Abstract
Background
The 2022 mpox outbreak has infected over 30 000 people in the USA, with cases declining since mid-August. Infections were commonly associated with sexual contact between men. Interventions to mitigate the outbreak included vaccination and a reduction in sexual partnerships. Understanding the contributions of these interventions to decreasing cases can inform future public health efforts.
Methods
We fit a dynamic network transmission model to mpox cases reported by Washington DC through 10 January 2023. This model incorporated both vaccine administration data and reported reductions in sexual partner acquisition by gay, bisexual or other men who have sex with men (MSM). The model output consisted of daily cases over time with or without vaccination and/or behavioural adaptation.
Results
We found that initial declines in cases were likely caused by behavioural adaptations. One year into the outbreak, vaccination and behavioural adaptation together prevented an estimated 84% (IQR 67% to 91%) of cases. Vaccination alone averted 79% (IQR 64% to 88%) of cases and behavioural adaptation alone averted 25% (IQR 10% to 42%) of cases. We further found that in the absence of vaccination, behavioural adaptation would have reduced the number of cases, but would have prolonged the outbreak.
Conclusions
We found that initial declines in cases were likely caused by behavioural adaptation, but vaccination averted more cases overall and was key to hastening outbreak conclusion. Overall, this indicates that outreach to encourage individuals to protect themselves from infection was vital in the early stages of the mpox outbreak, but that combination with a robust vaccination programme hastened outbreak conclusion.
INTRODUCTION
Since May 2022, over 30 000 cases of mpox have been reported, with daily cases peaking in mid-August 2022.1 The majority of infections have been associated with sexual contact among gay, bisexual and other men who have sex with men (MSM).2 3 In response to the outbreak, the Centers for Disease Control and Prevention (CDC) and other public health authorities recommended behavioural adaptations and preventive vaccination after the JYNNEOS (Modified Vaccinia Ankara vaccine, Bavarian Nordic) mpox vaccine became increasingly available. In June, the CDC developed messaging for individuals seeking to reduce their chances of acquiring mpox (updated on 5 August).4 The CDC additionally worked with partner organisations, media and digital apps to communicate this information to MSM.3 Later surveys reported that 50% of MSM who engaged in one-time sexual encounters had reduced their frequency of these encounters since the outbreak began.5 In addition to behavioural interventions, the JYNNEOS vaccine became available from the Strategic National Stockpile for postexposure prophylaxis shortly after the first confirmed case of mpox on 17 May,6 7 and for pre-exposure prophylaxis for impacted communities in late June.
Understanding the relative effects of behavioural adaptations and vaccination on mpox cases can inform public health efforts for future outbreaks. A previous model suggested that the mpox outbreak could have ended due to infection-driven herd immunity, but did not incorporate behavioural adaptation or vaccination.8 We used data from Washington DC, and a dynamic network model of mpox transmission to estimate the relative effects of behavioural adaptation and vaccination on the outbreak, and the theoretical impact if vaccines had been available earlier in the outbreak.
METHODS
Model overview
We adapted a dynamic network model of sexually transmitted infections in MSM9 10 to mpox transmission.11 The network is based on national surveys of MSM distributed from 2017 to 2019,9 supplemented with prior Atlanta based surveys.12 MSM were asked about their number and type of recent sexual activities. First, survey participants were asked how many ongoing sexual partners they had who ‘took priority over others’ (‘main’ sexual contacts), and how long these partnerships had lasted. Second, survey participants were asked how many ongoing sexual partners they had who did not ‘take priority over others’ (‘casual’ sexual partnerships), and how long those partnerships had lasted. Finally, survey participants reported how often they had sexual contact with an individual exactly one time, with no prior or future sexual contact (‘one-time’ sexual contacts). Based on these survey results, individuals in the model have a set probability of having 0 or 1 ‘main’ enduring partners with a mean relationship duration of 1900 days, and 0–3 ‘casual’ enduring partners with a mean relationship duration of 930 days. Additionally, individuals in the model are assigned to one of six sexual activity groups, depending on their probability per day of engaging in one-time sexual partnerships, with activity group 1 representing the lowest sexual activity level (never engaging in one-time partnerships) and activity group 6 representing the highest sexual activity level (30% daily chance of engaging in one-time partnership). Individuals choose partners based on age and preferred sexual position during intercourse. See online supplemental table S1 for network parameters. High-activity individuals do not actively seek other high-activity individuals, but are more likely to partner with other high-activity individuals than with low-activity individuals because high-activity individuals are over-represented in the sexual mixing pool.
