Abstract
Objectives
The reduction in recurrent Clostridium difficile–associated diarrhea (CDAD) with fidaxomicin therapy may reduce hospital readmissions and lead to lower overall CDAD costs. However, studies assessing the cost-effectiveness of fidaxomicin as first-line therapy from the U.S. hospital perspective are lacking. This study evaluated the costs associated with utilizing fidaxomicin or vancomycin as a first-line therapy for CDAD in specific patient populations from a U.S. hospital perspective.
Methods
A decision-analytic model was developed to estimate total costs (hospitalization and drug costs) associated with using fidaxomicin or vancomycin as first-line therapy for a first episode and up to two recurrences of CDAD in five patient populations: general population, elderly, patients receiving concomitant antibiotics, and patients with renal impairment or cancer.
Results
The total cost of CDAD treatment using fidaxomicin first line in the general population was $14,442 per patient versus $14,179 per patient with vancomycin first line. In subgroup analyses, fidaxomicin use resulted in total hospital cost savings of $616 per patient in patients with cancer and $312 in patients with concomitant antibiotic use; vancomycin use was associated with total hospital cost savings of $243 per patient in the elderly and $371 in patients with renal impairment.
Conclusions
Fidaxomicin as first-line CDAD therapy is associated with similar total costs as compounded vancomycin oral solution in the general population. In elderly and renally impaired patients, slight increases in hospital cost were observed with fidaxomicin therapy, and in patients with cancer or concomitant antibiotic use, hospital cost savings were observed.
Keywords: fidaxomicin, vancomycin, Clostridium difficile, costs
Clostridium difficile, a common cause of potentially life-threatening diarrheal disease, is the most prevalent pathogen among all health care–associated infections.1 This microorganism is classified by the Centers for Disease Control and Prevention as an urgent threat to public health.2 Clostridium difficile–associated diarrhea (CDAD) presents a significant burden to the U.S. health care system. CDAD-related hospitalizations nearly doubled between 2001 and 2010 while mortality and hospital length of stay (LOS) have yet to decline.3 Importantly, aggregate hospital costs among inpatients with CDAD exceed $8.2 billion annually.4
Although metronidazole and vancomycin have been the predominant CDAD therapies for decades, these antibiotics are associated with high rates of treatment failure and disease recurrence. Approximately 20–25% of individuals experience recurrent CDAD despite successful treatment of the initial episode.5–8 In those patients who have already experienced one recurrence, the risk of additional recurrences can be as high as 65%.9
The approval of fidaxomicin in 2011 marked the first new antibiotic indicated for CDAD in 25 years. In clinical trials, fidaxomicin was associated with significantly greater sustained clinical response and lower disease recurrence at 25 days follow-up as compared with vancomycin.5, 10, 11 Subsequent analyses of clinical trial data found that fidaxomicin may be more effective than vancomycin at preventing recurrent CDAD in certain high-risk subpopulations including the elderly, patients receiving concomitant antibiotics, and those with renal impairment or cancer.5, 10–14
Recurrent CDAD results in substantial costs to the health care system due to extended rehospitalizations and additional antibiotic courses. Despite this, health systems are reluctant to use fidaxomicin as first-line therapy because of higher drug acquisition costs as compared with metronidazole or vancomycin. The reduction in recurrent CDAD following fidaxomicin therapy likely reduces hospital readmissions, leading to lower overall CDAD costs; however, studies assessing the cost-effectiveness of fidaxomicin as first-line therapy from the hospital perspective are lacking.
The objective of this study was to determine the costs associated with utilizing fidaxomicin versus vancomycin as first-line therapy for CDAD in the general population, as well as in subpopulations at high risk for recurrent CDAD, from a U.S. hospital perspective.
Methods
Model Framework
A decision-analytic model was developed using Microsoft Excel to estimate the health outcomes and hospital-related inpatient costs associated with different CDAD treatment pathways. Figure 1 illustrates the structure of the decision model. In this model, initial CDAD treatment and subsequent treatment for up to two recurrences of CDAD were considered. The model takes the perspective of a U.S. hospital system and only includes direct costs incurred in the hospital.
Figure 1.
