Abstract
Objective.
To examine whether improved human immunodeficiency virus (HIV) treatment was associated with better survival after diagnosis of AIDS-defining opportunistic illnesses (AIDS-OIs) and how survival differed by AIDS-OI.
Design.
We used HIV surveillance data to conduct a survival analysis.
Methods.
We estimated survival probabilities after first AIDS-OI diagnosis among adult patients with AIDS in San Francisco during 3 treatment eras: 1981–1986; 1987–1996; and 1997–2012. We used Cox proportional hazards models to determine adjusted mortality risk by AIDS-OI in the years 1997–2012.
Results.
Among 20 858 patients with AIDS, the most frequently diagnosed AIDS-OIs were Pneumocystis pneumonia (39.1%) and Kaposi sarcoma (20.1%). Overall 5-year survival probability increased from 7% in 1981–1986 to 65% in 1997–2012. In 1997–2012, after adjustment for known confounders and using Pneumocystis pneumonia as the referent category, mortality rates after first AIDS-OI were highest for brain lymphoma (hazard ratio [HR], 5.14; 95% confidence interval [CI], 2.98–8.87) and progressive multifocal leukoencephalopathy (HR, 4.22; 95% CI, 2.49–7.17).
Conclusions.
Survival after first AIDS-OI diagnosis has improved markedly since 1981. Some AIDS-OIs remain associated with substantially higher mortality risk than others, even after adjustment for known confounders. Better prevention and treatment strategies are still needed for AIDS-OIs occurring in the current HIV treatment era.
Keywords: AIDS, AIDS-defining illness, mortality, HIV, survival
Throughout the 1980s and 1990s, AIDS-defining opportunistic illnesses (AIDS-OIs) were predominant causes of morbidity and mortality among human immunodeficiency virus (HIV)–infected persons in the United States [1]. During these early years, survival after AIDS diagnosis was estimated to be <1 year in some US cohorts [2, 3]. Despite steady improvements in AIDS-OI prophylaxis and treatment, and the development of antiretrovirals, starting with azidothymidine in 1987 and active combination antiretroviral therapy (cART) in 1996, AIDS-OIs remain a serious health threat [4–8]. The advent of cART substantially decreased the incidence of AIDS-OIs [9–12] and resulted in improved survival among HIV-infected persons [4, 7, 13, 14]. However, AIDS-OIs continue to contribute to HIV-related mortality in the era of cART [15–18].
The impact of specific AIDS-OIs on survival among HIV-infected persons has been investigated previously in the United States and other industrialized countries [19–29]. These studies have suggested that after controlling for clinical and demographic factors, persons with AIDS faced different outcomes based on the type of their first AIDS-OI. Most of the previous studies were hospital-based [22–24, 26–28], and few were population-based [19–21]. Recent estimates of survival after AIDS-OIs in the United States are scarce.
Estimating survival after AIDS-OI in the United States is a challenge. At the start of the epidemic in 1981, AIDS was diagnosed solely by the occurrence of AIDS-OIs, and these conditions have remained part of the national surveillance case definition since then [30]. However, following the revision of the AIDS case definition in 1993 to include immunologic criteria (ie, CD4 T-lymphocyte count <200 cells/mm3 or <14% of total lymphocytes [31, table 11]), AIDS cases have increasingly been reported based solely on low CD4 cell counts or percentages and not on AIDS-OIs [32]. CD4 cell counts and percentages are easier for HIV surveillance programs to capture than incident AIDS-OI diagnoses, particularly after clinical laboratories began routine electronic reporting of these test results to the health departments.
The San Francisco Department of Public Health is, to our knowledge, the only health department in the United States that has both continuously conducted complete AIDS-OIs surveillance of all initial and subsequent diagnoses through follow-up medical chart reviews and reported these AIDS-OIs to the national HIV/AIDS surveillance system. For all persons with a diagnosis of AIDS in San Francisco, the health department collects initial and subsequent AIDS-defining OIs as well as clinical and demographic characteristics by reviewing medical records at the time of diagnosis and approximately every 18–24 months thereafter to update medical history and determine vital status. We took advantage of San Francisco’s highly complete set of data to achieve 3 objectives: (1) to examine the frequency distribution of specific AIDS-OIs diagnosed from 1 January 1981 through 30 June 2012, calendar years during which HIV treatments evolved dramatically; (2) to compare differences in AIDS-OI-specific survival across 3 distinct HIV antiretroviral therapy (ART) eras; and (3) to assess the relative hazards of death after the first AIDS-OIs and risk factors for mortality among persons with an AIDS-OI diagnosed during the cART era.
MATERIALS AND METHODS
Study Population and Data Source
The study population included residents of San Francisco with a diagnosis of AIDS and ≥1 of the 26 AIDS-OIs included in the 1993 revised AIDS case definition for persons aged ≥13 years [31, table 11]. We used national HIV/AIDS surveillance system data through 1 October 2012 and excluded patients for whom the date of death preceded the last reported laboratory test by >1 month (because such a discrepancy would imply possibly erroneous dates, or a false match between case and death records), or in which the date of first AIDS-OI was missing.
The national HIV/AIDS surveillance system data included demographic characteristics, date of first AIDS-OI diagnosis, type of first AIDS-OI, dates and types of any subsequent AIDS-OIs, CD4 cell count and HIV viral load values and dates, date of death, and causes of death obtained from death certificates. Dates of AIDS-OI diagnoses generally specified the year and month of diagnosis but not the exact date. For our analyses, the San Francisco Department of Public Health provided additional data not routinely collected by the national HIV/AIDS surveillance system, including ART, prophylaxis against Pneumocystis pneumonia (PCP), disseminated Mycobacterium avium complex (MAC) infection, and date of latest clinical encounter (ie, physician visit or laboratory test).
This analysis was considered exempt from institutional review board review in accordance with the Code of Federal Regulations regarding protection of human subjects (45 CFR §46.101[b]) because the analyses were conducted using existing data collected through routine public health surveillance activities. The project was determined by the Centers for Disease Control and Prevention (CDC) to be a public health practice related to epidemic or endemic disease control activity that required no contact with human subjects or informed consent process. To protect the confidentiality of persons reported with HIV/AIDS, the HIV surveillance data housed at the CDC do not contain names and other personal identifying information. All analyses were conducted at the CDC using deidentified records.
Data Analyses
The first part of the analyses was based on data from patients with their first AIDS-OI diagnosed anytime during the study period. We used descriptive statistics to characterize the case population and the diagnosis frequencies of different AIDS-OIs in each treatment era (defined below). For patient with >1 first AIDS-OI diagnosed in the same month and year, each of the first AIDS-OIs was analyzed in its own category. We used the Kaplan–Meier method to estimate survival after first AIDS-OI and compared survival across 3 time periods corresponding to 3 distinct treatment eras: pre-ART (1981–1986), mono/dual ART (1987–1996), and cART (1997–2012). In these analyses, we examined survival after diagnosis for the 10 AIDS-OIs reported most frequently over the entire observation period. Each patient was assigned to a specific treatment era based on the year of first AIDS-OI diagnosis and followed up from the date of the first AIDS-OI diagnosis to death from any cause. Patients in whom death was not known to have occurred during the treatment era were censored at the date of the last clinical encounter.