Individuals in the model can be susceptible to infection (S), infected but presymptomatic and non-infectious (E), presymptomatic and infectious (P), symptomatic and infectious (I), recovered and resistant (R), and vaccinated with one (V1) or two (V2) doses of JYNNEOS. Symptom onset temporarily moves individuals to one lower sexual activity level and reduces the probability of sexual contact with main and casual partners by half (though our results do not qualitatively change if symptoms do not alter partnership, online supplemental fig. S10–S13). At each timestep, the probability that an infectious individual will infect a susceptible partner is the product of the probability of sexual contact per timestep in each relationship type and the per-exposure transmission probability. On infection, an individual enters the E state, after which they have a probability per timestep of subsequent infection states. Individuals have an 80% probability of seeking medical attention on symptom onset. This probability is based on the probability of individuals with symptomatic gonorrhoea seeking treatment,13 but slightly reduced due to the unfamiliarity of the population with mpox. Those who seek care become aware of their infectious status, and so do not engage in sexual partnerships until they recover. We assume that individuals who learn their infection status have a 10% chance of infecting their main partner over the duration of the infection, reflecting prior estimates of household transmission.14 Infected individuals who seek medical care are reported as diagnosed cases, hereafter referred to as cases.
We parameterised the model to represent the mpox outbreak in Washington DC as a case study. Thus, we set the population size to be 37 400, roughly equal to the population of MSM in Washington DC.15 We additionally ran our analysis for a population size of 20 000, as not all MSM may be in the relevant sexual mixing pool (online supplemental fig. S4). The model begins with five newly infectious individuals randomly selected from individuals in activity groups 5 and 6 on 2 June. This produced a median of five individuals reporting symptom onset on 6 June, matching DC data. The length of time between symptom onset and medical attention seeking is derived from the average time between symptom onset and orthopoxvirus test reported by Washington DC, and decreases over the course of the outbreak (online supplemental fig. S3).
Vaccination
We parameterised our model with first and second doses administered in Washington DC through 18 March (online supplemental table S2). Surveys of vaccine recipients indicate that 87.6% of all vaccination dose recipients identified as MSM. Thus, we multiply the number of administered doses by 0.876.
As vaccines were initially offered to MSM with multiple recent sexual partners,16 we limit vaccine distribution to the top two sexual activity groups for the first 4 weeks of vaccination, to the top four sexual activity groups for the next 4 weeks of vaccination, and then to any individuals with a non-zero probability of engaging in one-time sexual partnerships. In our model, we vaccinate susceptible (S) and presymptomatic (E,P) individuals, though we do not model postexposure receipt effects. For second dose vaccination, we draw individuals from those who have received their first dose at least 4 weeks in the past. We assume that vaccines have no efficacy until 2 weeks after administration. In the case of breakthrough infections, individuals enter the presymptomatic (E) class and prior vaccination has no further effect on subsequent contagiousness.
In the USA, JYNNEOS has a first dose effectiveness of 75.2% and second dose effectiveness of 85.9%.17 We set these values as the per-exposure reduction in transmission probability (hereafter referred to as vaccine efficacy). We additionally run a sensitivity analysis for lower first dose and second dose effectiveness of 35.8% and 66.0%, respectively, based on independent vaccine estimates18 (online supplemental fig. S7–S9).
Behavioral adaptation
Individuals may adapt their sexual behaviour in response to their perceived risk level.19 We assume that MSM reduced their probability of one-time sexual contacts per day by a time-varying relative percentage. We assume all individuals reduce their activity by the same relative amount.
Surveys of behavioural adaptations among MSM due to mpox generally did not capture adaptations in the early stages of the outbreak and did not survey with high temporal resolution.5 Thus, to measure risk perception, we identified LGBTQ+ focused communities on the social media discussion site Reddit that contained multiple discussions about mpox. We used the number of posts and comments in these ‘subreddits’ containing the terms ‘monkeypox’, ‘mpox’ or ‘mpx’ over time (hereafter ‘Reddit activity’) as a proxy for risk perception (appendix). The relative reduction in sexual activity per day in our model was set equal to relative Reddit activity. We fit maximum reductions in sexual activity to case data (described below).
Modelling process
We fit three parameters to incident daily cases in DC: the probability of transmission per contact (μ), the maximum per cent reduction in probability of one-time sexual contact per day in response to the mpox outbreak (ω), and the number of extra high-activity individuals (activity groups 5 and 6) infected per day by extra-network contacts during a 2-week ‘surge period’ from 11–25 June 2022 (ε). This ‘surge period’ represents a time of frequent gatherings of MSM. These gatherings of MSM may generate additional mpox infections by (A) attracting visitors from other jurisdictions who may import mpox, (B) increasing the frequency of group sex events, or (C) increasing the partner turnover rate among MSM. We additionally fit our model assuming no interventions to test the likelihood that the outbreak ended due to infection-driven herd immunity alone. See appendix for a detailed fitting procedure.