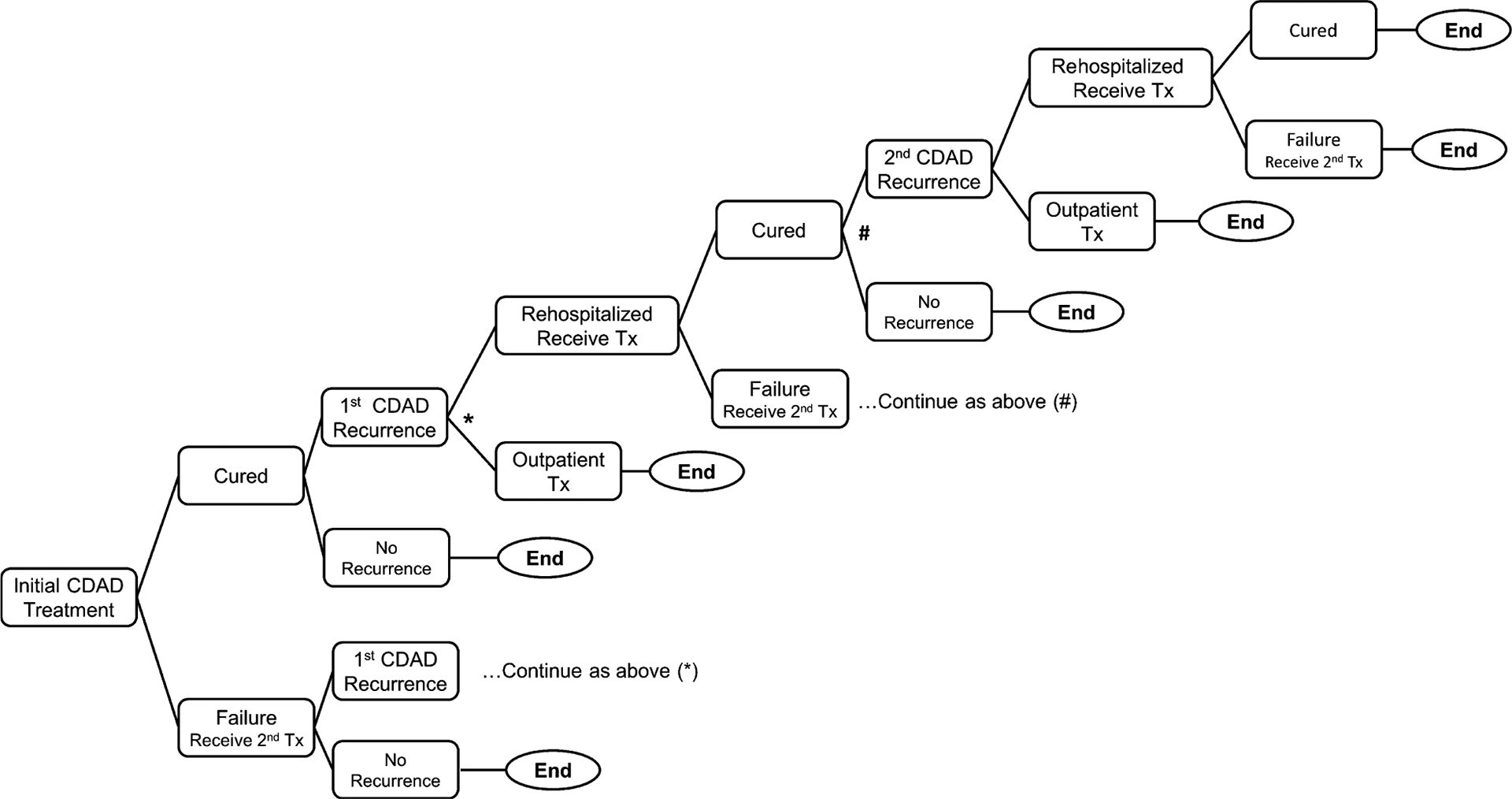
Model structure: decision tree used in the treatment pathway for Clostridium difficile–associated diarrhea (CDAD). Tx = treatment.
For this analysis, two treatment pathways were compared: the fidaxomicin pathway and the vancomycin pathway. In the fidaxomicin pathway, fidaxomicin (200 mg orally twice/day for 10 days) was administered as first-line therapy for the initial CDAD episode. In the vancomycin pathway, vancomycin (125 mg compounded oral solution 4 times/day for 10 days) was administered as first-line therapy for the initial CDAD episode. Both pathways used identical treatment regimens for failure of first-line CDAD therapy, first CDAD recurrence, or second CDAD recurrence treatment (vancomycin 250 mg compounded oral solution 4 times/day) and identical initial treatment regimens for each recurrence (vancomycin 125 mg compounded oral solution 4 times/day). The treatment pathways were developed based on expert opinion, given that the current Society for Healthcare Epidemiology of America/Infectious Diseases Society of America clinical practice guidelines for C. difficile infections stratify therapy recommendations by disease severity.15
The model considered five different patient groups: general population, elderly (65 yrs or older), patients with cancer (active solid tumor or hematologic malignancy), patients with concomitant antibiotic use (one or more doses of oral or intravenous antibiotics during treatment for CDAD or up to 25 days after the end of therapy), and patients with renal impairment (stage 3 or 4 chronic kidney disease).
Model Inputs and Assumptions
Table 1 presents all clinical inputs used in the model for the general population. The clinical cure and first recurrence rates were based on pooled findings from two head-to-head phase III clinical trials comparing fidaxomicin and vancomycin for CDAD first episode or first recurrence.5, 11, 16 Efficacy inputs for the subpopulations were obtained from published subanalyses describing specific patient populations within these trials (Table 2).5, 11–14
Table 1.
General Population Model Inputs
| Model parameter | Base value | Low value | High value |
|---|---|---|---|
| Fidaxomicin | |||
| Initial treatment failure rate5,11,16 | 12.06% | 9.05% | 15.08% |
| Rate of first CDAD recurrence5,11,16 | 14.14% | 10.61% | 17.68% |
| Rate of second CDAD recurrence17 | 20.30% | 15.23% | 25.38% |
| Daily drug acquisition cost | $235 | ||
| Vancomycin | |||
| Initial treatment failure rate5,11,16 | 13.78% | 10.34% | 17.23% |
| Rate of first CDAD recurrence5,11,16 | 26.02% | 19.52% | 32.53% |
| Rate of second CDAD recurrence17 | 32.30% | 24.23% | 40.38% |
| Daily drug acquisition cost, 125 mg | $20 | – | – |
| Daily drug acquisition cost, 250 mg | $40 | – | – |
| Nondrug specific | |||
| Cost of additional hospital days in cases with treatment failure4 | $5573 | $4179 | $6966 |
| Attributable hospitalization cost of initial CDAD4 | $11,145 | $8359 | $13,931 |
| Attributable hospitalization cost of recurrent CDAD18 | $12,627 | $9703 | $15,838 |
| No. of days of drug therapy received in hospital19,20 | 6.00 | 4.50 | 7.50 |
| % of patients with recurrent CDAD who are rehospitalized21 | 51.60 | 39.40 | 63.80 |
CDAD = Clostridium difficile–associated diarrhea.