For the second part of the analyses, we restricted data to patients with the first AIDS-OI diagnosed in the cART era (1997–2012) to permit assessment of important survival covariates, such as CD4 cell count and ART prescription, which were not routinely available in our data for the prior treatment eras. For statistical modeling purposes, we defined 2 new categories of AIDS-OI: (1) “multiple AIDS-OIs” for patients with >1 AIDS-OI diagnosed in the same month to differentiate these patients from those with a single first AIDS-OI and to ensure that all AIDS-OIs categories were mutually exclusive; and (2) “rare AIDS-OIs,” each of which occurred in ≤15 patients during this time period. We used Cox proportional hazards models to determine the unadjusted and adjusted mortality risk stratified by first AIDS-OI category, modeled ART and AIDS-OIs as time dependent covariates, and used an intent-to-treat approach. In the final model for the cART era, we adjusted for age at first AIDS-OI diagnosis, sex, race/ethnicity, HIV transmission category, the geometric mean of CD4 cell counts within 12 months before or after the first AIDS-OI diagnosis, ART, prophylaxis against PCP and against disseminated MAC during the follow-up, and subsequent AIDS-OIs. The proportional hazards assumption was tested using Kaplan–Meier curves and by creating interactions between predictors and survival time; there was no evidence of nonproportionality (P > .05). We used SAS software for Windows, version 9.3 (SAS Institute) for all statistical analyses and assessed statistical significance at P < .05 for 2-sided testing.
RESULTS
We identified 21 005 San Francisco patients with AIDS with ≥1 AIDS-OI diagnosed during the observation period. We excluded 147 patients: 109 were reported with a death date >1 month before the last reported laboratory test, 33 were aged <13 years, and 5 had no date associated with their first AIDS-OI. This left data from 20 858 San Francisco patients with a first diagnosed AIDS-OI available for analyses, including 3002 (14.4%) with diagnosis in the pre-ART era, 14 097 (67.6%) in the mono/dual ART era, and 3759 (18.0%) in the cART era.
The demographics of AIDS-OI case patients shifted over time toward a distribution consisting of fewer white persons, fewer gay, bisexual or other men who have sex with men (MSM), and more black and Hispanic men and women who acquired HIV through injection drug use or heterosexual transmission. Across the 3 treatment eras, the median ages at first AIDS-OI diagnosis were 37, 39, and 43 years, respectively, and the majority of patients were white non-Hispanic (85.3%, 75.7%, and 57.8%, respectively), male (99.4%, 97.1%, and 91.9%, respectively), and MSM (82.8%, 76.9%, and 59.6%, respectively) (Table 1). Owing to differential mortality, patients with a first AIDS-OI diagnosed in the cART era had a longer median follow-up (5.16 years; range, <1 to 15.6 years) than those diagnosed in pre-ART and mono/dual ART eras (1.00 years [range, <1 to 29.3 years] and 1.52 years [<1 to 25.5 years], respectively). Of the 20 858 patients with AIDS-OIs, death occurred in 17 099 (82.0%) by the end of observation: 2957 (98.4%), 12 569 (89.2%), and 1573(41.9%) in each treatment era, respectively. Within each era, the majority of decedents with death certificate information available had an AIDS-OI mentioned on the death certificate.
Table 1.
Demographic Characteristics of Patients With AIDS-OI (San Francisco, 1981—2012)a
| Characteristic | Patients, No. (%)b |
|||
|---|---|---|---|---|
| Pre-ART Era 1981–1986 (n = 3002) | Mono/Dual-ART Era 1987–1996 (n = 14 097) | cART Era 1997–2012 (n = 3759) | Total 1981–2012 (N = 20 858) | |
|
| ||||
| Age at AIDS-OI diagnosis, y | ||||
| 13–35 | 1268 (42.2) | 4691 (33.3) | 780 (20.8) | 6739 (32.3) |
| 36–45 | 1209 (40.3) | 6228 (44.2) | 1572 (41.8) | 9009 (43.2) |
| ≥46 | 525 (17.5) | 3178 (22.5) | 1407 (37.4) | 5110 (24.5) |
| Race/ethnicity | ||||
| Hispanic | 245 (8.2) | 1612 (11.4) | 629 (16.7) | 2486 (11.9) |
| Black, non-Hispanic | 166 (5.5) | 1625 (11.5) | 755 (20.1) | 2546 (12.2) |
| White, non-Hispanic | 2559 (85.3) | 10 665 (75.7) | 2171 (57.8) | 15 395 (73.8) |
| Other | 32 (1.1) | 195 (1.4) | 204 (5.4) | 431 (2.1) |
| Sex at birth | ||||
| Male | 2983 (99.4) | 13 685 (97.1) | 3453 (91.9) | 20 121 (96.5) |
| HIV transmission category | ||||
| MSM | 2485 (82.8) | 10 844 (76.9) | 2241 (59.6) | 15 570 (74.7) |
| IDU | 32 (1.1) | 876 (6.2) | 519 (13.8) | 1427 (6.8) |
| MSM and IDU | 448 (14.9) | 2102 (14.9) | 798 (21.2) | 3348 (16.1) |
| Heterosexual with HIV-infected or IDU/MSM sex partner | 11 (0.37) | 126 (0.89) | 129 (3.4) | 266 (1.3) |
| Other/unknown | 26 (0.87) | 149 (1.1) | 72 (1.9) | 247 (1.2) |
| Multiple first AIDS-OIs | 317 (10.6) | 1754 (12.4) | 376 (10.0) | 2447 (11.7) |
| Follow-up time, median (range) y | 1.00 (<1.0 to 29.3) | 1.52 (<1.0 to 25.5) | 5.16 (<1.0 to 15.6) | 1.6 (<1.0 to 29.3) |
| Vital status | ||||
| Dead | 2955 (98.4) | 12 569 (89.2) | 1573 (41.9) | 17 097 (82.0) |
| AIDS-OI mentioned on death certificate (n = 17 097) | 1660 (56.2) | 9335 (74.3) | 1160 (73.7) | 12 155 (71.1) |
Abbreviations: AIDS-OI, AIDS-defining opportunistic illness; ART, antiretroviral therapy; cART, combination ART; HIV, human immunodeficiency virus; IDU, injection drug user; MSM, men who have sex with men.