We estimated the proportion of cases averted by behavioural adaptation and vaccination. We ran the model with no vaccination and no behavioural adaptation. We then compared the cumulative cases in this model until 1 June 2023 to cases in models where we include behavioural adaptation, include vaccination or include both interventions. Based on our fitting procedure (appendix), we run each intervention scenario for 100 fit parameter sets. To control for variation in model parameters, we measure the difference in cumulative cases between intervention scenarios for each parameter set individually, and then report the median and IQRs of these individual estimates. We additionally estimated cumulative cases if vaccinations had been rolled out 14 days or 28 days earlier or later, and if available vaccine doses were increased or decreased by 25% or 50%.
RESULTS
Fit parameter sets had a 51% (IQR 43% to 61%) probability of transmission per sex act (μ), 11 (IQR 6 to 16) extranetwork daily infections during the ‘surge period’ (ε) and a 39% (IQR 19% to 57%) maximum reduction in probability of one-time sexual contacts in response to the mpox outbreak (ω) (figure 1A and C). We found that a model that incorporated infection-driven herd immunity but not behavioural adaptation or vaccination could not produce a good fit to the data (figure 1B and D). See online supplemental fig. S13 for infection risk by sexual activity group.
Figure 1.
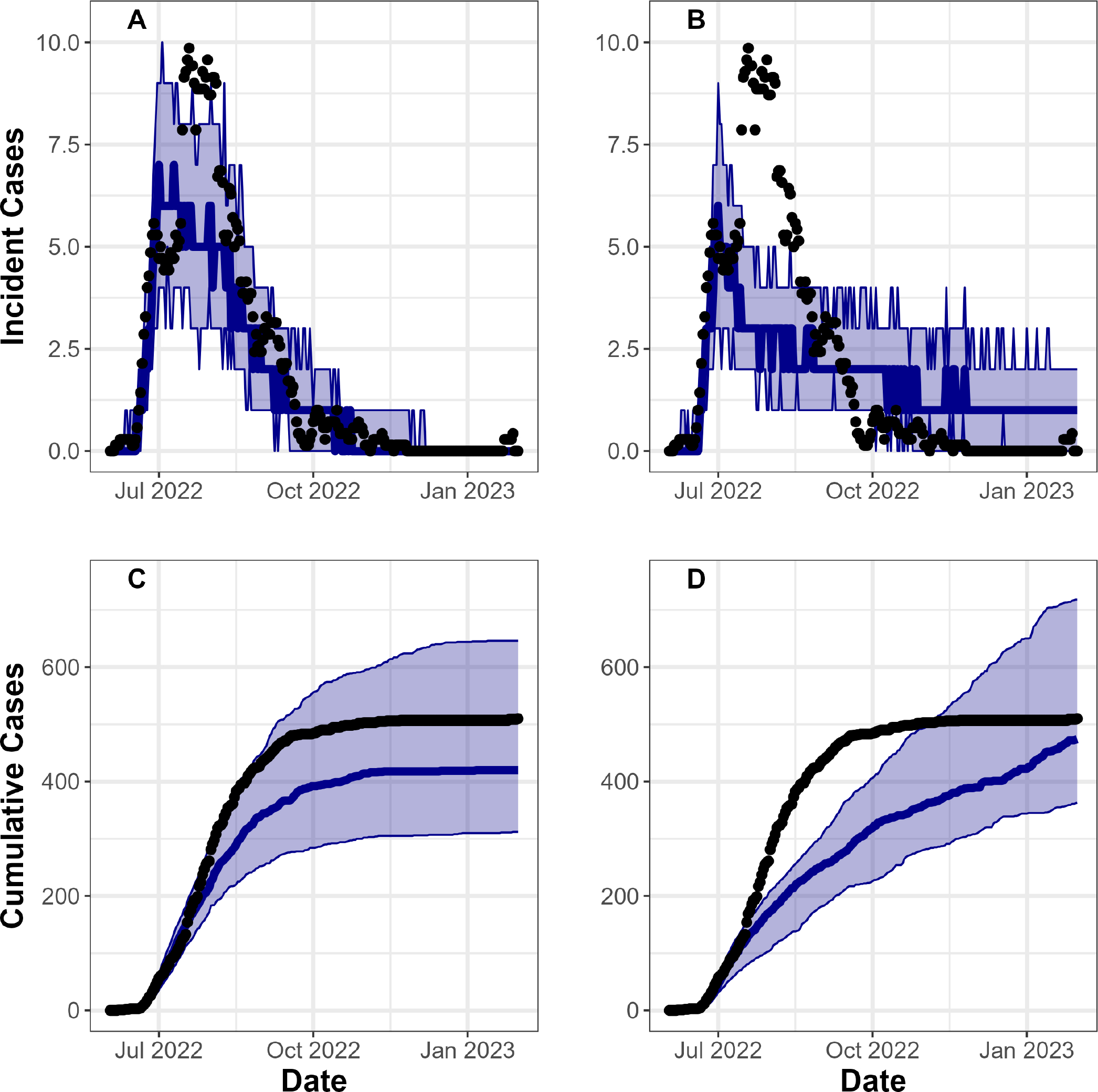
Model output fit to incident daily cases from Washington DC. Y-axis shows incident (A,B) and cumulative (C,D) cases, over date on the X-axis (Model likelihood was based on incident data in the model fitting process). Black dots represent case reports from Washington DC, while the blue line and band represent median cases and IQRs over 100 fit model parameter sets. For A and C we fit the model while incorporating vaccination and behavioural adaptations, while for B and D we fit the model without incorporating interventions.