Table 2.
Clinical Inputs for All Patient Subpopulations in Model
| Input | Elderly5,11, % | Cancer12, % | Concomitant antibiotics13, % | Renal impairment14, % |
|---|---|---|---|---|
| Initial treatment failure ratea,b | ||||
| Fidaxomicin | 15.18 | 14.94 | 10.00 | 22.20 |
| Vancomycin | 15.69 | 26.04 | 20.59 | 20.90 |
| Rate of first CDAD recurrence | ||||
| Fidaxomicin | 16.07 | 13.51 | 16.85 | 19.51 |
| Vancomycin | 29.29 | 29.58 | 29.17 | 32.56 |
| Rate of second CDAD recurrencec,17 | ||||
| Fidaxomicin | 20.30 | 20.30 | 20.30 | 20.30 |
| Vancomycin | 32.30 | 32.30 | 32.30 | 32.30 |
CDAD = Clostridium difficile–associated diarrhea.
Efficacy rates for vancomycin 125 mg and vancomycin 250 mg are assumed to be the same.
Initial treatment failure rates for subpopulations from published subset phase III data; fidaxomicin was shown to be statistically noninferior to vancomycin for initial cure in the overall population and superior in sustained clinical response (clinical response without recurrence or death) at 25 days.
Rate of second CDAD recurrence assumed to be the same for all subpopulations.
It was assumed that all patients who failed the initial treatment were prescribed vancomycin 250 mg orally 4 times/day and were cured. The treatment failure rate was assumed to be the same as the initial failure rate from the clinical trials for all recurrences. The rate of second recurrence was derived from patients who received fidaxomicin or vancomycin for a first recurrence in the general population within the clinical trials and subsequently had a recurrence.17 Because these data were not available in the subpopulations, the same rates were applied to all groups. Using data from one study,21 it was estimated that approximately half (51.6%) of patients who experienced recurrent CDAD were rehospitalized; patients not readmitted for their recurrent episode were assumed to be treated outside of the hospital and did not incur additional hospital costs.
Table 1 presents the daily drug costs. The cost of fidaxomicin was based on a standard 29% discount on the wholesale acquisition cost given in U.S. hospitals at the time of the analysis. It was assumed that vancomycin would be compounded from the intravenous solution in the hospital, and the cost was adjusted accordingly based on expert opinion and prior economic models.22–24
Initial hospitalization costs for the general population and model subpopulations were obtained from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (Table 1).4 The cost of recurrence was taken from a study that included costs from 2003–2009.18 Hospitalization costs were adjusted using the U.S. Consumer Price Index inflation calculator to reflect 2014 U.S. dollar values. Published cost data were not available for the subpopulations; therefore, hospitalization costs were assumed to be the same as for the general population.
The LOS for initial or recurrent CDAD was ~6 days based on published literature.19, 20 Patients failing initial treatment were estimated to be hospitalized for an additional 3 days.19, 22 Because LOS was estimated to be half of the initial episode, the cost of additional hospital days in cases of treatment failure for any CDAD episode was estimated as half the cost of an initial CDAD hospitalization. Because the indicated duration of CDAD therapy exceeds LOS (10 days for fidaxomicin and 10–14 days for vancomycin), patients were assumed to complete the remaining treatment outside of the hospital following discharge. Any treatment cost incurred in the outpatient setting was not included in this model.
The primary outcome assessed by the model was inpatient costs that represent the sum of hospitalization costs and drug costs. Costs were also analyzed by CDAD episode and segmented by cost components. Costs were calculated based on the probability of each patient progressing to the next step in the pathway. For example, CDAD recurrence costs accounted for the probability of recurrence given the initial treatment, as well as the probability of rehospitalization for the recurrent episode.
Sensitivity Analysis
A univariate sensitivity analysis was performed on cost and efficacy parameters for the general population across a predefined range of values to assess the impact of model uncertainties and the robustness of the analysis (Table 1). Low and high values for each parameter were set using the 95% confidence interval (CI) provided in the peer-reviewed study, if available.4, 18, 22 When a 95% CI was unavailable, the low and high values were set as ±25% of the base value provided in the corresponding study.5, 11, 16, 19, 20 The results are presented in the form of a tornado diagram, with variables stacked in decreasing order of impact on incremental total costs (cost of vancomycin pathway minus cost of fidaxomicin pathway).
In addition, a sensitivity analysis was conducted to determine the impact of fidaxomicin use for recurrent episodes. Specifically, total costs for initial episodes, first recurrences, and second recurrences were calculated for each pathway when fidaxomicin 200 mg orally twice/day was used for the first recurrence only and for both the first and second recurrence.