Patients had a total of 3411, 16 206, and 4190 first AIDS-OIs across the 3 treatment eras, respectively (some had multiple first AIDS-OIs). All characteristics were determined at the time of first AIDS-OI diagnosis, unless otherwise specified.
Data represent No. (%) of patients unless otherwise noted.
The annual number of AIDS-OIs diagnoses peaked in the mono/dual-ART era, followed by a pronounced decline in the cART era (Table 2). Across the entire study period, the 10 most frequently diagnosed AIDS-OIs in 1981–2012 were PCP (n = 8163), Kaposi sarcoma (KS; n = 4195), HIV wasting syndrome (n = 1864), esophageal candidiasis (n = 1381), MAC (n = 1069), HIV encephalopathy (n = 932), extrapulmonary cryptococcosis (n = 883), chronic cryptosporidiosis (n = 694), immunoblastic lymphoma (n = 676), and cytomegalovirus disease other than retinitis (n = 643). The 5 least frequently diagnosed AIDS-OIs were invasive cervical cancer (n = 12), recurrent Salmonella septicemia (n = 19), disseminated coccidioidomycosis (n = 32), chronic isosporiasis (n = 36), and disseminated histoplasmosis (n = 78).
Table 2.
Number of First AIDS-OIs Diagnoses Among Patients by Treatment Era (San Francisco, 1981–2012)
| AIDS-OIa | AIDS-OIs Diagnoses, No. (%) |
|||
|---|---|---|---|---|
| Pre-ART Era 1981–1986 (n = 3002) | Mono/Dual-ART Era 1987–1996 (n = 14 097) | cART Era 1997–2012 (n = 3759) | Total 1981–2012 (N = 20 858)b | |
|
| ||||
| PCP | 1640 (54.59) | 5379 (38.16) | 1144 (30.43) | 8163 (39.13) |
| KS | 952 (31.69) | 2768 (19.64) | 475 (12.64) | 4195 (20.11) |
| HIV wasting syndrome | 35 (1.17) | 1365 (9.68) | 464 (12.34) | 1864 (8.94) |
| Esophageal candidiasis | 100 (3.33) | 910 (6.46) | 371 (9.87) | 1381 (6.62) |
| MAC, disseminated | 80 (2.66) | 834 (5.92) | 155 (4.12) | 1069 (5.12) |
| HIV encephalopathy | 35 (1.17) | 715 (5.07) | 182 (4.84) | 932 (4.47) |
| Cryptococcosis | 106 (3.53) | 558 (3.96) | 219 (5.83) | 883 (4.23) |
| Cryptosporidiosis | 64 (2.13) | 503 (3.57) | 127 (3.38) | 694 (3.33) |
| Immunoblastic lymphoma | 72 (2.40) | 449 (3.19) | 155 (4.12) | 676 (3.24) |
| CMV, nonretinitis | 91 (3.03) | 455 (3.23) | 97 (2.58) | 643 (3.08) |
| Recurrent pneumonia | 8 (0.27) | 307 (2.18) | 226 (6.01) | 541 (2.59) |
| Toxoplasmosis, brain | 60 (2.00) | 334 (2.37) | 64 (1.70) | 458 (2.20) |
| CMV, retinitis | 19 (0.63) | 378 (2.68) | 56 (1.49) | 453 (2.17) |
| M. tuberculosis, infection | ||||
| Pulmonary | 5 (0.17) | 291 (2.06) | 135 (3.59) | 431 (2.07) |
| Disseminated | 14 (0.47) | 210 (1.49) | 54 (1.44) | 278 (1.33) |
| Burkett lymphoma | 26 (0.87) | 144 (1.02) | 69 (1.84) | 239 (1.15) |
| PML | 16 (0.53) | 109 (0.77) | 26 (0.69) | 151 (0.72) |
| Herpes simplex | 49 (1.63) | 148 (1.05) | 54 (1.44) | 241 (1.16) |
| Mycobacterium other, disseminated | 9 (0.30) | 94 (0.67) | 20 (0.53) | 123 (0.59) |
| Primary brain lymphoma | 14 (0.47) | 81 (0.57) | 24 (0.64) | 119 (0.57) |
| Tracheal candidiasis | 7 (0.23) | 49 (0.35) | 30 (0.80) | 86 (0.41) |
| Histoplasmosis | 3 (0.10) | 60 (0.43) | 15 (0.40) | 78 (0.37) |
| Isosporiasis | 1 (0.03) | 27 (0.19) | 8 (0.21) | 36 (0.17) |
| Coccidioidomycosis | 2 (0.07) | 20 (0.14) | 10 (0.27) | 32 (0.15) |
| Salmonella septicemia | 3 (0.10) | 14 (0.10) | 2 (0.05) | 19 (0.09) |
| Invasive cervical cancer | 0 (0.00) | 4 (0.03) | 8 (0.21) | 12 (0.06) |
Abbreviations: AIDS-OI, AIDS-defining opportunistic illness; ART, antiretroviral therapy; cART, combination ART; CMV, cytomegalovirus; HIV, human immunodeficiency virus; KS, Kaposi sarcoma; MAC, Mycobacterium avium complex; M. tuberculosis, Mycobacterium tuberculosis; n, number of individual patients; PCP, Pneumocystis pneumonia; PML, progressive multifocal leukoencephalopathy.
The AIDS-OIs numbered 3411, 16 206 and 4190 in the three treatment eras, respectively, for a total of 23 807. Patients may have >1 initial AIDS-OI and diagnoses shown in this table are sorted in descending order of their total count during 1981–2012.
There were a total of 23 807 OIs reported for 20 858 unique patients.
In Kaplan–Meier analyses of survival after diagnosis by AIDS-OI type, survival probabilities were significantly higher in the cART era than in previous treatment eras (P < .05; logrank test). The overall 5-year survival probability after first AIDS-OI was 7% in the pre-ART era but almost doubled to 18% in the mono/dual-ART era and showed the greatest improvement in the cART era, increasing to 65% (Figure 1).
Figure 1.
