 113216. 10.1016/j.physbeh.2020.113216 [DOI] [PubMed] [Google Scholar]
- McMullin SD, Shields GS, Slavich GM, & Buchanan TW (2021). Cumulative lifetime stress exposure predicts greater impulsivity and addictive behaviors. Journal of Health Psychology, 26(14), 2921–2936. 10.1177/1359105320937055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A (2013). Impulsivity and overeating: A closer look at the subscales of the Barratt Impulsiveness Scale. Frontiers in Psychology, 0. 10.3389/fpsyg.2013.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhail ME (2021). Affect Dysregulation in Context: Implications and Future Directions of Experience Sampling Research on Affect Regulation Models of Loss of Control Eating. Frontiers in Psychiatry, 12, 747854. 10.3389/fpsyt.2021.747854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Sichko S, Bui TQ, Libowitz MR, Shields GS, & Slavich GM (2023). Intergenerational transmission of lifetime stressor exposure in adolescent girls at differential maternal risk for depression. Journal of Clinical Psychology, 79(2), 431–448. 10.1002/jclp.23417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera Alvarez HA, Provencio-Vasquez E, Slavich GM, Laurent JGC, Browning M, McKee-Lopez G, Robbins L, & Spengler JD (2019). Stress and Health in Nursing Students: The Nurse Engagement and Wellness Study. Nursing Research, 68(6), 453–463. 10.1097/NNR.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, & Dallman MF (2004). Chronic Stress Promotes Palatable Feeding, Which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology, 145(8), 3754–3762. 10.1210/en.2004-0305 [DOI] [PubMed] [Google Scholar]
- Robinson E, Haynes A, Hardman CA, Kemps E, Higgs S, & Jones A (2017). The bogus taste test: Validity as a measure of laboratory food intake. Appetite, 116, 223–231. 10.1016/j.appet.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, & Laessle RG (2012). Stress-induced laboratory eating behavior in obese women with binge eating disorder. Appetite, 58(2), 457–461. 10.1016/j.appet.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Sinha R (2018). Role of addiction and stress neurobiology on food intake and obesity. Biological Psychology, 131, 5–13. 10.1016/j.biopsycho.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF (1963). Operant behavior. American Psychologist, 18(8), 503–515. 10.1037/h0045185 [DOI] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing Lifetime Stress Exposure Using the Stress and Adversity Inventory for Adults (Adult STRAIN): An Overview and Initial Validation. Psychosomatic Medicine, 80(1), 17–27. 10.1097/PSY.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, & Jacobs G (1983). Manual for the State-Trait Anxiety Inventory (Form Y1 – Y2). In Palo Alto, CA: Consulting Psychologists Press; Vol. IV. [Google Scholar]
- Telch CF, & Agras WS (1996). Do emotional states influence binge eating in the obese? The International Journal of Eating Disorders, 20(3), 271–279. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Finch LE, & Cummings JR (2015). Did That Brownie Do Its Job? Stress, Eating, and the Biobehavioral Effects of Comfort Food. In Emerging Trends in the Social and Behavioral Sciences (pp. 1–15). American Cancer Society. 10.1002/9781118900772.etrds0324 [DOI] [Google Scholar]
- Tryon MS, Carter CS, DeCant R, & Laugero KD (2013). Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiology & Behavior, 120, 233–242. 10.1016/j.physbeh.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Tryon MS, DeCant R, & Laugero KD (2013). Having your cake and eating it too: A habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiology & Behavior, 114–115, 32–37. 10.1016/j.physbeh.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Udo T, Grilo CM, & McKee SA (2014). Gender differences in the impact of stressful life events on changes in body mass index. Preventive Medicine, 69, 49–53. 10.1016/j.ypmed.2014.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk ES, Savas M, & van Rossum EFC (2018). Stress and Obesity: Are There More Susceptible Individuals? Current Obesity Reports, 7(2), 193–203. 10.1007/s13679-018-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wei N-L, Quan Z-F, Zhao T, Yu X-D, Xie Q, Zeng J, Ma F-K, Wang F, Tang Q-S, Wu H, & Zhu J-H (2019). Chronic stress increases susceptibility to food addiction by increasing the levels of DR2 and MOR in the nucleus accumbens. Neuropsychiatric Disease and Treatment, 15, 1211–1229. 10.2147/NDT.S204818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters S, Jacobs N, Duif M, Lechner L, & Thewissen V (2018). Negative affective stress reactivity: The dampening effect of snacking. Stress and Health, 34(2), 286–295. 10.1002/smi.2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau YHC, & Potenza MN (2013). Stress and eating behaviors. Minerva Endocrinologica, 38(3), 255–267. [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Blundell JE, & Leshem M (2004). Palatability: Response to nutritional need or need-free stimulation of appetite? The British Journal of Nutrition, 92 Suppl 1, S3–14. 10.1079/bjn20041134 [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, & Wolf A (2006). Food selection changes under stress. Physiology & Behavior, 87(4), 789–793. 10.1016/j.physbeh.2006.01.014 [DOI] [PubMed] [Google Scholar]