Results
In the general population, the total cost of CDAD treatment was $14,442 per patient for fidaxomicin first-line therapy versus $14,179 for vancomycin (Figure 2). The cost of the initial hospitalization, which was similar between treatment pathways, comprised most of the overall CDAD cost (range 82–84% of total costs) (Figure 2). Despite the substantially higher per patient drug costs with fidaxomicin in the initial CDAD episode, lower recurrence rates with fidaxomicin resulted in fewer rehospitalizations, offsetting most of the drug costs.
Figure 2.
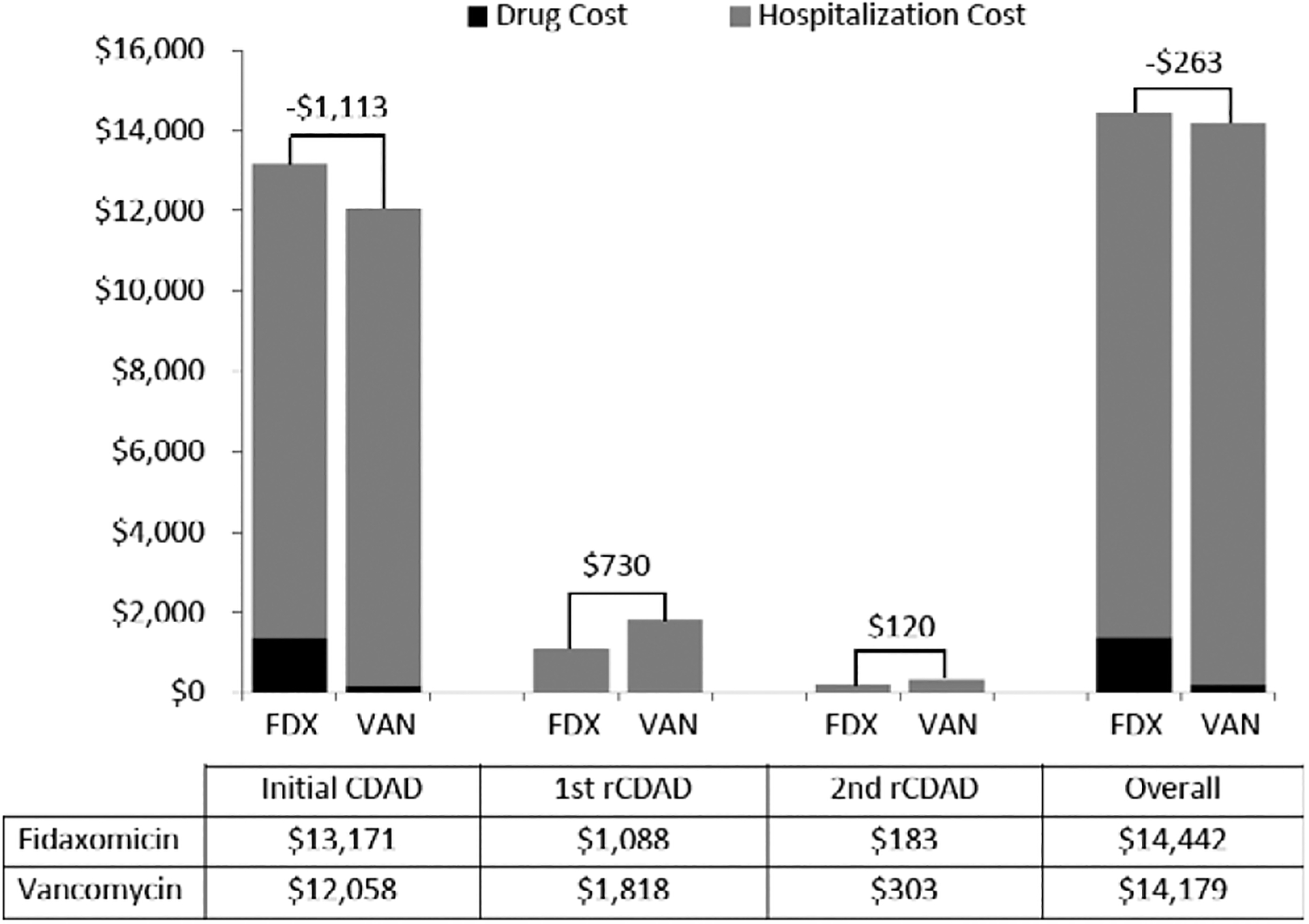
Per patient cost of Clostridium difficile–associated diarrhea (CDAD) treatment in the general population for two different treatment pathways (fidaxomicin first line vs vancomycin first line), by CDAD episode. FDX = fidaxomicin; rCDAD = recurrent C. difficile–associated diarrhea; VAN = vancomycin.
Subgroup Analysis
In addition to the overall population, the model determined costs associated with CDAD therapy among the elderly, cancer patients, patients with concomitant antibiotic use, and renally impaired patients treated in the hospital setting. Figure 3 provides the estimated costs for each of these patient subgroups.
Figure 3.