Cumulative cases
We estimate that initial reductions in mpox cases were due to behavioural adaptation. By mid-August, our ‘behavioural adaptation only’ scenario and our ‘vaccine and behaviour’ scenario had averted a similar per cent of cases: 13% (IQR −3.5% to 30%) and 15% (−2.0% to 30%), respectively. Our ‘vaccine only’ scenario, alternatively, had only averted 3.8% (IQR −8.3% to 19%) of cases (figure 2A). Behavioural adaptation has an earlier impact on cases than vaccination for two reasons. First, behavioural adaptation began when cases first appeared in DC (online supplemental fig. S1), whereas vaccine administration did not reach more than 400 doses per week until July. Second, behavioural adaptations immediately reduce transmission, whereas vaccines offer protection only 14 days after administration.
Figure 2.
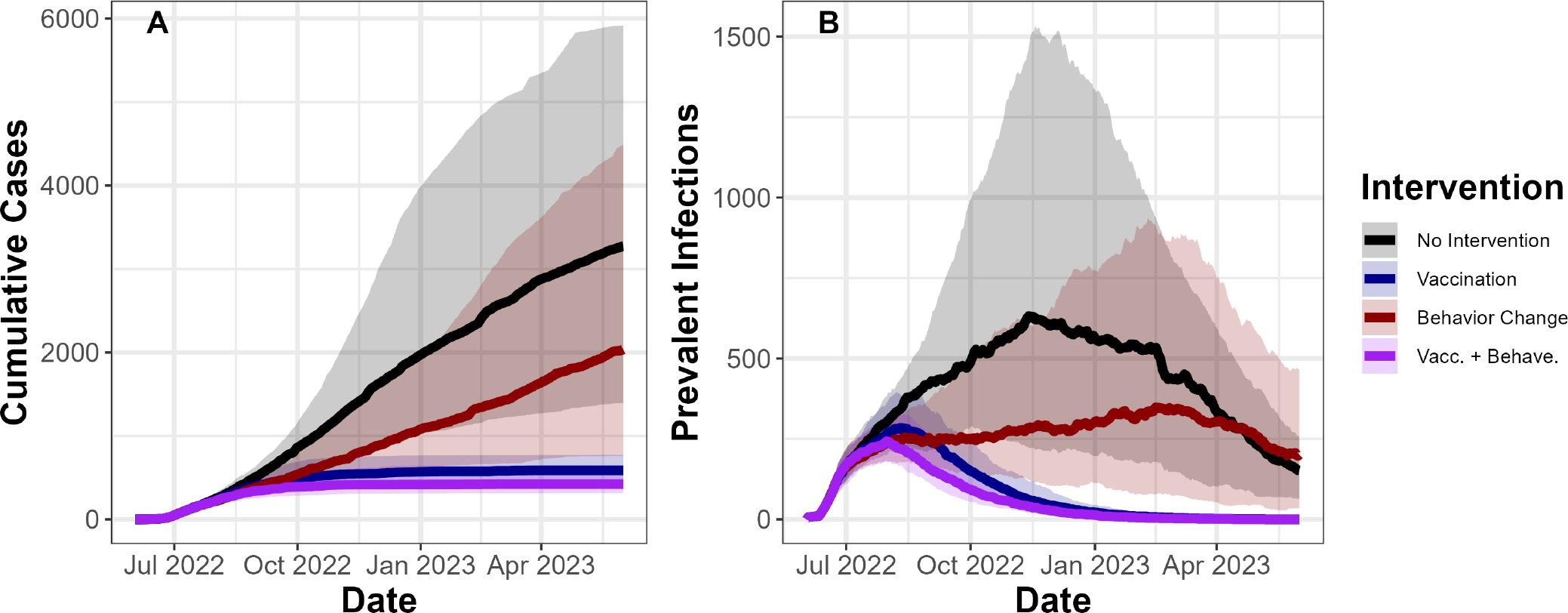
Vaccination and behavioural adaptation both reduce estimated cumulative cases and prevalent infections. (A) Y-axis shows model estimates of cumulative cases (ie, individuals who are diagnosed with mpox) over time on the X-axis from 2 June 2022 to 1 June 2023. (B) Y-axis shows prevalent infections over time on the X-axis. Solid lines indicate median values from 100 fit parameter sets, while transparent bands represent IQRs. Colours indicate intervention combinations.