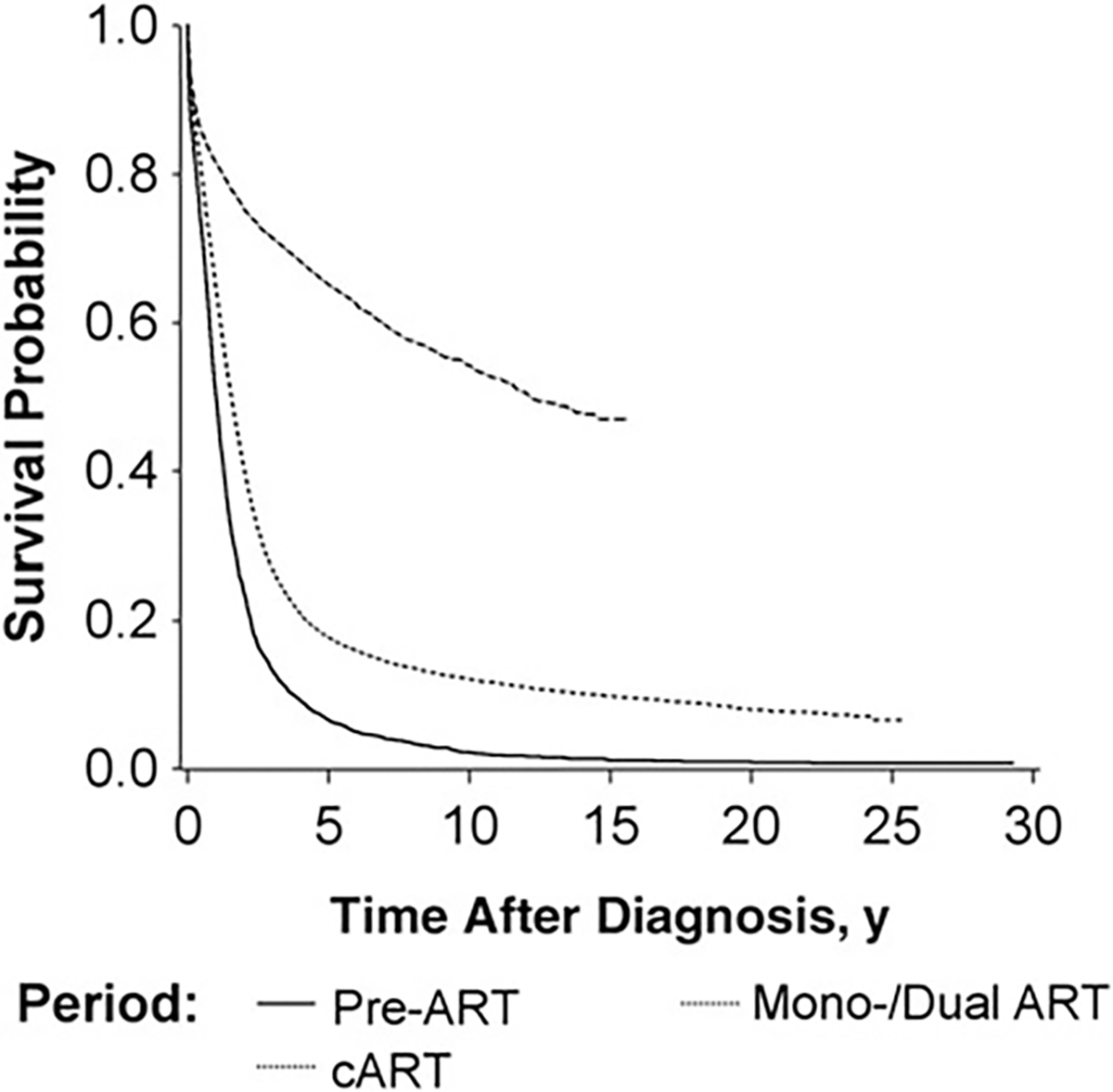
Overall survival time after diagnosis of the first AIDS-defining opportunistic illness (AIDS-OI) by human immunodeficiency virus (HIV) treatment era (1: pre– [ART], 1981–1986; 2: mono/dual ART, 1987–1996; and 3: combination ART [cART], 1997–2012) in San Francisco, 1981–2012. Abbreviation: ART, antiretroviral therapy.
When we analyzed survival after selected types of AIDS-OIs, we found that in the cART era, the median survival time after the diagnosis of PCP, KS, and cryptosporidiosis was >15 years, compared with <5 years in the previous treatment eras (Figure 2). Although there have been improvements in survival in the cART era for MAC and immunoblastic lymphoma, the median survival times for these AIDS-OIs remained <5 years.
Figure 2.
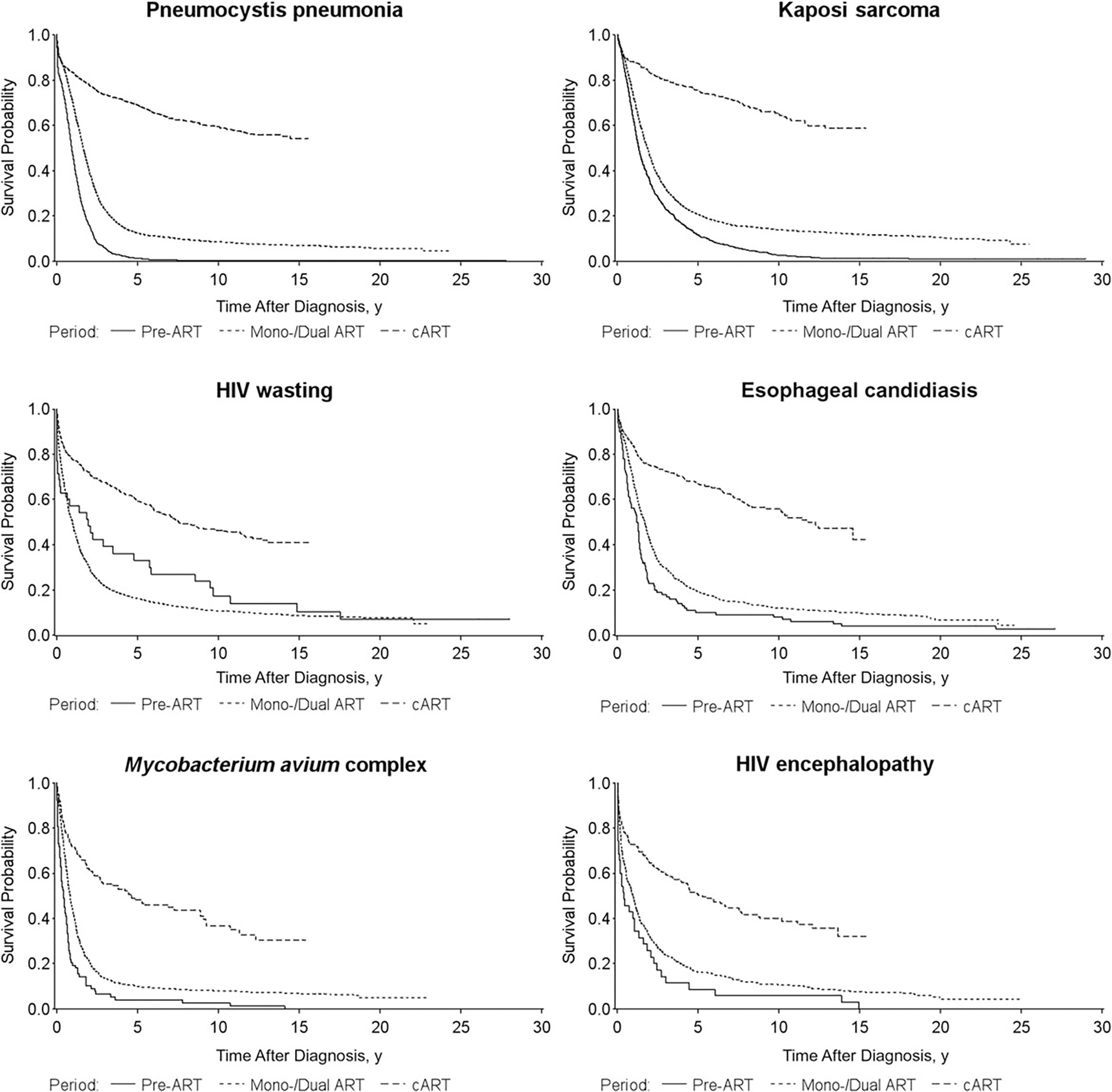
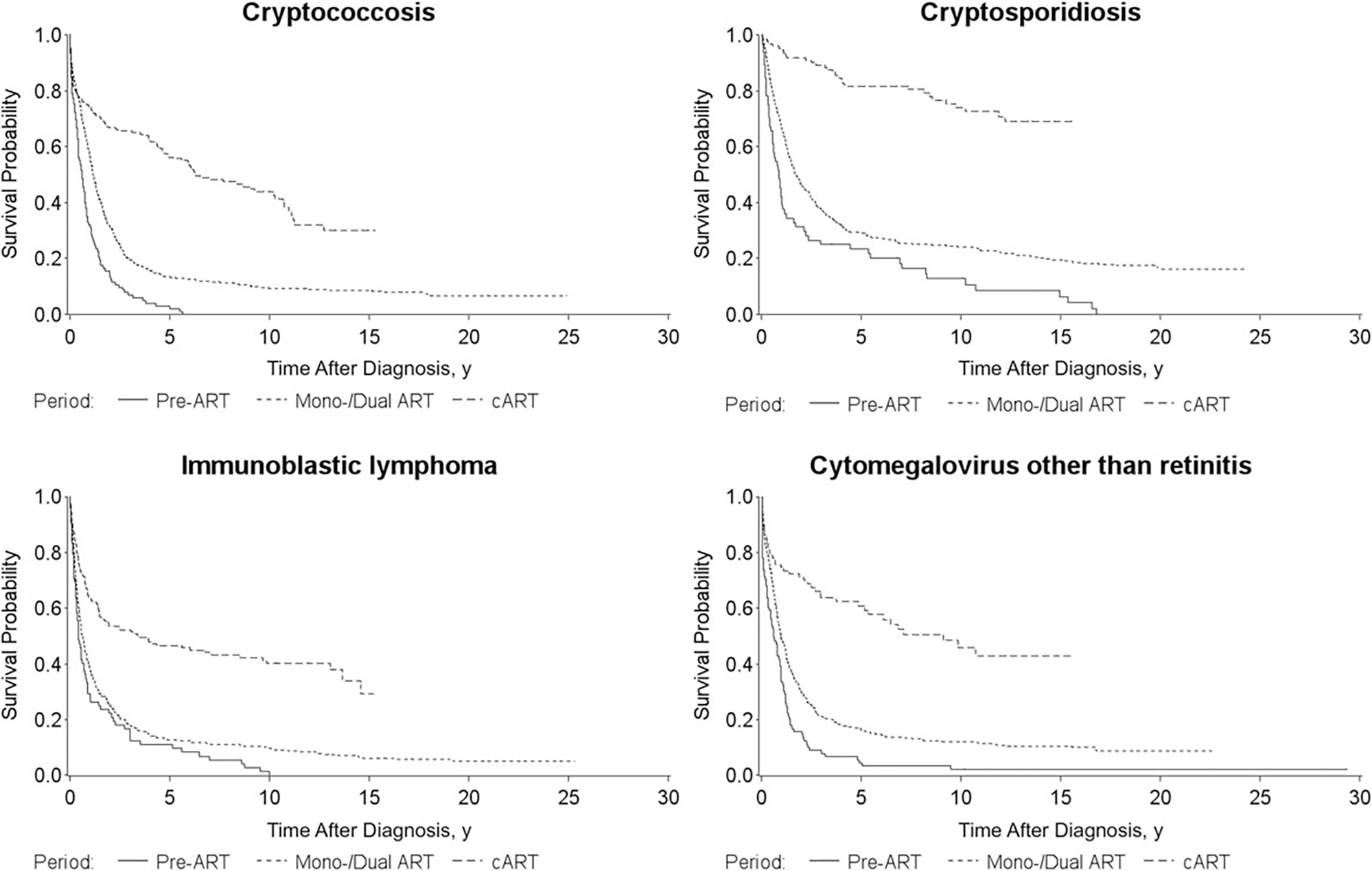
Survival time after diagnosis of the first AIDS-defining opportunistic illness (AIDS-OI) by human immunodeficiency virus (HIV) treatment era (1: pre– [ART], 1981–1986; 2: mono/dual ART, 1987–1996; and 3: combination ART [cART], 1997–2012) for persons with the ten most common AIDS-OIs in San Francisco, 1981–2012. Abbreviation: ART, antiretroviral therapy.
For each of the 10 most common AIDS-OIs during 1981–2012, the 5-year survival probabilities after diagnosis increased from the pre-ART era to the cART era, with the greatest survival gain for patients with PCP: from 1% in the pre-ART era to 69% in the cART era (Figure 2). In the cART era, patients with a diagnosis of chronic cryptosporidiosis had the highest 5-year survival probability (81%), followed by those with KS (75%); patients with immunoblastic lymphoma had the lowest 5-year survival probability (47%).