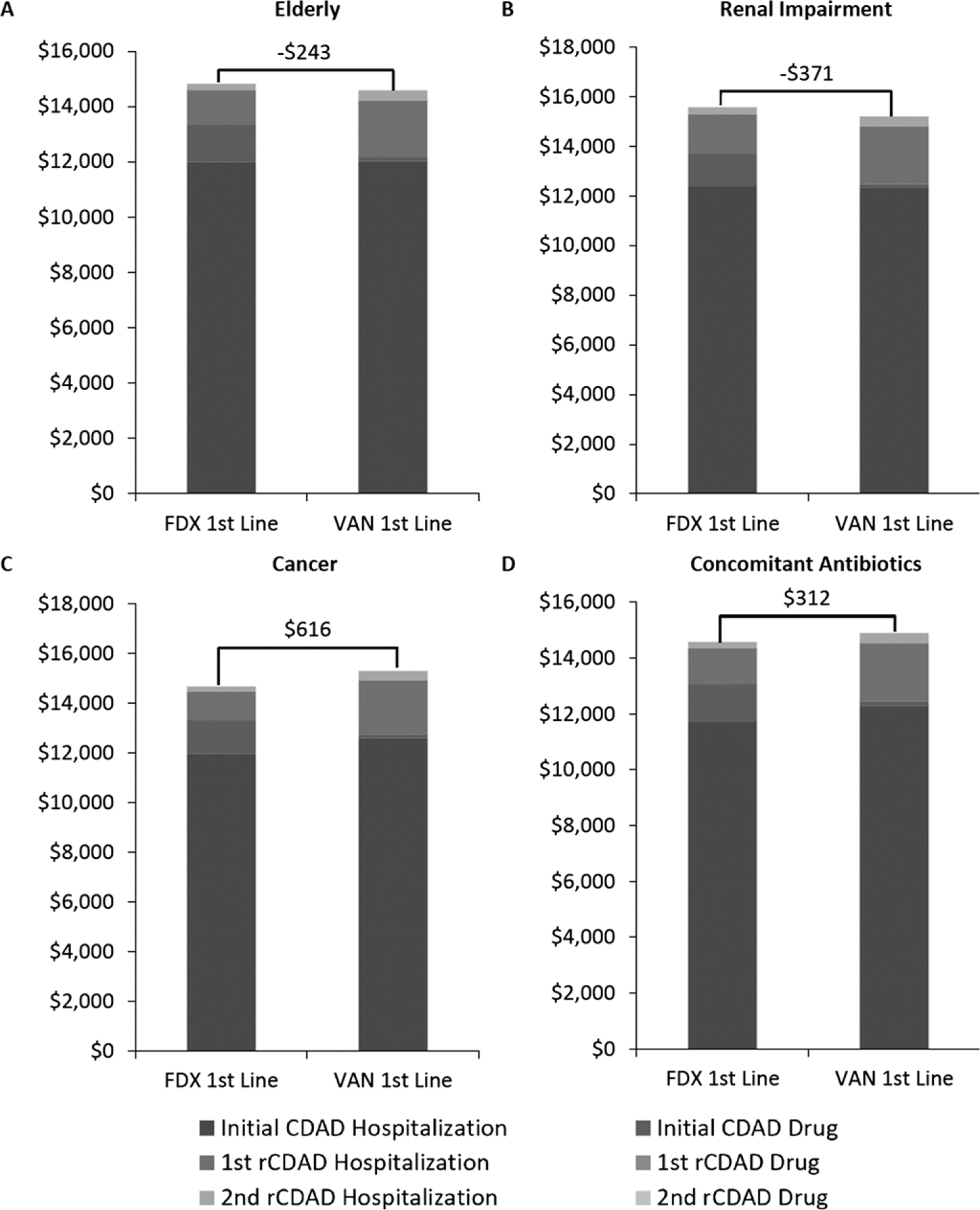
(A–D) Overall per patient cost of Clostridium difficile–associated diarrhea (CDAD) treatment in different patient populations. FDX = fidaxomicin; rCDAD = recurrent C. difficile–associated diarrhea; VAN = vancomycin.
In the elderly and renal impairment patient populations, vancomycin use resulted in total hospital cost savings of $243 and $371 per patient, respectively (Figure 3A, B). In contrast, fidaxomicin use resulted in total hospital cost savings of $616 and $312 per patient for cancer and concomitant antibiotic use subgroups, respectively (Figure 3C, D). When costs were analyzed by CDAD episode, a consistent pattern of cost reduction was observed during the treatment of recurrent CDAD infections across all patient groups in patients who received fidaxomicin for initial CDAD.
Sensitivity Analysis
The results of the univariate sensitivity analysis are illustrated by the tornado diagram in Figure 4. The base-case incremental total cost was sensitive to 8 of the 11 parameters analyzed. The parameter with the largest impact on the cost difference was the rate of first recurrence associated with vancomycin treatment. As the figure illustrates, the higher the rate of first recurrence associated with vancomycin, the more favorable the model is to fidaxomicin. In terms of incremental cost (defined as cost associated with vancomycin minus cost associated with fidaxomicin), higher first recurrence rates result in higher incremental costs associated with vancomycin use, and vice versa. Rate of second recurrence associated with fidaxomicin treatment, attributable hospitalization cost of initial CDAD, and number of days of drug therapy received in the hospital per successfully treated episode did not impact base-case results.
Figure 4.
