Abstract
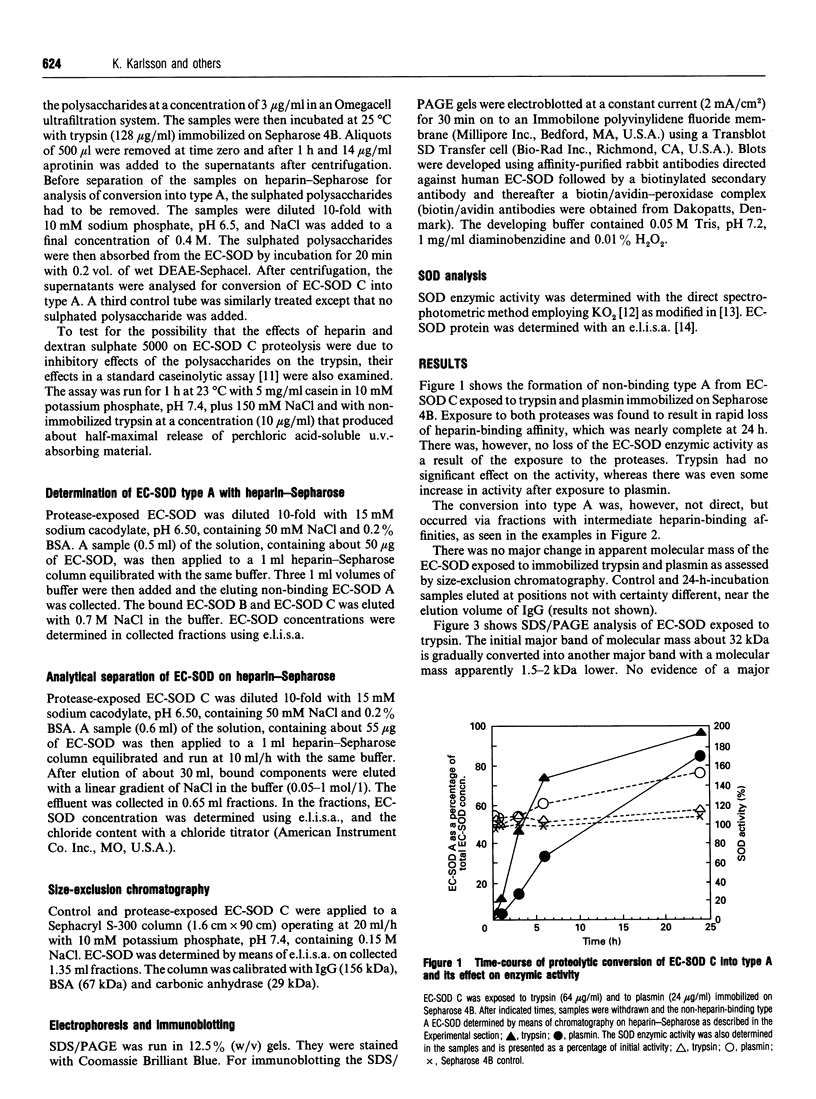
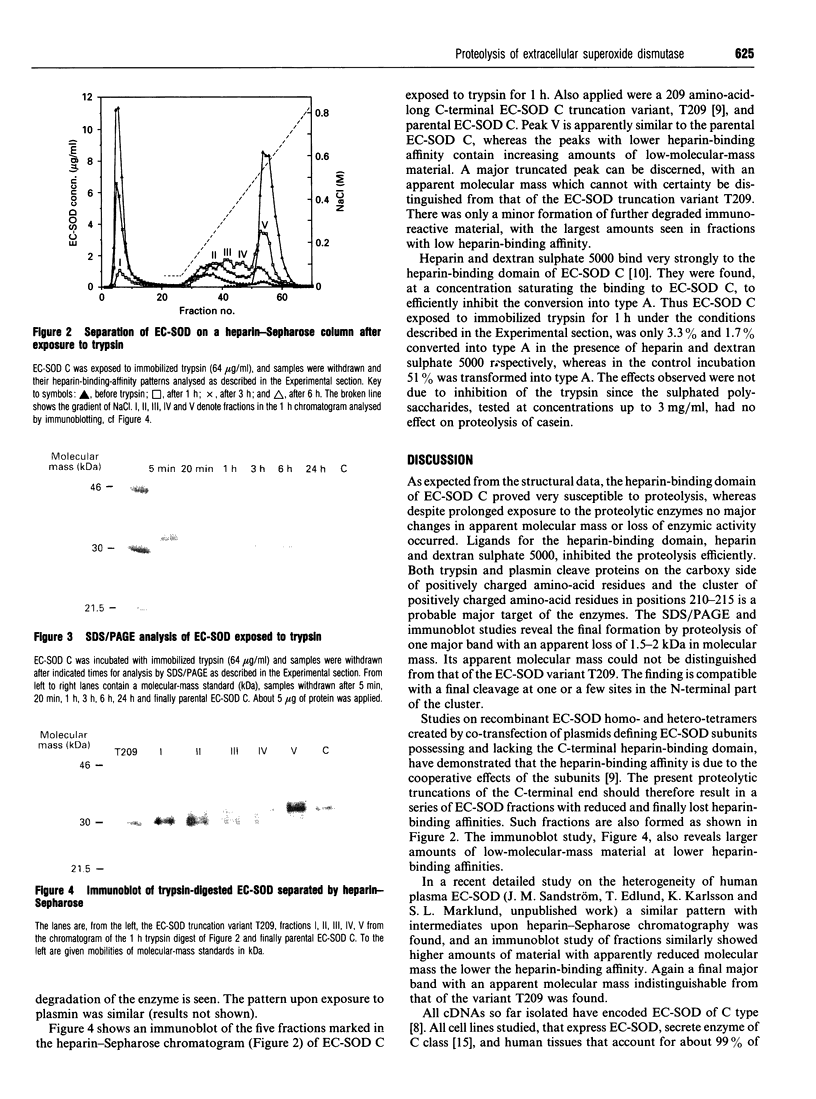
The heparin-binding affinity of the tetrameric extracellular superoxide dismutase (EC-SOD) is a result of the cooperative effect of the heparin-binding domains of the subunits, located in the hydrophilic, strongly positively charged C-terminal ends. EC-SOD C, the high-heparin-affinity type, exposed to immobilized trypsin and plasmin was found to rapidly lose its affinity for heparin, without any loss of enzymic activity or major change in molecular mass as judged by size-exclusion chromatography. Heparin and dextran sulphate 5000 inhibited the proteolysis, suggesting that EC-SOD C sequestered by heparan sulphate proteoglycan in vivo is partially protected against proteolysis. The loss of heparin-affinity occurred with the stepwise formation of intermediates, and the pattern upon chromatography on heparin-Sepharose and subsequent immunoblotting was compatible with the notion that the changes are due to sequential truncations of heparin-binding domains from subunits composing the EC-SOD tetramers. A similar pattern with intermediates and apparent truncations has previously been found with EC-SOD of human plasma. The findings show that the unique design of the heparin-binding domain of EC-SOD allows easy modification of the heparin-affinity by means of limited proteolysis, and suggest that such proteolysis is a major contributor to the heterogeneity in heparin-affinity of EC-SOD in mammalian plasma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Marklund S. L. Interactions between human extracellular superoxide dismutase C and sulfated polysaccharides. J Biol Chem. 1989 May 25;264(15):8537–8541. [PubMed] [Google Scholar]