We estimated that 1 year into the outbreak, vaccination will have averted more cases than behavioural adaptation. We estimated that by 2 June 2023, we would have had 3290 (IQR 1400 to 6000) cumulative case reports with no interventions. We estimated that behavioural adaptation alone would have prevented 25% (IQR 10% to 42%) of cases, vaccination alone would have prevented 79% (IQR 64% to 88%) of cases and both interventions together will have prevented 84% (IQR 67% to 91%) of cases (figure 2A).
Infection prevalence
Our analyses suggest that the outbreak would have only ended within a year with vaccination (figure 2B). After 1 year, our model estimates that there would be 151 (IQR 63 to 254) prevalent infections (defined as the number of infectious individuals on a given day) in the absence of any intervention, and 0 prevalent infections with vaccination, regardless of behavioural adaptation. Alternatively, behavioural adaptation alone would increase prevalent infection by 14% (IQR −41% to 140%) after 1 year. Behavioural adaptation can increase prevalence late in the outbreak because while vaccination replaces infection-driven herd immunity with vaccine-driven herd immunity, behavioural adaptation only delays herd immunity. Thus, 1 year into the outbreak, fewer individuals are resistant to infection in the ‘behavioural adaptation only’ scenario than in the ‘no intervention’ scenario, resulting in higher transmission and infection prevalence. If vaccinations were not available, individuals may have reduced their sexual activity for longer periods of time than we observed. However, our results hold even when we extend the period of maximum behavioural adaptation by an additional 2 months (online supplemental fig. S6).
Varying vaccine administration and efficacy
We estimated that by 1 year into the outbreak, moving the vaccination timeline earlier by 28 days would have decreased cumulative cases by 34% (IQR 24% to 46%), and moving the vaccination timeline later by 28 days would have increased cumulative cases by 27% (IQR 1.5%–65%) (figure 3A). Increasing available vaccine doses by 50% would have decreased cumulative cases by 14% (IQR −6.4% to 27%), and decreasing doses by 50% would have increased cumulative cases by 31% (IQR 5.4%–68%) (figure 3B). Thus, distributing vaccines earlier would linearly decrease cases, whereas increasing available vaccine doses would have diminishing returns.
Figure 3.
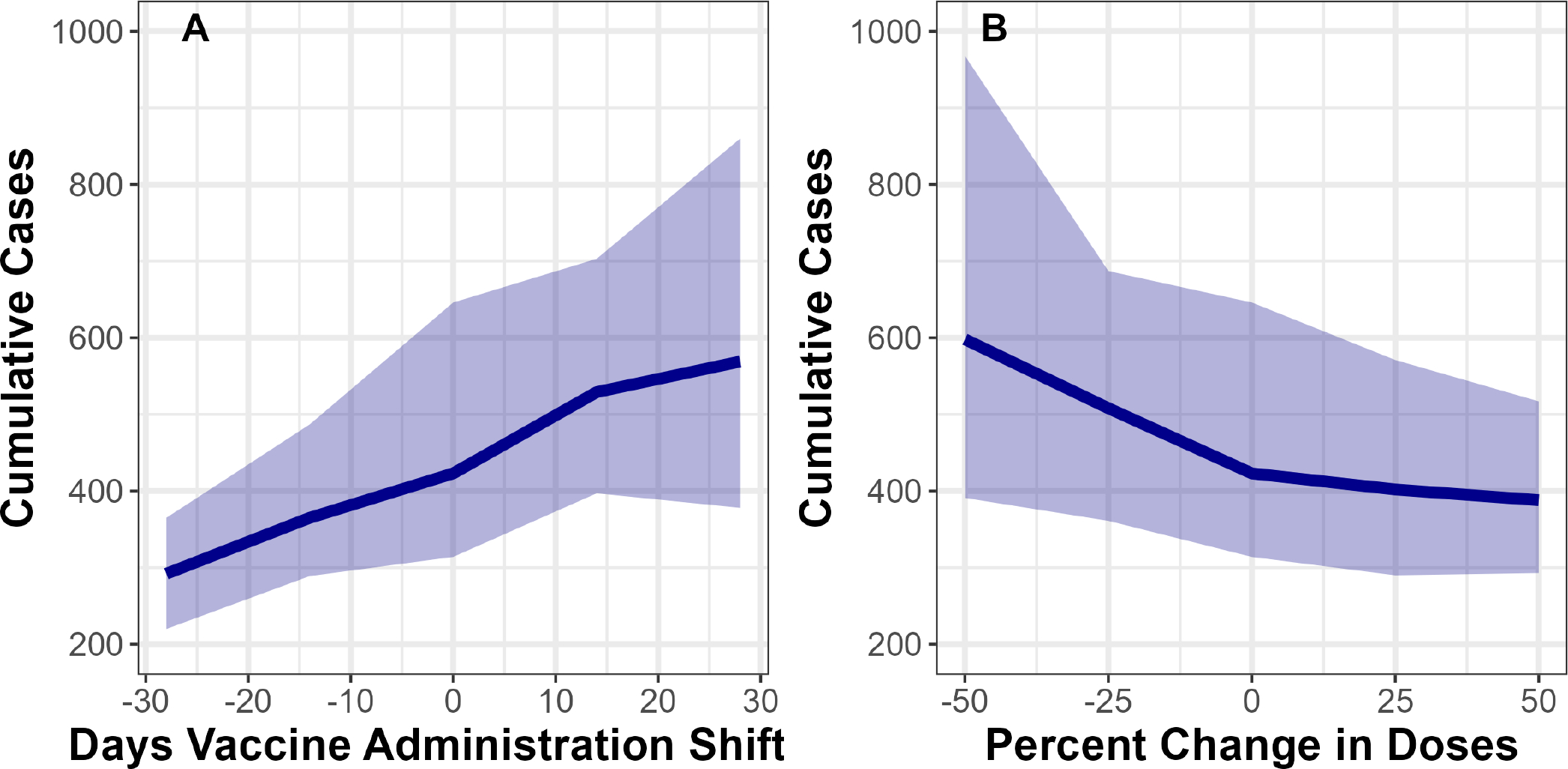
Changing number of administered doses and timing of vaccine administration can influence case reports. Y-axis shows model estimates of cumulative case counts 1 year after cases were introduced to DC, over (A) shifts in the timing of vaccine administration, and (B) per cent change in vaccine doses administered. Solid lines indicate median values from 100 fit parameter sets, while transparent bands represent IQRs. All scenarios shown here include behavioural adaptation.
We find that assuming a first and second dose vaccine efficacy of 35.8% and 66.0%, respectively, would increase the per cent of cases averted by behavioural adaptation alone 1 year into the outbreak (to 46% (IQR 27% to 59%)), decrease the per cent of cases averted by vaccination alone (to 55% (IQR 37% to 68%)) and decrease the per cent of cases averted by both interventions combined (to 69% (IQR 57% to 82%)).
DISCUSSION