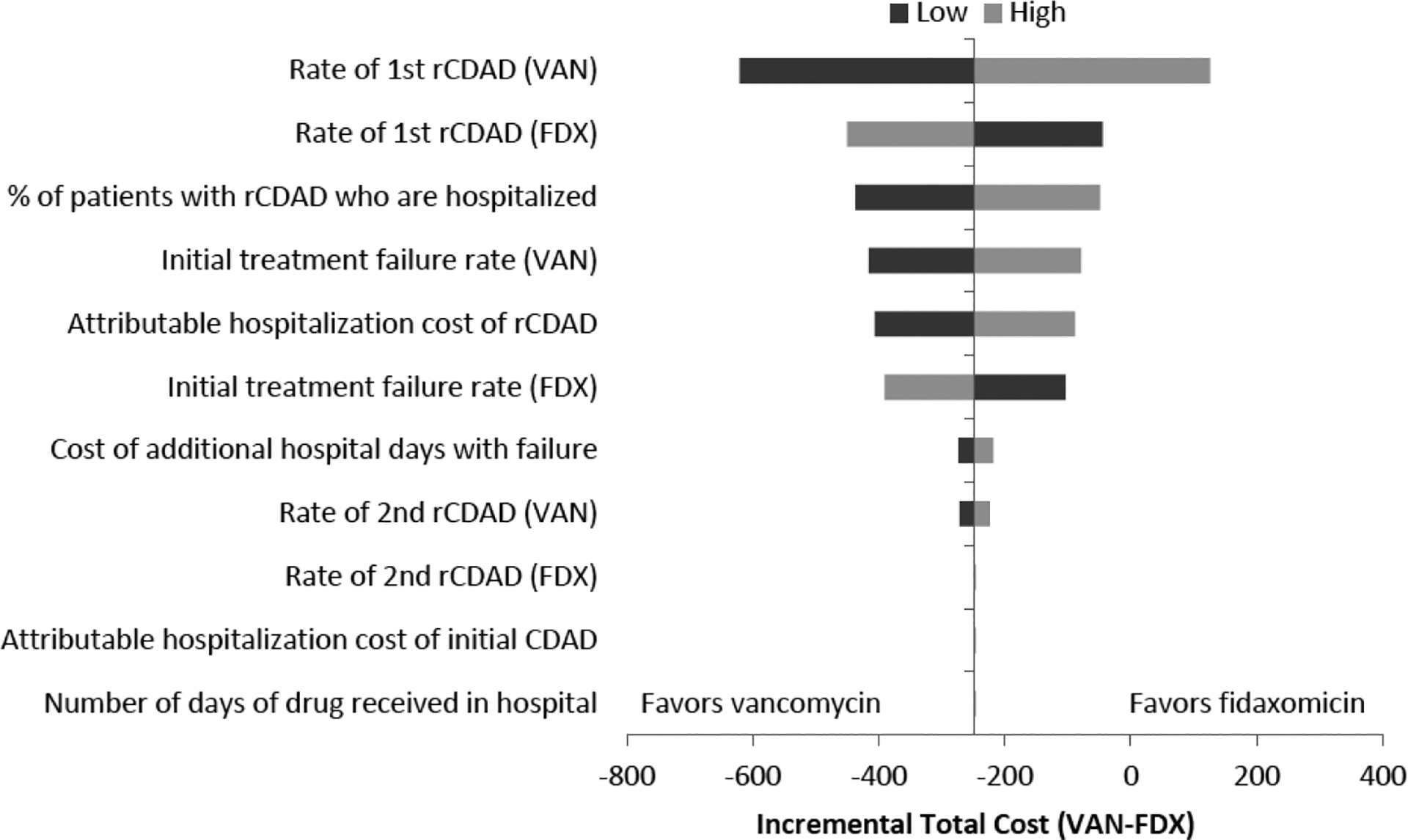
Univariate sensitivity analysis. CDAD = Clostridium difficile–associated diarrhea; FDX = fidaxomicin; rCDAD = recurrent C. difficile–associated diarrhea; VAN = vancomycin.
In the general population, fidaxomicin use for recurrences had little impact on total costs. When oral vancomycin was used first line, but fidaxomicin was used for the first recurrence, total costs increased from $14,179–$14,229. When fidaxomicin was used for the first and second recurrences, total costs increased to $14,246. In the fidaxomicin pathway, fidaxomicin use for the first recurrence only or for first and second recurrences increased total costs from $14,442–$14,471 or $14,481, respectively.
Discussion
CDAD contributes to poor patient health and high health care resource utilization; thus it is imperative to identify therapeutic strategies that not only improve patient health outcomes but also reduce costs. This is one of the first models to evaluate the total costs associated with treating CDAD from a hospital perspective. Importantly, this study also evaluated the costs of treating CDAD in subpopulations of patients traditionally at higher risk for recurrence. Using a decision tree model that included up to two CDAD recurrences, the total costs associated with treating CDAD were found to be similar between treatment pathways that included first-line fidaxomicin or first-line compounded oral vancomycin. Despite higher drug costs in the fidaxomicin pathway, overall costs were offset by reducing rehospitalizations due to recurrences. Hospitalization costs for the initial episode and recurrences were the major cost drivers, comprising a large proportion of the total cost of CDAD, whereas overall drug costs account for a small fraction of the total cost (range 1–9% of total costs). These findings highlight the importance of assessing hospital and drug costs and health outcomes simultaneously, with the goal of providing the most cost-effective therapies for patients.