Among the 3759 patients with their first AIDS-OI in the cART era (1997–2012), 1,579 (41.2%) died. The median follow time was 5.0 years, and the median time to death was 1.5 years. In addition, 42.6% of patients had a CD4 cell count of 50–199 cells/mm3, 84.9% were prescribed ART during the follow-up, 78.1% were prescribed PCP prophylaxis, and 36.5% were prescribed MAC prophylaxis (Table 3). Using PCP as the first AIDS-OI for the referent group and adjusting for age, HIV transmission category, CD4 cell count, use of any ART, and prophylaxis against MAC and PCP, mortality rates were the highest for brain lymphoma (hazard ratio [HR], 5.14; 95% confidence interval [CI], 2.98–8.87), progressive multifocal leukoencephalopathy (HR, 4.22; CI, 2.49–7.17), multiple AIDS-OIs (HR, 3.22; CI, 2.75–3.77), and immunoblastic lymphoma (HR, 2.56; CI, 1.90–3.42) (Table 3). Burkitt lymphoma (HR, 2.40; CI, 1.58–3.65), HIV encephalopathy (HR, 2.23; CI, 1.67–2.96), HIV wasting (HR, 1.81; CI, 1.44–2.29), and recurrent pneumonia (HR, 1.56; CI, 1.17–2.08) were also associated with significantly higher mortality rates than PCP. In contrast, chronic cryptosporidiosis was associated with lower mortality rates than PCP (HR, 0.44; 95% CI, .24–.82). Mortality rates after diagnosis of the other AIDS-OIs did not differ significantly compared with those for PCP.