We compared the relative importance of vaccination and reduction in sexual activity among MSM for averting mpox cases. We found that the majority of averted cases through mid-August were averted by behavioural adaptation, but that vaccination averted more cases overall. These results have several implications. First, national vaccine distribution is logistically time-consuming, and vaccines are not immediately effective. Thus, behavioural interventions can temporarily slow transmission, protecting communities from infection before vaccination takes effect. Partnering with affected communities is key to effective communication on behavioural interventions.20 Second, without vaccine administration, temporary behavioural interventions could have delayed, but not eliminated, mpox transmission. This means that vaccinating areas with low mpox vaccine coverage is important for both preventing future outbreaks,21 and controlling those outbreaks if they occur. Third, at early stages of an outbreak, administering vaccines sooner will provide substantial benefit in preventing cases over time and potentially have more impact than delayed administration of broader vaccination efforts that might lead to higher overall vaccine coverage.
Consistent with the current mpox outbreak, our model indicated that a combination of vaccination and behavioural adaptation would end the outbreak within a year. This may not have been the case if (A) vaccines did not reach certain segments of the population, and (B) individuals who did not or could not access vaccination preferentially partner with one another. We assumed in our model that only age and sexual positioning determines who individuals choose to partner with, and that neither of these factors influenced vaccination or treatment seeking. However, MSM partner with same-race individuals roughly 90% of the time,22 and initial administration of the mpox vaccine was lower in black individuals than in other racial groups.5 Thus, mpox transmission might persist at a low but steady level in certain populations, mirroring our ‘behavioural adaptation only’ scenario (figure 2B), if vaccine equity is not improved. This persistent transmission may be undetected if populations with ongoing transmission face barriers to testing.
Our results suggest that the mpox outbreak in DC ended in large part due to vaccination, in addition to behavioural adaptation. This contrasts with prior modelling studies showing that outbreaks ended due to a combination of infection-driven herd immunity and behavioural adaptation, with vaccination only preventing resurgence.8 23–25 Differences between these model conclusions could come from four sources. First, three of these prior studies examined outbreak dynamics in the Netherlands and the UK, respectively.23–25 Both sexual network structure and the relative timing of vaccination within the mpox outbreak differed between countries. Therefore, behavioural adaptation and vaccination may have had different roles in ending the outbreak in the Netherlands and the UK than in DC. Second, modelling varied in assumptions about the natural history of mpox. For instance, the per-act sexual transmission probability in the study by Endo et al8 is assumed to be within the range of household transmission,14 much lower than our fitted parameter. Third, variation in model conclusions may be due to how each model characterised sexual network structure. Models have explicitly simulated the most active 11.9%,25 1%23 and 0.01%24 of MSM. Thus, these studies use both courser and finer population divisions than our study, which models the most active 5% of MSM. Finally, our model may come to different conclusions about the importance of infection-driven herd immunity than Endo et al8 because Endo et al did not explicitly consider vaccination or behavioural adaptations. Future work should identify the source of differing model conclusions by applying disparate model methods to shared data sets.