- Adachi T., Ohta H., Hirano K., Hayashi K., Marklund S. L. Non-enzymic glycation of human extracellular superoxide dismutase. Biochem J. 1991 Oct 1;279(Pt 1):263–267. doi: 10.1042/bj2790263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Cabelli D. E., Fisher C. L., Parge H. E., Viezzoli M. S., Banci L., Hallewell R. A. Faster superoxide dismutase mutants designed by enhancing electrostatic guidance. Nature. 1992 Jul 23;358(6384):347–351. doi: 10.1038/358347a0. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Lindahl U., Marklund S. L. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem J. 1988 Nov 15;256(1):29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Binding of human extracellular-superoxide dismutase C to cultured cell lines and to blood cells. Lab Invest. 1989 May;60(5):659–666. [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988 Oct 1;255(1):223–228. [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Plasma clearance of human extracellular-superoxide dismutase C in rabbits. J Clin Invest. 1988 Sep;82(3):762–766. doi: 10.1172/JCI113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLERTZ S. Formation and properties of the activator of plasminogen and of human and bovine plasmin. Biochem J. 1955 Nov;61(3):424–434. doi: 10.1042/bj0610424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990 Feb 15;266(1):213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Properties of extracellular superoxide dismutase from human lung. Biochem J. 1984 May 15;220(1):269–272. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Sandström J., Carlsson L., Marklund S. L., Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem. 1992 Sep 5;267(25):18205–18209. [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P., Iwanaga S. Differences between plasmic and tryptic digests of human S-sulfo-fibrinogen. Biochim Biophys Acta. 1968 Feb 19;154(2):414–417. doi: 10.1016/0005-2795(68)90114-1. [DOI] [PubMed] [Google Scholar]