Table 3.
Unadjusted and Adjusted Mortality Risk Associated With First AIDS-OI (San Francisco, 1997–2012)a
| First AIDS-OIb | Patients, No. (%) | HR (95% CI) |
|
|---|---|---|---|
| Unadjusted | Adjusted | ||
|
| |||
| PCP | 949 (25.25) | Referent | Referent |
| Brain lymphoma | 21 (0.56) | 5.75 (3.35–9.89) | 5.14 (2.98–8.87) |
| Progressive multifocal leukoencephalopathy | 25 (0.67) | 4.58 (2.71–7.73) | 4.22 (2.49–7.17) |
| Multiple AIDS-OIsc | 376 (10.00) | 3.50 (2.99–4.09) | 3.22 (2.75–3.77) |
| Immunoblastic lymphoma | 139 (3.70) | 2.19 (1.64–2.93) | 2.56 (1.90–3.42) |
| Burkitt lymphoma | 63 (1.68) | 1.95 (1.29–2.96) | 2.40 (1.58–3.65) |
| HIV encephalopathy | 153 (4.07) | 1.97 (1.49–2.61) | 2.23 (1.67–2.96) |
| HIV wasting | 379 (10.08) | 1.40 (1.12–1.76) | 1.81 (1.44–2.29) |
| Recurrent pneumonia | 204 (5.43) | 1.56 (1.18–2.05) | 1.56 (1.17–2.08) |
| Cryptococcosis | 173 (4.60) | 1.46 (1.06–1.99) | 1.34 (.98–1.83) |
| M. tuberculosis infection, pulmonary | 106 (2.82) | 1.32 (.90–1.96) | 1.30 (.87–1.92) |
| Tracheal candidiasis | 15 (0.40) | 1.03 (.33–3.23) | 1.20 (.38–3.75) |
| MAC, disseminated | 104 (2.77) | 1.46 (.97–2.20) | 1.17 (.78–1.76) |
| CMV, nonretinitis | 38 (1.01) | 1.23 (.65–2.32) | 1.10 (.58–2.08) |
| M. tuberculosis infection, disseminated | 36 (0.96) | 1.09 (.54–2.21) | 1.07 (.53–2.18) |
| Rare AIDS-OIsd | 78 (2.08) | 0.94 (.56–1.59) | 1.05 (.62–1.77) |
| Toxoplasmosis | 56 (1.49) | 0.93 (.51–1.71) | 1.03 (.56–1.89) |
| KS | 418 (11.12) | 0.73 (.56–.95) | 0.92 (.70–1.20) |
| Esophageal candidiasis | 265 (7.05) | 0.85 (.62–1.18) | 0.78 (.56–1.08) |
| CMV, retinitis | 40 (1.06) | 0.44 (.16–1.18) | 0.48 (.18–1.29) |
| Cryptosporidiosis | 121 (3.22) | 0.34 (.18-.62) | 0.44 (.24–0.80) |
| Age at AIDS-OI diagnosis, y | |||
| 13–35 | 780 (20.8) | Referent | Referent |
| 36–45 | 1572 (41.8) | 1.35 (1.16–1.56) | 1.44 (1.24–1.67) |
| ≥46 | 1407 (37.4) | 1.84 (1.59–2.13) | 2.05 (1.77–2.37) |
| CD4 cell count, cells/mm3 | |||
| ≥350 | 583 (16.1) | Referent | Referent |
| 200–349 | 742 (20.5) | 1.41 (1.15–1.74) | 1.57 (1.26–1.95) |
| 50–199 | 1544 (42.6) | 1.84 (1.53–2.22) | 2.12 (1.73–2.59) |
| <50 | 752 (20.8) | 4.44 (3.68–5.36) | 4.47 (3.61–5.52) |
| ART anytime during follow-up | |||
| Yes | 3192 (84.9) | Referent | Referent |
| No | 567 (15.1) | 3.76 (3.30–4.30) | 2.50 (2.15–2.91) |
| Injection drug use as HIV risk factor | |||
| No | 2370 (59.6) | Referent | Referent |
| Yes | 1317 (13.8) | 1.58 (1.43–1.75) | 1.42 (1.29–1.55) |
| PCP prophylaxis anytime during follow-up | |||
| Yes | 2937 (78.1) | Referent | Referent |
| No | 822 (21.9) | 1.16 (1.05–1.29) | 1.33 (1.15–1.53) |
| MAC prophylaxis anytime during follow-up | |||
| Yes | 1373 (36.5) | Referent | Referent |
| No | 2386 (63.5) | 1.30 (1.16–1.46) | 1.42 (1.26–1.59) |
Abbreviations: AIDS-OI, AIDS-defining opportunistic illness; ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; HIV, human immunodeficiency virus; HR, hazard ratio; KS, Kaposi sarcoma; MAC, Mycobacterium avium complex; M. tuberculosis, Mycobacterium tuberculosis; PCP, Pneumocystis pneumonia.
Mortality risk was adjusted for age at first AIDS-OI diagnosis, sex, race/ethnicity, HIV transmission category, CD4 cell count measured anytime between 1 year before and 1 year after the first AIDS-OI diagnosis, ART use anytime during follow-up, use of PCP and MAC prophylaxis anytime during follow-up, and subsequent AIDS-OIs occurring during the observation period. ART and OIs modeled as time dependent covariates.
AIDS-OIs are ranked in descending order based on the mortality risk associated with the first AIDS-OI, controlling for subsequent AIDS-OIs and covariates.
Patients with >1 AIDS-OI diagnosis in the same month as the first AIDS-OI diagnosis.
Rare AIDS-OIs include invasive cervical cancer, disseminated coccidioidomycosis, disseminated histoplasmosis, chronic herpes simplex, chronic isosporiasis, recurrent Salmonella septicemia, and disseminated mycobacteriosis with species other than avium complex, intracellulare, or kansasii.
Persons with a first AIDS-OI diagnosed in the cART era were more likely to die during follow-up if they were not prescribed prophylaxis against PCP (HR, 1.33; CI, 1.15–1.53) or MAC (HR, 1.42; CI, 1.26–1.59) or were not prescribed ART (HR, 2.50; CI, 2.15–2.91). Compared with persons who had geometric mean CD4 cell counts ≥350 cells/mm3, those with lower CD4 counts were more likely to die (HR, 4.47 [CI, 3.61–5.52], 2.12 [1.73–2.59], and 1.57 [1.26–1.95] for CD4 cell counts of <50, 50–199, and 200–349 cells/mm3, respectively). Compared with persons in all other HIV transmission categories (combined), those who acquired HIV through injection drug use were also more likely to die (HR, 1.42; CI, 1.29–1.55).