We used Washington DC as a case study to examine the relative effects of vaccination and behavioural adaptation on mpox cases. Washington DC was one of the first US cities to report a high number of mpox cases. As a result, it received a higher number of vaccine doses relative to the number of sexually active MSM compared with other US cities. Therefore, the absolute and relative contributions of behavioural adaptation and vaccination to averting mpox cases may differ between Washington DC and other US cities, or other regions globally. For instance, while our model shows that vaccination was key to ending the outbreak, mpox outbreaks occurred and ended in regions with little to no vaccination.8 However, different regions likely have different sexual network structures among MSM, and vaccination may not have been necessary to end outbreaks in regions where MSM have a smaller partner turnover rate, or less contact heterogeneity. Ultimately, the results we present here are most applicable to US regions where significant transmission occured before the deployment of vaccines, but where vaccine uptake was strong.
LIMITATIONS
The sexual network used here was primarily parameterised by surveys distributed nationally from 2017 to 2019.9 These surveys thus do not capture possible ongoing impacts of the COVID-19 pandemic on sexual networks. Nor do they capture seasonal fluctuations in the sexual network due to the summer festival season, or larger group sex events which may have contributed to mpox transmission. Thus, collecting updated, temporally specific data on sexual networks of MSM and other populations experiencing high rates of sexually associated infections would improve modelling capabilities in future outbreaks.
Although substantial adaptations in sexual behaviour by MSM in response to mpox have been documented,5 we were unable to directly measure changes in one-time partnership behaviour among MSM. We used social media posts as a proxy measurement of sexual behaviour adaptation because social media posts have been empirically linked to offline behaviour,26 27 and because sexual minorities often use online spaces to to communicate with their peers.28 However, the quantitative relationship between social media posts about sexual behaviour adaptations and offline adaptations has not been measured, and may take a different form than assumed in our model.
CONCLUSIONS
This study indicates synergistic effects of sexual behavioural adaptation and vaccination in controlling the mpox outbreak. By adapting their behaviour, gay, bisexual and other men who have sex with men MSM reduced transmission before vaccines were widely available, and those reductions in sexual behaviour continue to protect individuals who are not vaccinated. We estimated that vaccination in turn will more effectively reduce infections later in the outbreak by providing long-term protection. The synergies observed when combining behavioural harm reduction and vaccination serve as a reminder for those administering mpox vaccination to use this clinical encounter to reinforce the importance of behavioural harm reduction strategies in a culturally appropriate way.4
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
Over 30 000 individuals have been infected by mpox in the USA since May of 2022, with the largest outbreaks occurring among men who have sex with men (MSM) in large cities.
Interventions to mpox transmission included behavioural adaptations among MSM and administration of the JYNNEOS vaccine.
Prior modelling work suggests that the mpox outbreak could have ended due to infection-driven herd immunity alone, but did not take into account the potential impact of behavioural adaptations or vaccination.
WHAT THIS STUDY ADDS
Using a dynamic modelling framework, we estimate that initial decreases in daily mpox cases were due to behavioural adaptation, and that vaccination was key to ending the mpox outbreak.
We further estimate that the mpox outbreak could have infected five times as many individuals in the absence of vaccination or behavioural adaptation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
By understanding the relative roles of vaccination, behavioural adaptation and infection-driven herd immunity in ending the mpox outbreak, we can better inform behavioural messaging and vaccine administration strategy in potential future mpox outbreaks.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centres for Disease Control and Prevention or the National Cancer Institute.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data and code are available upon reasonable request.