Prior studies evaluated the cost-effectiveness of fidaxomicin for CDAD with mixed results.22, 23, 25, 26 One group22 developed a decision-analytic model to test the cost utility of fidaxomicin compared with oral vancomycin from the third-party payer perspective. This model, which considered up to two CDAD recurrences, found fidaxomicin to be cost-effective at a willingness-to-pay threshold of $100,000. In addition, a single-center retrospective cohort study27 assessed the economic impact of a hospital protocol encouraging first-line fidaxomicin use using Medicare reimbursement values for CDAD. Similar to the current findings, hospital costs were the primary cost driver, and fidaxomicin use resulted in overall cost savings of $3047 per patient. In contrast, other studies have found fidaxomicin to be cost prohibitive for the treatment of certain subpopulations, such as those with severe or recurrent CDAD.23, 25 In particular, one model made a similar comparison of fidaxomicin and vancomycin from the Canadian health care system perspective in patients with severe CDAD. This study found the incremental cost per recurrence avoided was $13,202. The authors concluded that fidaxomicin for the treatment of severe CDAD was associated with a cost increase to the Canadian health care system.25 Cost and clinical outcome input selections should be considered when comparing findings among studies, including this study.
In contrast to the general population, fidaxomicin use as first-line therapy resulted in cost savings for certain CDAD subpopulations, namely patients with cancer and those receiving concomitant antibiotics during or after CDAD therapy. This finding may be attributed to the more pronounced differences in initial clinical cure favoring fidaxomicin in these populations. Specifically, composite data from clinical trials found that fidaxomicin was associated with ~11% absolute risk reduction of treatment failure compared with vancomycin.16 In the current model, this would lead to reduced downstream resource utilization and shorter hospital stays. In addition, the use of fidaxomicin results in significantly lower recurrence rates, which likely leads to fewer rehospitalizations. Despite significantly reduced recurrence rates in clinical trials, elderly patients with CDAD or those with renal impairment experienced similar cure rates with vancomycin and fidaxomicin. This could have been responsible for the comparable overall costs associated with treating CDAD in these subpopulations.
There is a paucity of data describing costs and rates of recurrence and rehospitalization in CDAD patient subpopulations. In this model, a conservative assumption was made that the cost associated with treating CDAD in these subpopulations was the same as the general population. However, it is likely that these patients incur increased costs attributable to CDAD during their hospital stay due to the complexity of their underlying condition; therefore, this model may underestimate the cost savings associated with first-line fidaxomicin therapy. In addition, because these subpopulations tend to have higher rates of recurrence compared with the general population, they are likely rehospitalized more frequently. Given the potential of fidaxomicin to reduce recurrences, it is likely that first-line use in these subpopulations would result in decreased downstream resource utilization.
Conservative clinical and cost inputs were selected so as to not bias the results toward fidaxomicin. Oral vancomycin compounded from intravenous powder was evaluated for the alternative treatment pathway and for treatment failures and recurrences. Hospitals or health systems that use commercially available oral vancomycin capsules might find a cost benefit with fidaxomicin beyond what was found in this study. Similarly, health systems that use higher vancomycin doses or combination vancomycin plus metronidazole therapy might consider these cost differences when selecting initial treatments. In addition, an assumption was made that the rate of second recurrence was the same as the general population in all subpopulations. The second recurrence rate was taken from a small subset of data from phase III clinical trials, and it may not be representative of patients in the real world.5 Prior observational studies found second recurrent CDAD rates as high as 31–65%, especially when considering recurrences that occur beyond 30 days of follow-up.9,28–30 These analyses emphasize the importance of evaluating the rate of CDAD recurrences in individual hospitals or health systems. Next, it was assumed that only 51.6% of patients who experience recurrent CDAD were readmitted in all of the subpopulations based on a single study.21 Data describing the proportion of patients with recurrence who are readmitted are scarce, and this estimate may be conservative given the various high-risk subpopulations considered in this model. Finally, a recent publication31 reported average direct hospital costs ($21,448) and hospital LOS (11 days) attributable to C. difficile infection management higher than that used in the current model; therefore, these findings might underestimate the cost-effectiveness of fidaxomicin.
Reducing CDAD recurrences through aggressive infection control and effective antimicrobial therapy could impact both the patient and the health care system beyond the findings presented here. CDAD recurrence is detrimental to the patient because it increases morbidity and mortality, and reduces quality of life associated with repeated episodes of diarrhea.32, 33 Patients with recurrent CDAD experience prolonged symptoms and require repeated courses of antibiotics.33 This can lead to increased risk of adverse effects, rehospitalizations, and development of multidrug-resistant pathogens. Recurrent CDAD patients also continue to serve as a reservoir that can lead to infection in other vulnerable patients.34 Under the Centers for Medicare and Medicaid Services’ Hospital Read-missions Reduction Program, hospitals are penalized for excess hospital readmissions and specifically for patients with certain medical conditions who are readmitted to the hospital within 30 days.35 Although CDAD is not specified under this policy, patients with CDAD often have multiple comorbid conditions or concomitant infections that might fall under these policies. For example, if a patient who was initially hospitalized for pneumonia develops CDAD or recurrent CDAD and has to be readmitted within 30 days, the hospital will be penalized. Finally, although patient quality of life and symptom burden were not considered in this model, it is critical to consider the potential burden of recurrence when making initial treatment decisions in these high-risk patient populations.