DISCUSSION
In the large population of persons with AIDS in San Francisco, survival after first AIDS-OIs has increased markedly from the pre-ART to cART treatment era, but it has remained poor for certain AIDS-OIs. Diagnoses of all AIDS-OIs declined in cART era, consistent with results reported in previous studies [9–11]. In earlier analyses (from 1993 to 2008), Schwarcz et al [10] showed that the incidence of AIDS-OIs among San Francisco patients with AIDS declined since the introduction of cART, with the incidence rate reductions for individual AIDS-OI events ranging from 84% to 99% comparing the earliest cART (1996–2000) and the latest cART (2001–2008) period. Although the incidence of AIDS-OIs in San Francisco has been declining since the 1990s [10], the ranking of first AIDS-OIs by frequency has not changed meaningfully since the beginning of HIV epidemic. PCP and KS consistently remained the 2 most frequently reported AIDS-OIs in all eras. The predominance of PCP among patients with AIDS-OIs diagnosed in the cART era has been reported in previous studies [21, 33], although declines in PCP incidence have been noted with the introduction of PCP prophylaxis in the early 1990s [10].
Our findings of improvement in survival after AIDS-OI diagnosis are consistent with those from previous studies in industrialized countries from pre-ART and cART treatment eras [20, 23, 27]. These survival improvements, especially in the most recent treatment era, probably reflect a combination of factors, including use of cART, improved prophylaxis and treatment of AIDS-OIs, earlier diagnosis of HIV infection, and better linkage to effective HIV care [34–36]. Despite these overall improvements, changes were not uniform, and there remained significant disparities in survival across AIDS-OIs in the cART era in our analyses, which were also noted in aforementioned studies, although respective results cannot be directly compared, owing to varying study populations, time frames, and analytic designs [20, 23, 27]. Survival after PCP diagnosis demonstrated the largest improvement. The use of PCP prophylaxis to prevent the acquisition of PCP among HIV-infected persons with a CD4 cell count <200 cells/mm3 has been recommended since 1989 and has improved survival among HIV-infected persons [37, 38]. Prophylaxis has also been used to prevent MAC infection, but in these analyses the improvement in survival after a diagnosis of MAC infection as the first AIDS-OI was not as great as that after PCP, possibly owing to the more profound immunosuppression at which MAC typically occurs.
Although the AIDS-OIs of some persons may have resolved before death, and persons may have died of causes other than AIDS-OIs, 71% of decedents for whom death certificates were available had AIDS-OI mentioned on those certificates. After adjusting for clinical and demographic variables, we found that survival after first AIDS-OIs was poorest for persons with AIDS-defining cancers or AIDS-defining OIs associated with very low CD4 counts, such as progressive multifocal leukoencephalopathy, and diagnosis of multiple AIDS-OIs. Our findings support previous studies finding that survival remains poor after diagnosis of lymphomas, notably brain lymphoma, in the cART era [19–21, 27, 29, 39]. In analyses similar to ours, brain lymphoma was associated with the worst survival among persons with AIDS in New York City [21].
In the cART era, AIDS-OIs that had effective treatments generally were associated with survival similar to that after a first AIDS-OI diagnosis of PCP. Persons with chronic cryptosporidiosis as their first AIDS-OI were less likely to die than those with PCP, possibly because the definitive treatment for this condition with comparatively low mortality in the pre-ART and mono/dual-ART era (Figure 2) is effective cART. We found that higher CD4 cell counts within a year of the first AIDS-OI diagnosis, as well as cART and prophylaxis against PCP and MAC, were all associated with better survival, highlighting the continued importance of cART and OI prophylaxis. We also found that the risk of dying after first AIDS-OI diagnosis was higher among persons with a history of injection drug use than among those with a history of likely exposure to HIV through sexual contact but not injection drug use. As reported in other HIV-infected populations, the reasons for this decreased survival may be poorer health, less access, and adherence to cART, initiation of ART at a more advanced stage of HIV infection, or accidents and overdose [39–43].
Our analyses had several limitations. We relied on routinely collected HIV surveillance data with a limited number of predefined variables. For example, our ART data included only the date of treatment initiation but no information on treatment interruption or discontinuation, and the CD4 cell count data were routinely collected only in the cART period. Importantly, HIV diagnoses (vs AIDS diagnoses only) were not subject to mandatory reporting to the San Francisco Department of Public Health until 2002, and universal reporting of CD4 cell counts to HIV surveillance was introduced in 2008; thus, we could not systematically quantify whether and how earlier HIV diagnosis and cART may have contributed to improved survival after first AIDS-OI.
Because we used an intent-to-treat approach in our analyses, our estimates of the effect of ART on mortality are conservative. We did not have information on coexisting non–AIDS-related conditions, which may have contributed to mortality risk. In addition, the population of San Francisco is not representative of the US general population. The population in our analyses was predominately MSM and therefore provided a rich source of data on KS (which is more common among MSM than among persons in other HIV transmission categories), but with so few women in the study population, we could not study cervical cancer. The San Francisco Department of Public Health surveillance staff conducts follow-up chart reviews at clinical sites that serve large numbers of HIV-infected persons, but we may have missed AIDS-OIs among persons who were seen at smaller private practices or had moved outside San Francisco after their HIV diagnosis.
Some AIDS-OIs that require documentation of recurrence (eg, recurrent pneumonia) or fulfillment of other specific clinical criteria (eg, HIV wasting) may have been missed or incorrectly diagnosed by some reporting providers, and the extent of such overreporting or underreporting is not clear. We expect this misclassification to be nondifferential; thus, our overall estimates remain valid. In addition, our adjustment for clinical factors was limited to variables collected as part of routine AIDS surveillance and lacked more complete measures of whether or not persons were receiving or adhering to HIV care. Finally, our data are from a city with widespread and early treatment programs, so the survival estimates we found may not be generalizable to patients with AIDS in other areas of the United States with different levels of care.
In conclusion, using active HIV surveillance data from San Francisco, we have shown that survival after a first AIDS-OI diagnosis, especially for the most frequently diagnosed AIDS-OIs, has improved markedly since 1981. Despite these improvements, 35% of persons with an AIDS-OI diagnosed in the cART era died within 5 years. Some AIDS-OIs remain associated with substantial mortality risk even after adjustment for CD4 cell count. Better prevention and treatment strategies, including earlier HIV diagnosis, are still needed for AIDS-OIs in the cART era.
Financial support.