REFERENCES
- 1.US Monkeypox Case Trends Reported to CDC | Monkeypox | Poxvirus | CDC, Available: https://www.cdc.gov/poxvirus/monkeypox/response/2022/mpx-trends.html [Accessed 25 Sep 2022]. [Google Scholar]
- 2.U.S. Map & Case Count | Monkeypox | Poxvirus | CDC,. 2022Available: https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html [Accessed 25 Aug 2022]. [Google Scholar]
- 3.Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of Monkeypox cases — United States, may 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1018–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safer Sex, Social Gatherings, and Monkeypox | Monkeypox | Poxvirus | CDC, Available: https://www.cdc.gov/poxvirus/monkeypox/prevention/sexual-health.html [Accessed 25 Sep 2022]. [Google Scholar]
- 5.Delaney KP, Sanchez T, Hannah M, et al. Strategies adopted by gay, Bisexual, and other men who have sex with men to prevent Monkeypox virus transmission — United States. MMWR Morb Mortal Wkly Rep 2022;71:1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak — nine States, may 2022. MMWR Morb Mortal Wkly Rep 2022;71:764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriss JL, Boersma PM, Martin E, et al. Receipt of first and second doses of JYNNEOS vaccine for prevention of Monkeypox — United States, may 22–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo A, Murayama H, Abbott S, et al. Heavy-tailed sexual contact networks and Monkeypox epidemiology in the global outbreak. Science 2022;378:90–4. [DOI] [PubMed] [Google Scholar]
- 9.Weiss KM, Goodreau SM, Morris M, et al. Egocentric sexual networks of men who have sex with men in the United States: results from the Artnet study. Epidemics 2020;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenness SM, Goodreau SM, Rosenberg E, et al. Impact of the centers for disease control’s HIV Preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis 2016;214:1800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spicknall IH, Pollock ED, Clay PA, et al. Modeling the impact of sexual networks in the transmission of Monkeypox virus among gay, Bisexual, and other men who have sex with men — United States. MMWR Morb Mortal Wkly Rep 2022;71:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of Gonorrhea and Chlamydia following human immunodeficiency virus Preexposure prophylaxis among men who have sex with men: A modeling study. Clin Infect Dis 2017;65:712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Preventive Medicine 2003;36:502–9. [DOI] [PubMed] [Google Scholar]
- 14.Beer EM, Rao VB. A systematic review of the epidemiology of human Monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 2019;13:e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grey JA, Bernstein KT, Sullivan PS, et al. n.d. Estimating the population sizes of men who have sex with men in US States and counties using data from the American community survey. JMIR Public Health Surveill;2:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FACT SHEET: Biden-Harris Administration’s Monkeypox Outbreak Response | The White House, Available: https://www.whitehouse.gov/briefing-room/statements-releases/2022/06/28/fact-sheet-biden-harris-administrations-monkeypox-outbreak-response/ [Accessed 1 Nov 2022]. [Google Scholar]
- 17.Dalton AF, Diallo AO, Chard AN, et al. Estimated effectiveness of JYNNEOS vaccine in preventing Mpox: A Multijurisdictional case-control study — United States. MMWR Morb Mortal Wkly Rep 2022;72:553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deputy NP, Deckert J, Chard AN, et al. Vaccine effectiveness of JYNNEOS against Mpox disease in the United States. N Engl J Med 2023;388:2434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton LA, Kalichman SC. Risk compensation in HIV prevention: implications for vaccines, Microbicides, and other BIOMEDICAL HIV prevention Technologies. Curr HIV/AIDS Rep 2007;4:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC’s Response to the 2022 Monkeypox Outbreak | Monkeypox | Poxvirus | CDC, Available: https://www.cdc.gov/poxvirus/monkeypox/about/cdc-response.html [Accessed 9 Nov 2022]. [Google Scholar]
- 21.Pollock ED, Clay PA, Keen A, et al. Potential for recurrent Mpox outbreaks among gay, Bisexual, and other men who have sex with men — United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Romieu AC, Sullivan PS, Rothenberg R, et al. Heterogeneity of HIV prevalence among the sexual networks of black and white MSM in Atlanta: illuminating a mechanism for increased HIV risk for young black MSM. Sex Transm Dis 2015;42:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiridou M, Miura F, Adam P, et al. The fading of the mpox outbreak among men who have sex with men: a mathematical modelling study. Public and Global Health [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand SPC, Cavallaro M, Cumming F, et al. The role of vaccination and public awareness in forecasts of Mpox incidence in the United Kingdom. Nat Commun 2023;14:4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X-S, Mandal S, Mohammed H, et al. Transmission Dynamics and effect of control measures on the 2022 outbreak of Mpox among gay, Bisexual, and other men who have sex with men in England: a mathematical Modelling study. The Lancet Infectious Diseases September 2023. [DOI] [PubMed] [Google Scholar]
- 26.Youyou W, Kosinski M, Stillwell D. Computer-based personality judgments are more accurate than those made by humans. Proc Natl Acad Sci U S A 2015;112:1036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guntuku SC, Yaden DB, Kern ML, et al. Detecting depression and mental illness on social media: an integrative review. Current Opinion in Behavioral Sciences 2017;18:43–9. [Google Scholar]
- 28.Wignall L The sexual use of a social networking site: the case of pup Twitter. Sociological Research Online 2017;22:21–37. 10.1177/1360780417724066 Available: https://doi.org/101177/1360780417724066 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Data and code are available upon reasonable request.