This study has potential limitations, in addition to the inherent limitations of economic modeling. This model differs from other published models in that it does not consider patient quality of life but rather considers only costs and clinical outcomes. No formal studies have evaluated quality of life associated with CDAD or recurrences; therefore, it was not included in these analyses so as not to bias the results. Model stratification for CDAD severity or the presence of the NAP1/BI/027 strain was not included. The costs associated with treating severe CDAD have not been clearly defined, and testing for specific C. difficile strains is not routinely conducted in clinical practice; not including these factors should not invalidate these findings. CDAD costs vary by patient and institution, particularly in regard to hospital reimbursement for patient complications and comorbid conditions. Thus these results might not be generalizable to all individual patients or health care facilities. Because of limited data, it was necessary to use two separate data sources for initial and recurrent episode hospitalization cost estimates. The cost of recurrences at individual facilities should be compared with that used in this study to better inform first-line CDAD therapy choices. Costs might also be affected by the use of newer CDAD drug therapies, like bezlotoxumab; however, further economic analyses are needed to determine the most cost-effective use of this drug in combination with existing antimicrobials. CDAD mortality rates could also affect total treatment costs because those who die during a CDAD episode would be removed from the risk pool for subsequent recurrences. Lastly, this model does not consider indirect medical, nonmedical costs, or productivity loss. Therefore, these results likely underestimate the full economic burden of treating CDAD.
Fidaxomicin as first-line CDAD therapy is associated with similar total costs compared with compounded vancomycin oral solution in the general population, elderly patients, and patients with renal impairment. In addition, hospital cost savings were observed in patients with cancer and concomitant antibiotic use who received fidaxomicin as first-line therapy. Cost savings associated with fidaxomicin are largely attributable to lower recurrence rates leading to fewer rehospitalizations. The results of this economic evaluation emphasize the importance of selecting initial CDAD therapy in the hospital setting based on recurrence risk, rather than solely on drug acquisition costs.
Funding:
This work was funded by Merck and Co., Inc., Kenilworth, NJ.
Footnotes
Meeting Presentation: Making a Difference in Infectious Diseases (MAD-ID), Orlando, Florida, May 7–9, 2015.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;13:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic resistant threats in the United States, 2013. Available from http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed March 6, 2015.
- 3.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. Am J Infect Control 2014;10:1028–32. [DOI] [PubMed] [Google Scholar]
- 4.Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009. HCUP Statistical Brief 124. January 2012. Rockville, MD: Agency for Healthcare Research and Quality. Available from http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. Accessed December 27, 2011. [PubMed] [Google Scholar]
- 5.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;4:281–9. [DOI] [PubMed] [Google Scholar]
- 6.Wenisch JM, Schmid D, Tucek G, et al. A prospective cohort study on hospital mortality due to Clostridium difficile infection. Infection 2012;5:479–84. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 1989;2:340–3. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014;3:345–54. [DOI] [PubMed] [Google Scholar]
- 9.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002;7:1769–75. [DOI] [PubMed] [Google Scholar]
- 10.Cornely OA, Nathwani D, Ivanescu C, Odufowora-Sita O, Retsa P, Odeyemi IA. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother 2014;11:2892–900. [DOI] [PubMed] [Google Scholar]
- 11.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;5:422–31. [DOI] [PubMed] [Google Scholar]
- 12.Cornely OA, Miller MA, Fantin B, Mullane K, Kean Y, Gorbach S. Resolution of Clostridium difficile-associated diarrhea in patients with cancer treated with fidaxomicin or vancomycin. J Clin Oncol 2013;19:2493–9. [DOI] [PubMed] [Google Scholar]
- 13.Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis 2011;5:440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullane KM, Cornely OA, Crook DW, et al. Renal impairment and clinical outcomes of Clostridium difficile infection in two randomized trials. Am J Nephrol 2013;1:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;5:431–55. [DOI] [PubMed] [Google Scholar]
- 16.Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis 2012;55: S93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012;55:S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubberke ER, Schaefer E, Reske KA, Zilberberg M, Hollenbeak CS, Olsen MA. Attributable inpatient costs of recurrent Clostridium difficile infections. Infect Control Hosp Epidemiol 2014;11:1400–7. [DOI] [PubMed] [Google Scholar]
- 19.Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol 2008;9:823–8. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007;11:1219–27. [DOI] [PubMed] [Google Scholar]
- 21.Aitken SL, Joseph TB, Shah DN, et al. Healthcare resource utilization for recurrent Clostridium difficile infection in a large university hospital in Houston, Texas. PLoS One 2014;7: e102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health 2013;2:297–304. [DOI] [PubMed] [Google Scholar]
- 23.Konijeti GG, Sauk J, Shrime MG, Gupta M, Ananthakrishnan AN. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis 2014;11:1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis Clin Infect Dis 2013;4:555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol 2014;2:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathwani D, Cornely OA, Van Engen AK, Odufowora-Sita O, Retsa P, Odeyemi IA. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother 2014;11:2901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher JC, Reilly JP, Navalkele B, Downham G, Haynes K, Trivedi M. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother 2015;11:7007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;3:197–205. [DOI] [PubMed] [Google Scholar]
- 29.Pepin J, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis 2006;6:758–64. [DOI] [PubMed] [Google Scholar]
- 30.McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999;1:43–50. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis 2016;1:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 2009;6:403–10. [DOI] [PubMed] [Google Scholar]
- 33.DuPont HL. The search for effective treatment of Clostridium difficile infection. N Engl J Med 2011;5:473–5. [DOI] [PubMed] [Google Scholar]
- 34.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis 1997;3:324–33. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Medicare and Medicaid Services. Readmissions Reduction Program. Available from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed March 6, 2015.