CDC provided funds to the San Francisco Department of Public Health to support routine HIV/AIDS surveillance, under which the data used in this project were collected.
Disclaimer.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Footnotes
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Annual Epidemic Intelligence Service Conference, Atlanta, 29 May 2014.
References
- 1.Jones JL, Hanson DL, Dworkin MS, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. JAMA Dermatology 1999; 135:897–902. [PubMed] [Google Scholar]
- 2.Lemp GF, Payne SF, Neal D, Temelso T, Rutherford GW. Survival trends for patients with AIDS. JAMA 1990; 263:402–6. [PubMed] [Google Scholar]
- 3.Moore RD, Hidalgo J, Sugland BW, Chaisson RE. Zidovudine and the natural history of the acquired immunodeficiency syndrome. N Engl J Med 1991; 324:1412–6. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 5.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. BMJ 1997; 315:1194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 1998; 352:1725–30. [DOI] [PubMed] [Google Scholar]
- 7.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 1998; 280:1497–503. [DOI] [PubMed] [Google Scholar]
- 8.Dorrucci M, Balducci M, Pezzotti P, Sinicco A, Alberici F, Rezza G. Temporal changes in the rate of progression to death among Italians with known date of HIV seroconversion: estimates of the population effect of treatment. Italian HIV Seroconversion Study (ISS). J Acquir Immune Defic Syndr 1999; 22:65–70. [DOI] [PubMed] [Google Scholar]
- 9.Buchacz K, Baker RK, Palella FJ, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 2010; 24:1549–59. [DOI] [PubMed] [Google Scholar]
- 10.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. AIDS 2013; 27:597–605. [DOI] [PubMed] [Google Scholar]
- 11.Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART) in a London clinic. J Infect 2001; 42:134–9. [DOI] [PubMed] [Google Scholar]
- 12.Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination anti-retroviral therapy. AIDS 2001; 15:629–33. [DOI] [PubMed] [Google Scholar]
- 13.Lima VD, Hogg RS, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS 2007; 21:685–92. [DOI] [PubMed] [Google Scholar]
- 14.Hogg R, Lima V, Sterne JA, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 16.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS 2006; 20:741–9. [DOI] [PubMed] [Google Scholar]
- 17.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalité 2000 and 2005” surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr 2008; 48:590–8. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet F, Lewden C, May T, et al. Opportunistic infections as causes of death in HIV-infected patients in the HAART era in France. Scand J Infect Dis 2005; 37:482–7. [DOI] [PubMed] [Google Scholar]
- 19.Conti S, Masocco M, Pezzotti P, et al. Differential impact of combined antiretroviral therapy on the survival of Italian patients with specific AIDS-defining illnesses. J Acquir Immune Defic Syndr 2000; 25:451–8. [DOI] [PubMed] [Google Scholar]
- 20.Dore GJ, Li Y, McDonald A, Ree H, Kaldor JM; National HIV Surveillance Committee. Impact of highly active antiretroviral therapy on individual AIDS-defining illness incidence and survival in Australia. J Acquir Immune Defic Syndr 2002; 29:388–95. [DOI] [PubMed] [Google Scholar]
- 21.Fordyce EJ, Singh TP, Nash D, Gallagher B, Forlenza S. Survival rates in NYC in the era of combination ART. J Acquir Immune Defic Syndr 2002; 30:111–8. [DOI] [PubMed] [Google Scholar]
- 22.Friedland GH, Saltzman B, Vileno J, Freeman K, Schrager LK, Klein RS. Survival differences in patients with AIDS. J Acquir Immune Defic Syndr 1991; 4:144–53. [PubMed] [Google Scholar]
- 23.Grabar S, Lanoy E, Allavena C, et al. Causes of the first AIDS-defining illness and subsequent survival before and after the advent of combined antiretroviral therapy. HIV Med 2008; 9:246–56. [DOI] [PubMed] [Google Scholar]
- 24.Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA 2001; 285:1308–15. [DOI] [PubMed] [Google Scholar]
- 25.Mocroft A, Johnson MA, Phillips AN. Factors affecting survival in patients with the acquired immunodeficiency syndrome. AIDS 1996; 10:1057–65. [PubMed] [Google Scholar]
- 26.Mocroft AJ, Lundgren JD, Monforte AD, et al. Survival of AIDS patients according to type of AIDS-defining event. Int J Epidemiol 1997; 26:400–7. [DOI] [PubMed] [Google Scholar]
- 27.Mocroft A, Sterne JA, Egger M, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis 2009; 48:1138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaisson RE, Gallant JE, Keruly JC, Moore RD. Impact of opportunistic disease on survival in patients with HIV infection. AIDS 1998; 12:29–33. [DOI] [PubMed] [Google Scholar]
- 29.Luo K, Law M, Kaldor JM, McDonald AM, Cooper DA. The role of initial AIDS-defining illness in survival following AIDS. AIDS 1995; 9:57–63. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Current trends update on acquired immune deficiency syndrome (AIDS)—United States. MMWR Morb Mortal Wkly Rep 1982; 31:507–8, 513–4. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. http://www.cdc.gov/hiv/pdf/statistics_hivsur92.pdf. Accessed 31 March 2014.
- 32.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 33.Hanna DB, Gupta LS, Jones LE, Thompson DM, Kellerman SE, Sackoff JE. AIDS-defining opportunistic illnesses in the HAART era in New York City. AIDS Care 2007; 19:264–72. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 30(suppl 1):S5–14. [DOI] [PubMed] [Google Scholar]
- 35.Gopal S, Patel MR, Yanik EL, et al. Association of early HIV viremia with mortality after HIV-associated lymphoma. AIDS 2013; 27:2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS 2013; 27:442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim PL, Zhou J, Ditangco RA, et al. Failure to prescribe Pneumocystis prophylaxis is associated with increased mortality, even in the cART era: results from the Treat Asia HIV Observational Database. J Int AIDS Soc 2012; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0. Accessed 12 May 2015.
- 39.Zucchetto A, Bruzzone S, De Paoli A, et al. AIDS and injecting drug use: survival determinants in the highly active antiretroviral therapy era. Epidemiol Prev 2009; 33:184–9. [PubMed] [Google Scholar]
- 40.Poundstone KE, Chaisson RE, Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active antiretroviral therapy. AIDS 2001; 15:1115–23. [DOI] [PubMed] [Google Scholar]
- 41.Grigoryan A, Hall HI, Durant T, Wei X. Late HIV diagnosis and determinants of progression to AIDS or death after HIV diagnosis among injection drug users, 33 US States, 1996–2004. PLoS One 2009; 4:e4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hessol NA, Kalinowski A, Benning L, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis 2007; 44:287–94. [DOI] [PubMed] [Google Scholar]
- 43.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr 2005; 38:96–103. [DOI] [PubMed] [Google Scholar]


